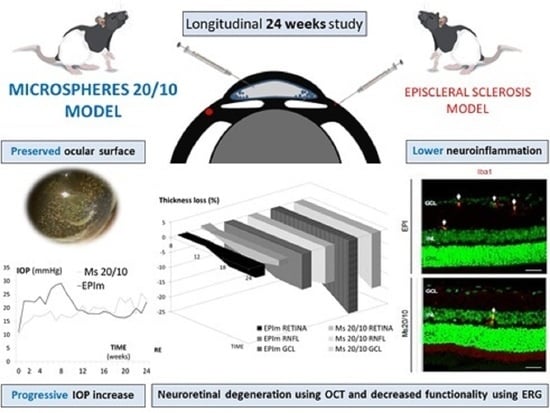
Chronic Glaucoma Using Biodegradable Microspheres to Induce Intraocular Pressure Elevation. Six-Month Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods, Intervention, or Testing
2.2. Animal Welfare and Anesthesia
2.3. Injection Procedure for Induced Ocular Hypertension
2.4. Clinical Signs and Intraocular Pressure Measurements
2.5. Neuroretinal Examination
2.5.1. In Vivo Optical Coherence Tomography
2.5.2. In Vivo Electroretinography
2.5.3. Histology
2.6. Statistical Analysis
3. Results
3.1. Ophthalmological Signs and IOP
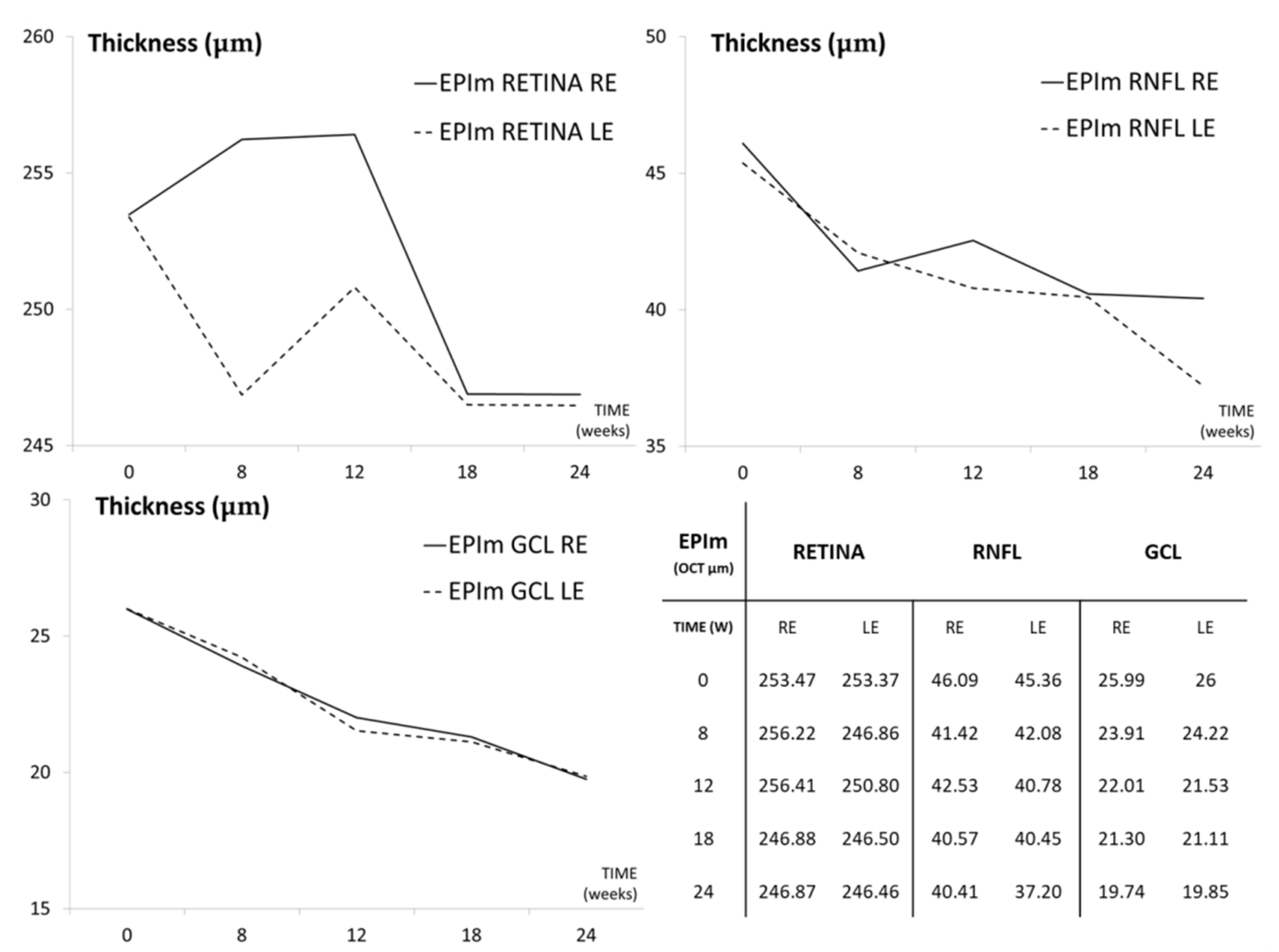
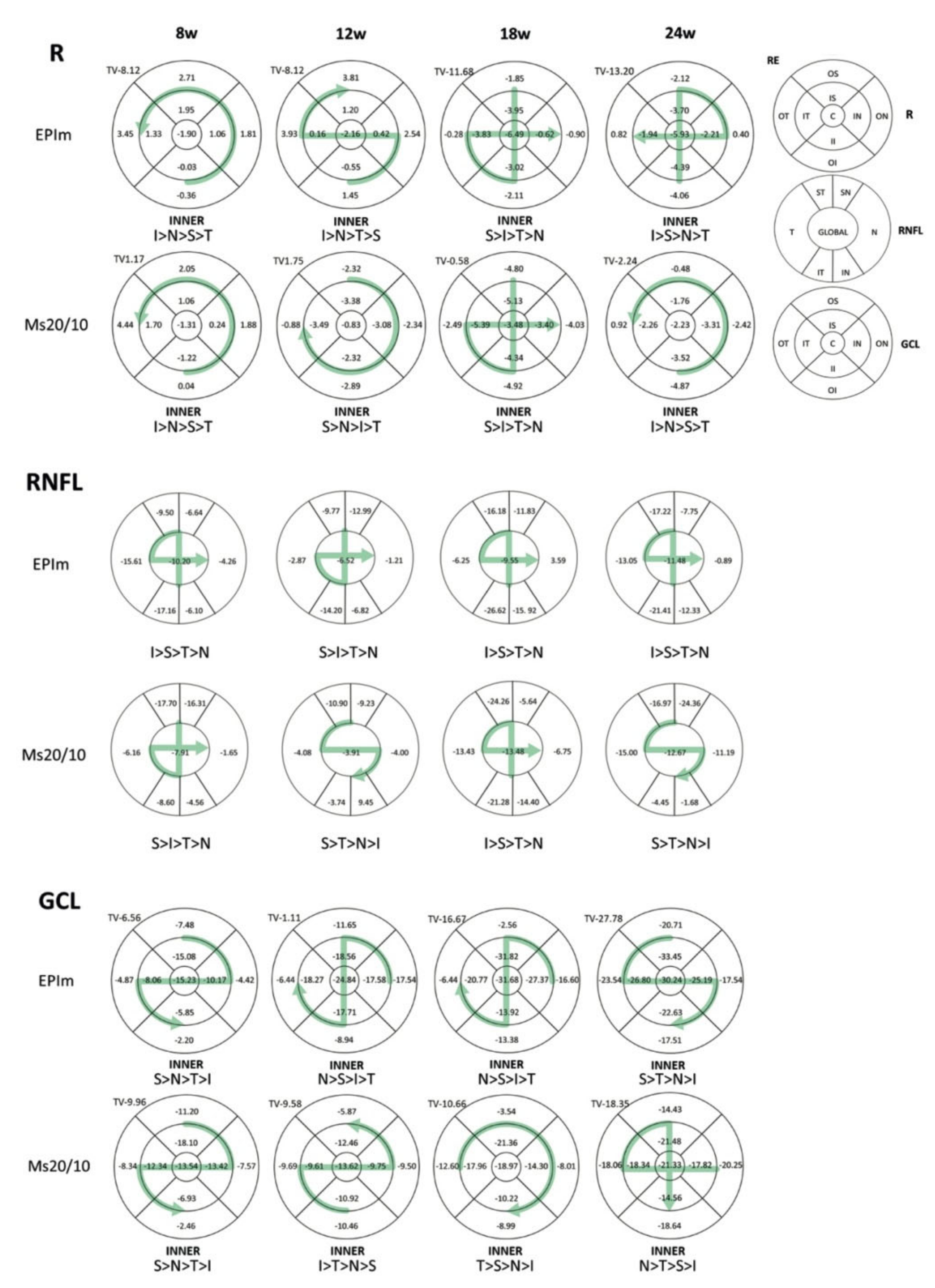
3.2. In Vivo Neuroretinal Analysis by Optical Coherence Tomography
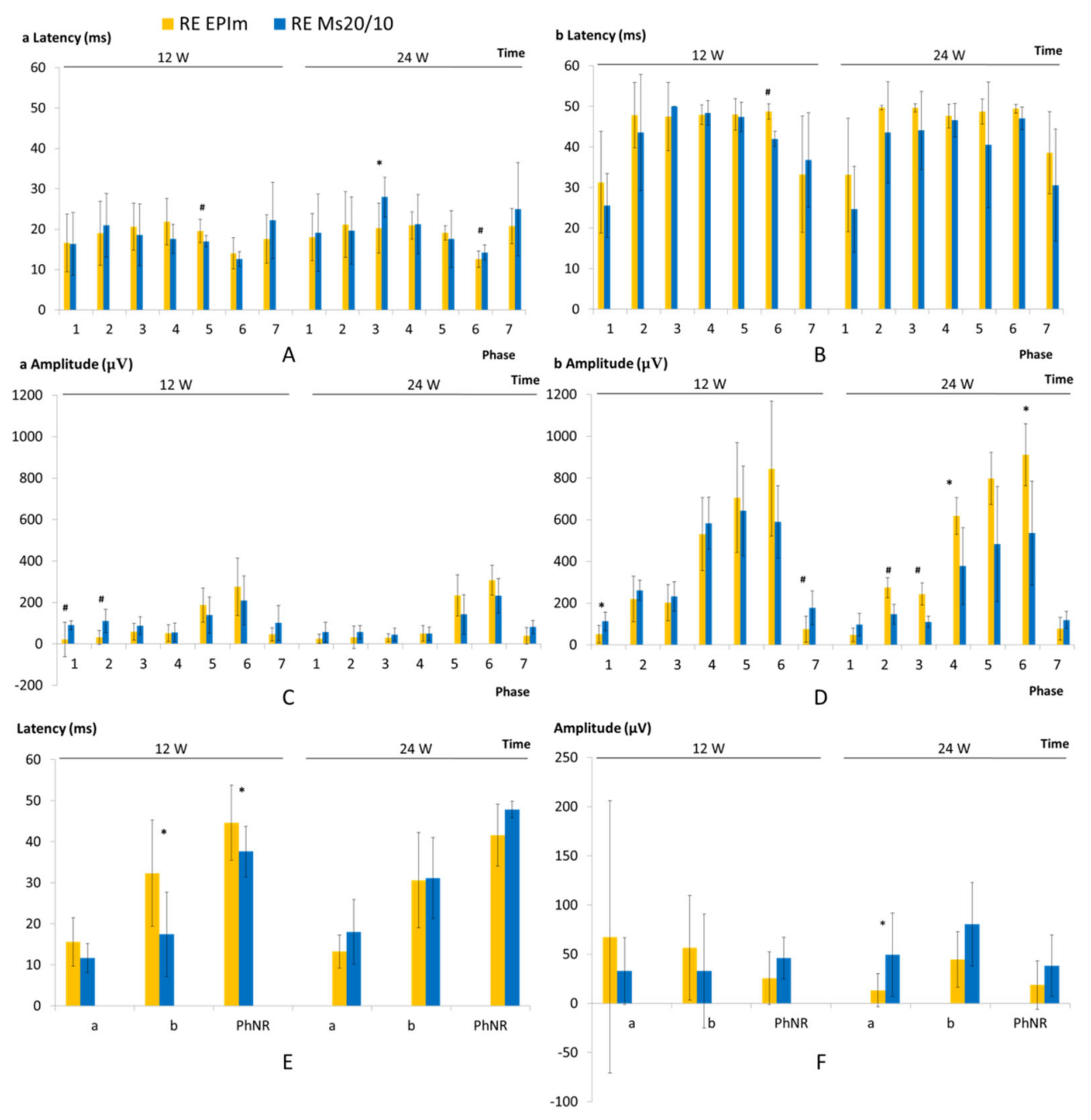
3.3. In Vivo Electroretinogram
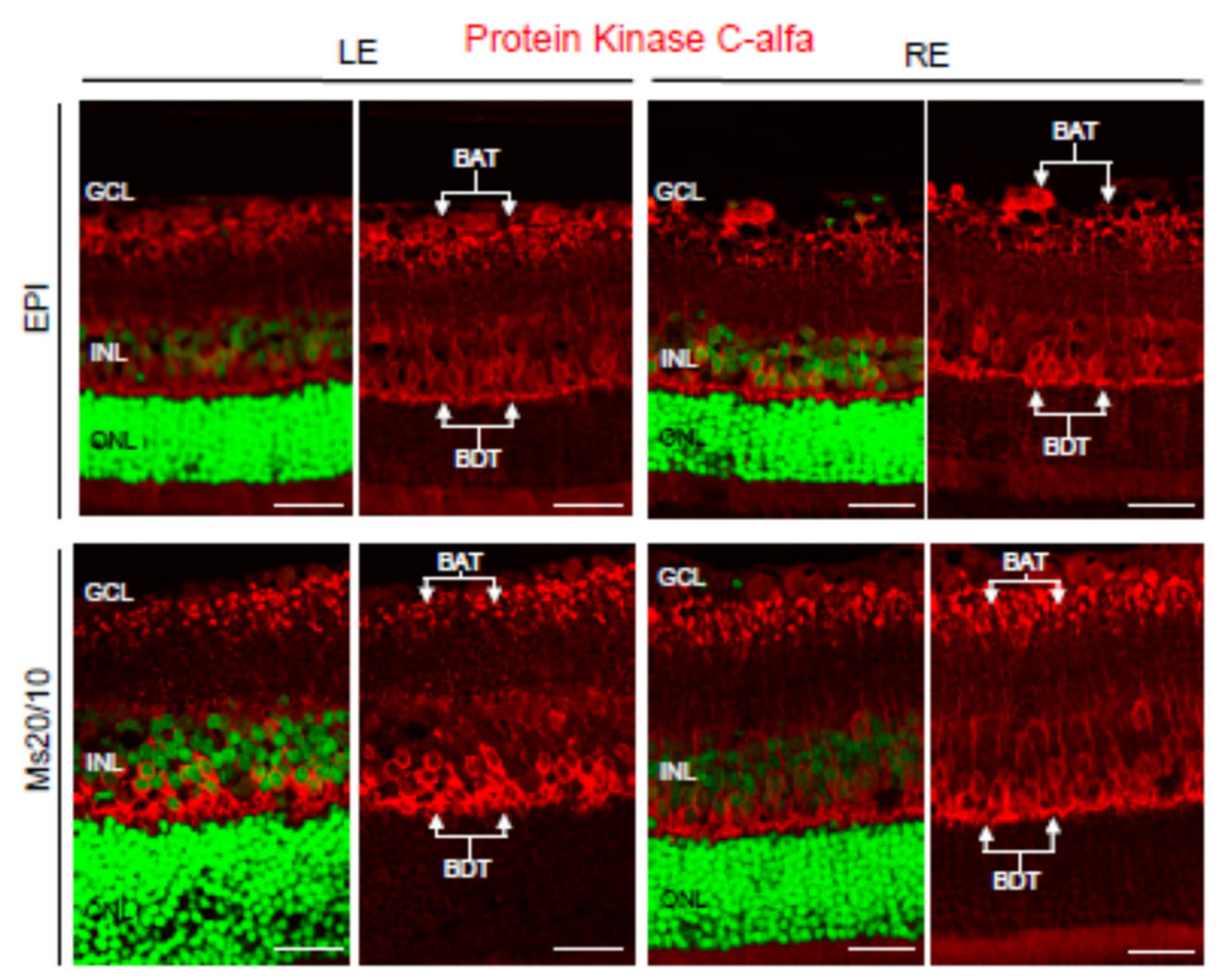
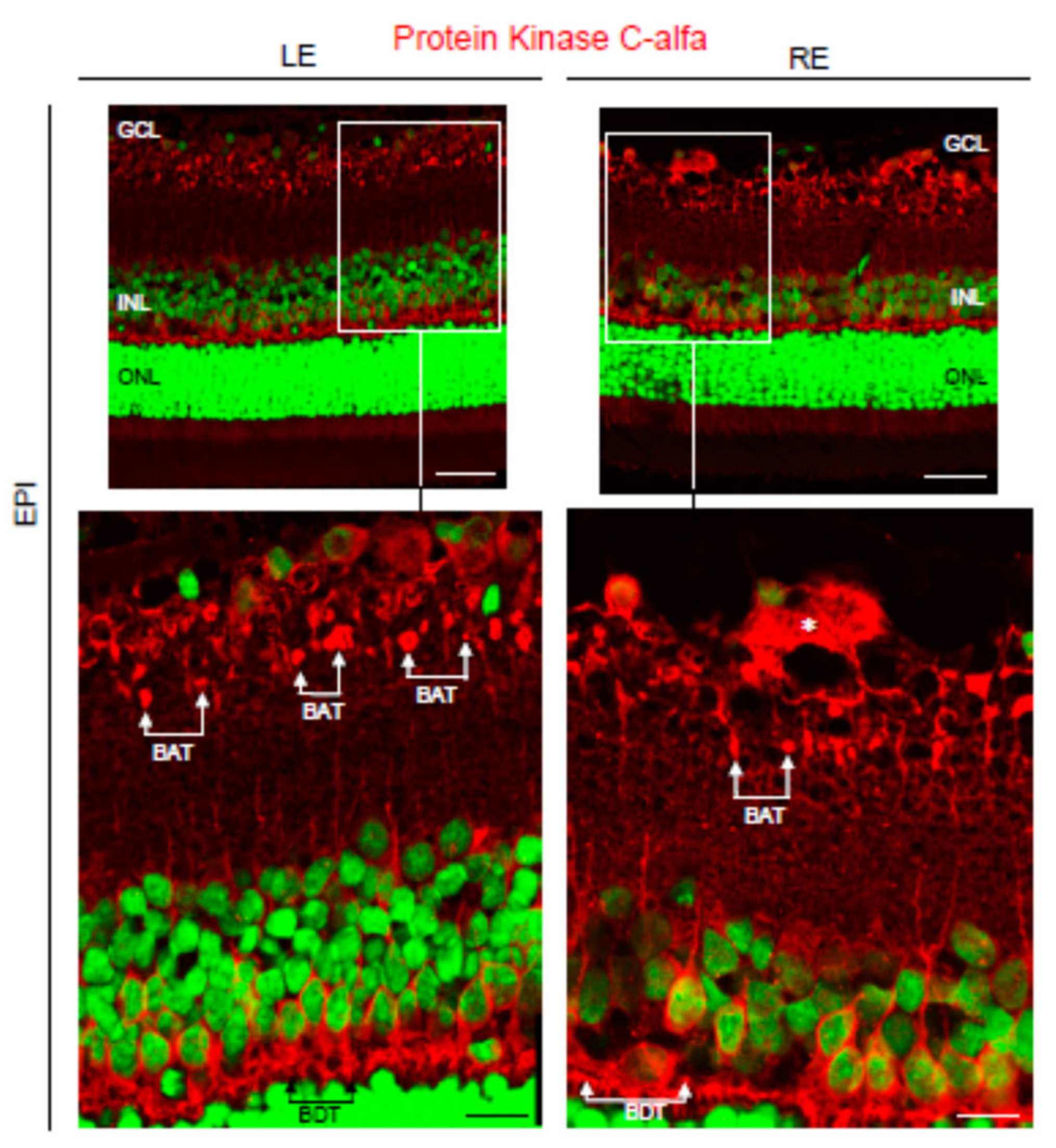
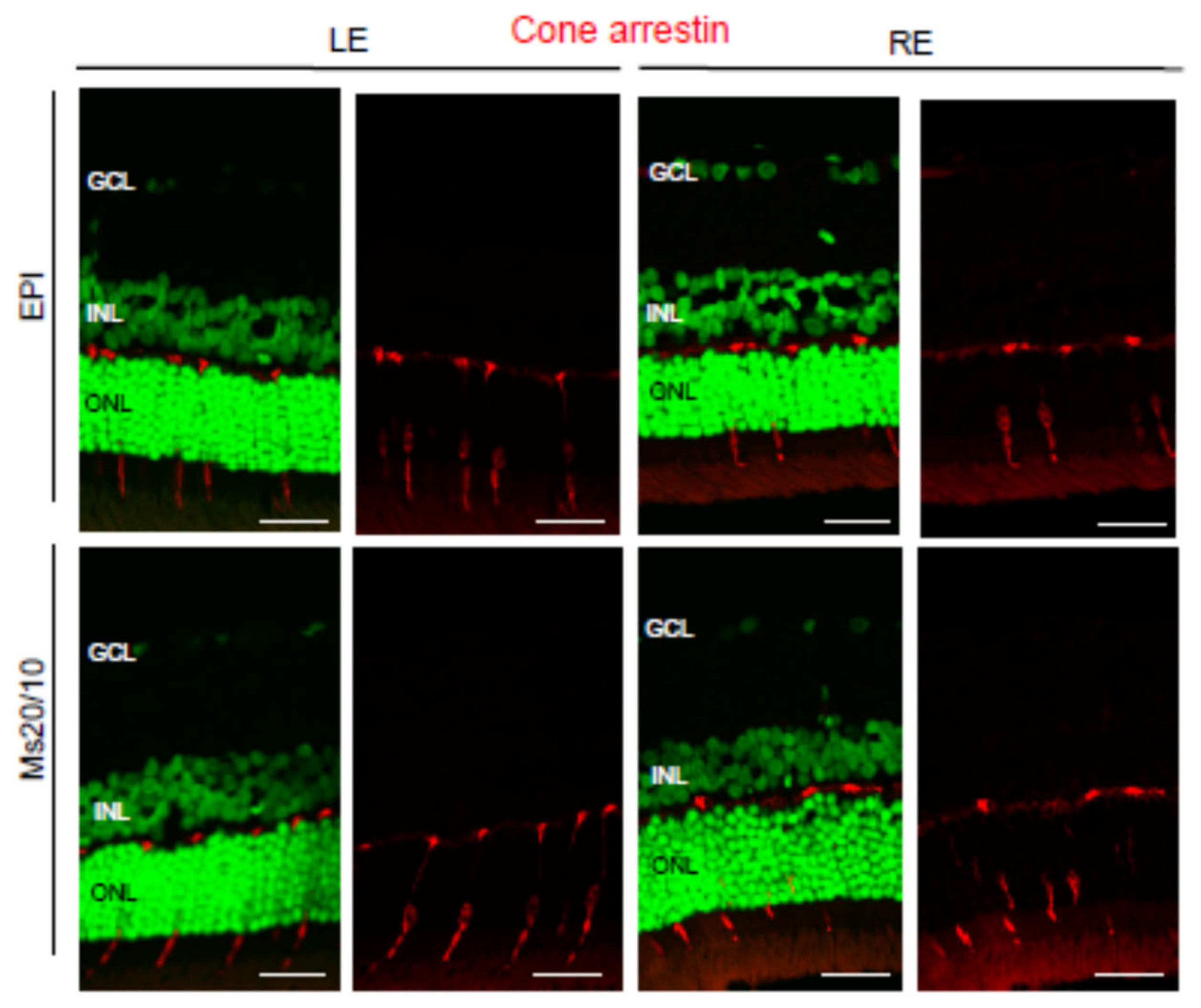
3.4. Pathological Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quigley, H.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- Lau, L.I.; Liu, C.J.L.; Chou, J.C.K.; Hsu, W.M.; Liu, J.H. Patterns of visual field defects in chronic angle-closure glaucoma with different disease severity. Ophthalmology 2003, 110, 1890–1894. [Google Scholar] [CrossRef]
- Ederer, F.; Face Gaasterland, D.; Sullivan, E.K.; Beck, A.; E Prum, B.; Cyrlin, M.N.; Weiss, H. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration.The AGIS Investigators. Am. J. Ophthalmol. 2000, 130, 429–440. [Google Scholar] [CrossRef]
- Choi, D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988, 1, 623–634. [Google Scholar] [CrossRef]
- Lucas, D.R.; Newhouse, J.P. The toxic effect of sodium L-glutamate on the inner layers of the retina. AMA. Arch. Ophthalmol. 1957, 58, 193–201. [Google Scholar] [CrossRef]
- Neufeld, A.H. Nitric oxide: A potential mediator of retinal ganglion cell damage in glaucoma. Surv. Ophthalmol. 1999, 43 (Suppl. 1), S129–S135. [Google Scholar] [CrossRef]
- Lee, D.; Shim, M.S.; Kim, K.-Y.; Noh, Y.H.; Kim, H.; Kim, S.Y.; Weinreb, R.N.; Ju, W.-K. Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 993–1005. [Google Scholar] [CrossRef]
- Yang, L.; Li, S.; Miao, L.; Huang, H.; Liang, F.; Teng, X.; Xu, L.; Wang, Q.; Xiao, W.; Ridder, W.H.; et al. Rescue of glaucomatous neurodegeneration by differentially modulating neuronal endoplasmic reticulum stress molecules. J. Neurosci. 2016, 36, 5891–5903. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Smith, R.S.; Hawes, N.L.; Anderson, M.G.; Zabaleta, A.; Savinova, O.; Roderick, T.H.; Heckenlively, J.R.; Davisson, M.T.; John, S.W.M. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nat. Genet. 1999, 21, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Schlamp, C.L.; Li, Y.; Dietz, J.A.; Janssen, K.T.; Nickells, R.W. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci 2006, 7, 66. [Google Scholar] [CrossRef]
- Dey, A.; Manthey, A.L.; Chiu, K.; Do, C.W. Methods to Induce Chronic Ocular Hypertension: Reliable Rodent Models as a Platform for Cell Transplantation and Other Therapies. Cell Transplant. 2018, 27, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.E.; Tribble, J.R. Microbead models in glaucoma. Exp. Eye Res. 2015, 141, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.C.; Moore, C.G.; Deppmeier, L.M.H.; Gold, B.G.; Meshul, C.K.; Johnson, E.C. A rat model of chronic pressure-induced optic nerve damage. Exp. Eye Res. 1997, 64, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Samsel, P.A.; Kisiswa, L.; Erichsen, J.T.; Cross, S.D.; Morgan, J.E. A novel method for the induction of experimental glaucoma using magnetic microspheres. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1671–1675. [Google Scholar] [CrossRef] [PubMed]
- Smedowski, A.; Pietrucha-Dutczak, M.; Kaarniranta, K.; Lewin-Kowalik, J. A rat experimental model of glaucoma incorporating rapid-onset elevation of intraocular pressure. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Mukai, R.; Park, D.H.; Okunuki, Y.; Hasegawa, E.; Klokman, G.; Kim, C.B.; Krishnan, A.; Gregory-Ksander, M.; Husain, D.; Miller, J.W.; et al. Mouse model of ocular hypertension with retinal ganglion cell degeneration. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Davis, B.M.; Crawley, L.; Pahlitzsch, M.; Javaid, F.; Cordeiro, M.F. Glaucoma: The retina and beyond. Acta Neuropathol. 2016, 132, 807–826. [Google Scholar] [CrossRef]
- Garcia-Herranz, D.; Rodrigo, M.J.; Subias, M.; Martinez-Rincon, T.; Mendez-Martinez, S.; Bravo-Osuna, I.; Bonet, A.; Ruberte, J.; Garcia-Feijoo, J.; Pablo, L.; et al. Novel Use of PLGA Microspheres to Create an Animal Model of Glaucoma with Progressive Neuroretinal Degeneration. Pharmaceutics 2021, 13, 237. [Google Scholar] [CrossRef]
- Morrison, J.C.; Cepurna, W.O.; Johnson, E.C. Modeling glaucoma in rats by sclerosing aqueous outflow pathways to elevate intraocular pressure. Exp. Eye Res. 2015, 141, 23–32. [Google Scholar] [CrossRef]
- Sappington, R.M.; Carlson, B.J.; Crish, S.D.; Calkins, D.J. The microbead occlusion model: A paradigm for induced ocular hypertension in rats and mice. Investig. Ophthalmol. Vis. Sci. 2010, 51, 207–216. [Google Scholar] [CrossRef]
- Lozano, D.C.; Hartwick, A.T.E.; Twa, M.D. Circadian rhythm of intraocular pressure in the adult rat. Chronobiol. Int. 2015, 32, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Photocoagulation for Diabetic Macular Edema. Arch. Ophthalmol. 1985, 103, 1796. [CrossRef]
- Ding, C.; Wang, P.; Tian, N. Effect of general anesthetics on IOP in elevated IOP mouse model. Exp. Eye Res. 2011, 92, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Sarthy, P.V.; Fu, M.; Huang, J. Developmental expression of the Glial fibrillary acidic protein (GFAP) gene in the mouse retina. Cell. Mol. Neurobiol. 1991, 11, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Gerhardinger, C.; Lorenzi, M. Muller cell changes in human diabetic retinopathy. Diabetes 1998, 47, 445–449. [Google Scholar] [CrossRef]
- Hernandez, M.R.; Miao, H.; Lukas, T. Astrocytes in glaucomatous optic neuropathy. Prog. Brain Res. 2008, 173, 353–373. [Google Scholar] [PubMed]
- Chen, C.T.; Alyahya, K.; Gionfriddo, J.R.; Dubielzig, R.R.; Madl, J.E. Loss of glutamine synthetase immunoreactivity from the retina in canine primary glaucoma. Vet. Ophthalmol. 2008, 11, 150–157. [Google Scholar] [CrossRef]
- Gionfriddo, J.R.; Freeman, K.S.; Groth, A.; Scofield, V.L.; Alyahya, K.; Madl, J.E. α-Luminol prevents decreases in glutamate, glutathione, and glutamine synthetase in the retinas of glaucomatous DBA/2J mice. Vet. Ophthalmol. 2009, 12, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Pannicke, T.; Biedermann, B.; Francke, M.; Iandiev, I.; Grosche, J.; Wiedemann, P.; Albrecht, J.; Reichenbach, A. Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochem. Int. 2009, 54, 143–160. [Google Scholar] [CrossRef]
- Imai, Y.; Ibata, I.; Ito, D.; Ohsawa, K.; Kohsaka, S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem. Biophys. Res. Commun. 1996, 224, 855–862. [Google Scholar] [CrossRef]
- Haverkamp, S.; Ghosh, K.K.; Hirano, A.A.; Wässle, H. Immunocytochemical description of five bipolar cell types of the mouse retina. J. Comp. Neurol. 2003, 455, 463–476. [Google Scholar] [CrossRef]
- Shen, Y.; Luo, X.; Liu, S.; Shen, Y.; Nawy, S.; Shen, Y. Rod bipolar cells dysfunction occurs before ganglion cells loss in excitotoxin-damaged mouse retina. Cell Death Dis. 2019, 10, 905. [Google Scholar] [CrossRef]
- Biswas, S.; Wan, K.H. Review of rodent hypertensive glaucoma models. Acta Ophthalmol. 2019, 97, e331–e340. [Google Scholar] [CrossRef]
- Ito, Y.A.; Belforte, N.; Cueva Vargas, J.L.; di Polo, A. A magnetic microbead occlusion model to induce ocular hypertension-dependent glaucoma in mice. J. Vis. Exp. 2016, 2016, 53731. [Google Scholar] [CrossRef]
- Herrero-Vanrell, R.; Bravo-Osuna, I.; Andrés-Guerrero, V.; Vicario-de-la-Torre, M.; Molina-Martínez, I.T. The potential of using biodegradable microspheres in retinal diseases and other intraocular pathologies. Prog. Retin. Eye Res. 2014, 42, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef]
- Arranz-Romera, A.; Esteban-Pérez, S.; Garcia-Herranz, D.; Aragón-Navas, A.; Bravo-Osuna, I.; Herrero-Vanrell, R. Combination therapy and co-delivery strategies to optimize treatment of posterior segment neurodegenerative diseases. Drug Discov. Today 2019, 24, 1644–1653. [Google Scholar] [CrossRef]
- Guo, L.; Normando, E.M.; Nizari, S.; Lara, D.; Francesca Cordeiro, M. Tracking longitudinal retinal changes in experimental ocular hypertension using the cSLO and spectral domain-OCT. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6504–6513. [Google Scholar] [CrossRef]
- Porciatti, V. Electrophysiological assessment of retinal ganglion cell function. Exp. Eye Res. 2014, 141, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Jeffrey, B.G.; Messias, A.M.V.; Robson, A.G. ISCEV extended protocol for the stimulus–response series for the dark-adapted full-field ERG b-wave. Doc. Ophthalmol. 2019, 138, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, N.; Fernández-Sánchez, L.; Sauvé, Y.; Segura, F.J.; Martínez-Navarrete, G.; Tamarit, J.M.; Fuentes-Broto, L.; Sanchez-Cano, A.; Pinilla, I. Correlation between SD-OCT, immunocytochemistry and functional findings in an animal model of retinal degeneration. Front. Neuroanat. 2014, 8, 1–20. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Martinez-Rincon, T.; Subias, M.; Mendez-Martinez, S.; Luna, C.; Pablo, L.E.; Polo, V.; Garcia-Martin, E. Effect of age and sex on neurodevelopment and neurodegeneration in the healthy eye: Longitudinal functional and structural study in the Long–Evans rat. Exp. Eye Res. 2020, 200, 108208. [Google Scholar] [CrossRef]
- Nadal-Nicolás, F.M.; Jiménez-López, M.; Sobrado-Calvo, P.; Nieto-López, L.; Cánovas-Martinez, I.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo, M. Brn3a as a marker of retinal ganglion cells: Qualitative and quantitative time course studies in naïve and optic nerve-injured retinas. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3860–3868. [Google Scholar] [CrossRef]
- Chen, H.; Wei, X.; Cho, K.S.; Chen, G.; Sappington, R.; Calkins, D.J.; Chen, D.F. Optic neuropathy due to microbead-induced elevated intraocular pressure in the mouse. Investig. Ophthalmol. Vis. Sci. 2011, 52, 36–44. [Google Scholar] [CrossRef]
- Sapienza, A.; Raveu, A.-L.; Reboussin, E.; Roubeix, C.; Boucher, C.; Dégardin, J.; Godefroy, D.; Rostène, W.; Goazigo, R.-L.A.; Baudouin, C.; et al. Bilateral neuroinflammatory processes in visual pathways induced by unilateral ocular hypertension in the rat. J. Neuroinflammation 2016, 13, 44. [Google Scholar] [CrossRef]
- Ramirez, A.I.; de Hoz, R.; Salobrar-Garcia, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; López-Cuenca, I.; Rojas, P.; Triviño, A.; Ramírez, J.M. The role of microglia in retinal neurodegeneration: Alzheimer’s disease, Parkinson, and glaucoma. Front. Aging Neurosci. 2017, 9, 1–21. [Google Scholar] [CrossRef]
- Downs, J.C. Optic nerve head biomechanics in aging and disease. Exp. Eye Res. 2015, 133, 19–29. [Google Scholar] [CrossRef]
- Howell, G.R.; Libby, R.T.; Jakobs, T.C.; Smith, R.S.; Phalan, F.C.; Barter, J.W.; Barbay, J.M.; Marchant, J.K.; Mahesh, N.; Porciatti, V.; et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J. Cell Biol. 2007, 179, 1523–1537. [Google Scholar] [CrossRef]
- Lin, B.; Wang, S.W.; Masland, R.H. Retinal ganglion cell type, size, and spacing can be specified independent of homotypic dendritic contacts. Neuron 2004, 43, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Shou, T.; Liu, J.; Wang, W.; Zhou, Y.; Zhao, K. Differential dendritic shrinkage of α and β retinal ganglion cells in cats with chronic glaucoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3005–3010. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Thanos, S. Differential increases in rat retinal ganglion cell size with various methods of optic nerve lesion. Neurosci. Lett. 1996, 207, 117–120. [Google Scholar] [CrossRef]
- de Hoz, R.; Ramírez, A.I.; González-Martín, R.; Ajoy, D.; Rojas, B.; Salobrar-Garcia, E.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; et al. Bilateral early activation of retinal microglial cells in a mouse model of unilateral laser-induced experimental ocular hypertension. Exp. Eye Res. 2018, 171, 12–29. [Google Scholar] [CrossRef]
- Gallego, B.I.; Salazar, J.J.; de Hoz, R.; Rojas, B.; Ramírez, A.I.; Salinas-Navarro, M.; Ortín-Martínez, A.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Perez, M.P.; et al. IOP induces upregulation of GFAP and MHC-II and microglia reactivity in mice retina contralateral to experimental glaucoma. J. Neuroinflammation 2012, 9, 586. [Google Scholar] [CrossRef]
- Chen, H.; Cho, K.S.; Vu, T.H.K.; Shen, C.H.; Kaur, M.; Chen, G.; Mathew, R.; McHam, M.L.; Fazelat, A.; Lashkari, K.; et al. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Lawlor, M.; Danesh-Meyer, H.; Levin, L.A.; Davagnanam, I.; De Vita, E.; Plant, G.T. Glaucoma and the brain: Trans-synaptic degeneration, structural change, and implications for neuroprotection. Surv. Ophthalmol. 2018, 63, 296–306. [Google Scholar] [CrossRef]
- Tezel, G. The immune response in glaucoma: A perspective on the roles of oxidative stress. Exp. Eye Res. 2011, 93, 178–186. [Google Scholar] [CrossRef]
- Pronin, A.; Pham, D.; An, W.; Dvoriantchikova, G.; Reshetnikova, G.; Qiao, J.; Kozhekbaeva, Z.; Reiser, A.E.; Slepak, V.Z.; Shestopalov, V.I. Inflammasome Activation Induces Pyroptosis in the Retina Exposed to Ocular Hypertension Injury. Front. Mol. Neurosci. 2019, 12, 36. [Google Scholar] [CrossRef]
- Bosco, A.; Romero, C.O.; Breen, K.T.; Chagovetz, A.A.; Steele, M.R.; Ambati, B.K.; Vetter, M.L. Neurodegeneration severity can be predicted from early microglia alterations monitored in vivo in a mouse model of chronic glaucoma. Dis. Model. Mech. 2015, 8, 443–455. [Google Scholar] [CrossRef]
- Damisah, E.C.; Hill, R.A.; Rai, A.; Chen, F.; Rothlin, C.V.; Ghosh, S.; Grutzendler, J. Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Sci Adv. 2020, 6, eaba3239. [Google Scholar] [CrossRef]


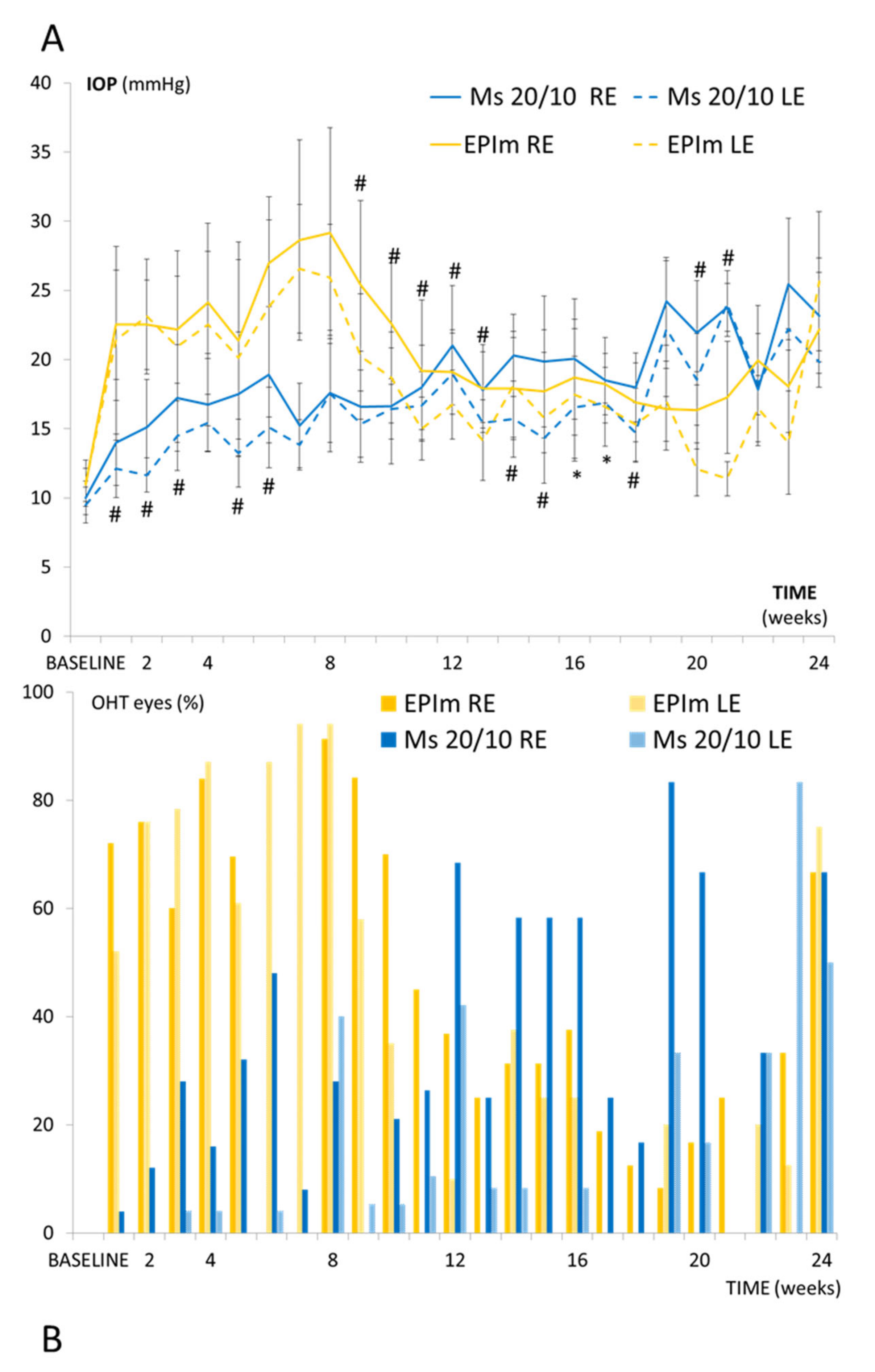
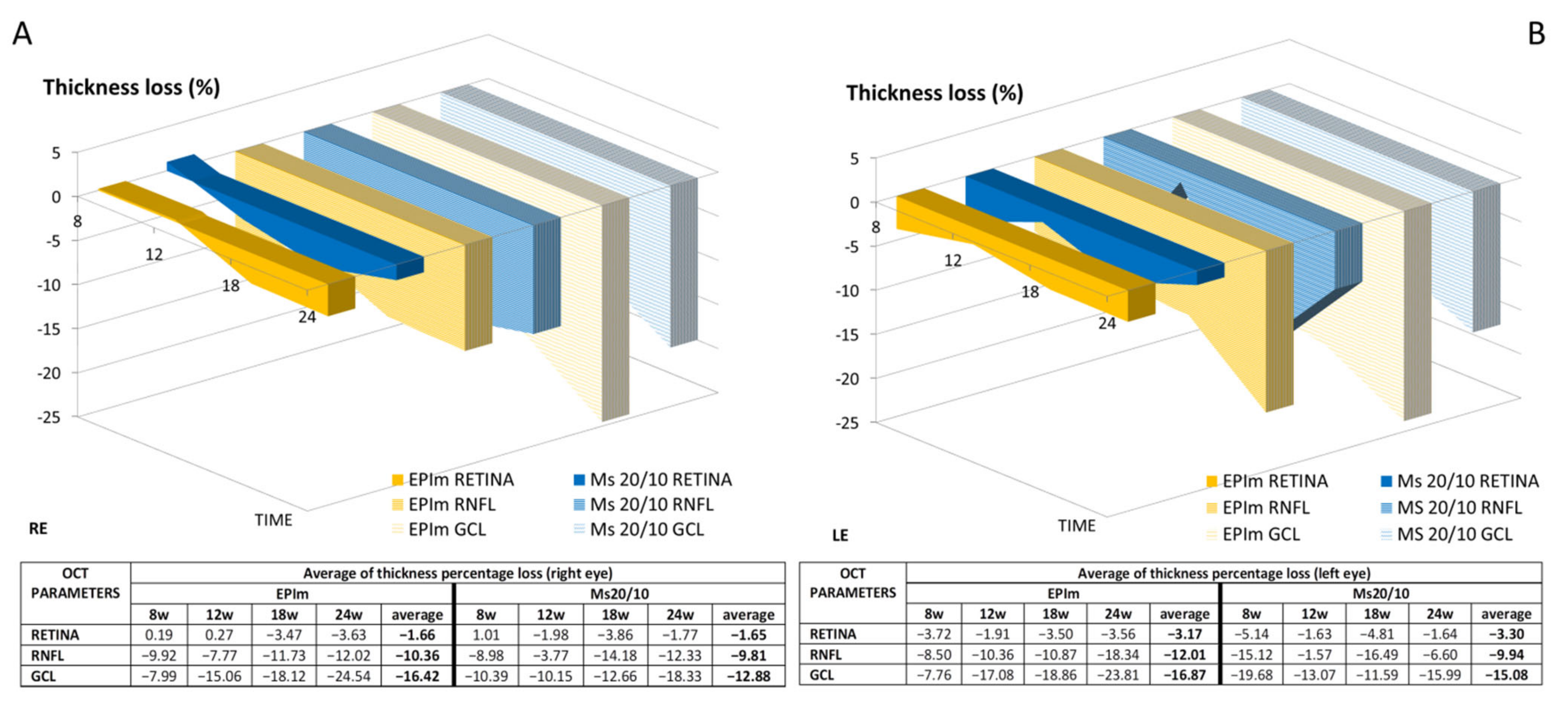



| Right Eye, Microsphere 20/10 Model | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OCT Parameters (μm) | Baseline | 8 w | 12 w | 18 w | 24 w | ||||||||
| Mean ± SD | Mean ± SD | %Ch | p | Mean ± SD | %Ch | p | Mean ± SD | %Ch | p | Mean ± SD | %Ch | p | |
| Retinal thickness | ↑ | ↓ | ↓ | ↑ | |||||||||
| central | 271.04 ± 13.48 | 267.50 ± 10.68 | −1.31 | 0.347 | 268.80 ± 17.72 | −0.83 | 0.5 | 261.60 ± 9.91 | −3.48 | 0.893 | 265.00 ± 22.45 | −2.23 | 0.6 |
| inner inferior | 256.54 ± 7.39 | 253.42 ± 8.59 | −1.22 | 0.237 | 250.60 ± 8.50 | −2.32 | 0.221 | 245.40 ± 10.13 | −4.34 | 0.043 | 247.50 ± 14.48 | −3.52 | 0.116 |
| outer inferior | 246.33 ± 5.94 | 246.42 ± 10.71 | 0.04 | 0.802 | 239.20 ± 6.09 | −2.89 | 0.345 | 234.20 ± 2.58 | −4.92 | 0.042 | 234.33 ± 10.25 | −4.87 | 0.028 |
| inner superior | 251.08 ± 7.70 | 253.75 ± 25.36 | 1.06 | 0.846 | 242.60 ± 15.50 | −3.38 | 0.345 | 238.20 ± 6.68 | −5.13 | 0.041 | 246.67 ± 16.23 | −1.76 | 0.752 |
| outer superior | 250.21 ± 6.37 | 255.33 ± 20.02 | 2.05 | 0.573 | 244.40 ± 7.43 | −2.32 | 0.581 | 238.20 ± 6.18 | −4.80 | 0.043 | 249.00 ± 10.67 | −0.48 | 0.753 |
| inner nasal | 253.21 ± 6.93 | 253.83 ± 15.81 | 0.24 | 0.931 | 245.40 ± 9.39 | −3.08 | 0.144 | 244.60 ± 7.63 | −3.40 | 0.043 | 244.83 ± 15.80 | −3.31 | 0.027 |
| outer nasal | 248.00 ± 6.03 | 252.67 ± 16.90 | 1.88 | 0.608 | 242.20 ± 9.09 | −2.34 | 0.683 | 238.00 ± 5.61 | −4.03 | 0.043 | 242.00 ± 10.90 | −2.42 | 0.116 |
| inner temporal | 253.04 ± 10.20 | 257.33 ± 28.91 | 1.7 | 0.928 | 244.20 ± 8.10 | −3.49 | 0.345 | 239.40 ± 5.45 | −5.39 | 0.043 | 247.33 ± 14.41 | −2.26 | 0.078 |
| outer temporal | 248.38 ± 7.42 | 259.42 ± 26.63 | 4.44 | 0.365 | 246.20 ± 10.08 | −0.88 | 0.225 | 242.20 ± 2.86 | −2.49 | 0.078 | 250.67 ± 12.43 | 0.92 | 0.5 |
| total volume | 1.71 ± 0.36 | 1.73 ± 0.05 | 1.17 | 0.038 | 1.74 ± 0.06 | 1.75 | 0.126 | 1.71 ± 0.36 | −0.58 | 0.5 | 1.75 ± 0.08 | 2.24 | 0.027 |
| RNFL thickness | ↓ | ↑ | ↓ | ↑ | |||||||||
| global | 46.00 ± 4.40 | 42.36 ± 6.26 | −7.91 | 0.04 | 44.20 ± 3.27 | −3.91 | 0.345 | 39.80 ± 2.77 | −13.48 | 0.042 | 40.17 ± 8.65 | −12.67 | 0.043 |
| inferior temporal | 46.75 ± 6.52 | 42.73 ± 7.86 | −8.60 | 0.008 # | 45.00 ± 6.96 | −3.74 | 0.99 | 36.80 ± 4.65 | −21.28 | 0.042 | 44.67 ± 12.12 | −4.45 | 0.207 |
| inferior nasal | 46.96 ± 6.19 | 44.82 ± 5.87 | −4.56 | 0.814 | 51.40 ± 6.80 | 9.45 | 0.99 | 40.20 ± 5.58 | −14.40 | 0.068 | 46.17 ± 10.77 | −1.68 | 0.458 |
| superior temporal | 49.38 ± 8.15 | 40.64 ± 11.73 | −17.70 | 0.012 # | 44.00 ± 9.43 | −10.90 | 0.08 | 37.40 ± 4.09 | −24.26 | 0.043 | 41.00 ± 12.61 | −16.97 | 0.046 |
| superior nasal | 39.00 ± 8.20 | 32.64 ± 16.72 | −16.31 | 0.548 | 35.40 ± 4.03 | −9.23 | 0.042 | 36.80 ± 7.53 | −5.64 | 0.043 | 29.50 ± 9.99 | −24.36 | 0.046 |
| nasal | 43.54 ± 6.15 | 42.82 ± 6.33 | −1.65 | 0.878 | 41.80 ± 6.90 | −4.00 | 0.144 | 40.60 ± 5.94 | −6.75 | 0.042 | 38.67 ± 12.06 | −11.19 | 0.075 |
| temporal | 49.21 ± 8.21 | 46.18 ± 9.71 | −6.16 | 0.095 | 47.20 ± 6.09 | −4.08 | 0.683 | 42.60 ± 5.89 | −13.43 | 0.042 | 41.83 ± 8.32 | −15.00 | 0.027 |
| GCL thickness | ↓ | ≈↓ | ≈↓ | ↓ | |||||||||
| central | 22.46 ± 2.10 | 19.42 ± 3.23 | −13.54 | 0.033 | 19.40 ± 2.70 | −13.62 | 0.066 | 18.20 ± 2.49 | −18.97 | 0.043 | 17.67 ± 3.55 | −21.33 | 0.027 |
| inner inferior | 28.29 ± 1.57 | 26.33 ± 2.10 | −6.93 | 0.002 # | 25.20 ± 1.64 | −10.92 | 0.131 | 25.40 ± 1.51 | −10.22 | 0.042 | 24.17 ± 3.25 | −14.56 | 0.042 |
| outer inferior | 27.25 ± 1.35 | 26.58 ± 3.50 | −2.46 | 0.441 | 24.40 ± 1.67 | −10.46 | 0.066 | 24.80 ± 0.83 | −8.99 | 0.042 | 22.17 ± 4.79 | −18.64 | 0.027 |
| inner superior | 26.96 ± 1.92 | 22.08 ± 3.82 | −18.10 | 0.006 # | 23.60 ± 3.64 | −12.46 | 0.042 | 21.20 ± 1.09 | −21.36 | 0.043 | 21.17 ± 3.97 | −21.48 | 0.042 |
| outer superior | 25.71 ± 2.57 | 22.83 ± 3.71 | −11.20 | 0.037 | 24.20 ± 2.77 | −5.87 | 0.285 | 24.80 ± 0.83 | −3.54 | 0.492 | 22.00 ± 5.21 | −14.43 | 0.168 |
| inner nasal | 26.38 ± 2.33 | 22.83 ± 2.03 | −13.42 | 0.004 # | 23.80 ± 2.38 | −9.75 | 0.257 | 22.60 ± 1.81 | −14.30 | 0.042 | 21.67 ± 5.78 | −17.82 | 0.027 |
| outer nasal | 26.96 ± 1.75 | 24.92 ± 2.02 | −7.57 | 0.013 # | 24.40 ± 2.07 | −9.50 | 0.059 | 24.80 ± 0.83 | −8.01 | 0.066 | 21.50 ± 3.93 | −20.25 | 0.027 |
| inner temporal | 26.33 ± 2.18 | 23.08 ± 4.83 | −12.34 | 0.044 | 23.80 ± 1.92 | −9.61 | 0.063 | 21.60 ± 4.61 | −17.96 | 0.039 | 21.50 ± 4.18 | −18.34 | 0.058 |
| outer temporal | 27.46 ± 1.56 | 25.17 ± 3.38 | −8.34 | 0.061 | 24.80 ± 2.86 | −9.69 | 0.066 | 24.00 ± 2.82 | −12.60 | 0.042 | 22.50 ± 3.14 | −18.06 | 0.026 |
| total volume | 0.19 ± 0.01 | 0.17 ± 0.01 | −9.96 | 0.047 | 0.17 ± 0.01 | −9.58 | 0.059 | 0.17 ± 0.01 | −10.66 | 0.042 | 0.15 ± 0.03 | −18.35 | 0.042 |
| Re Structural Neuroretinal Measurements According to the Oht Model (EPIm vs. Ms20/10) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OCT Parameters (μm) | Baseline | 8 w | 12 w | 18 w | 24 w | |||||
| Mean ± SD | p | Mean ± SD | p | Mean ± SD | p | Mean ± SD | p | Mean ± SD | p | |
| Retinal Thickness | ||||||||||
| central | 271.08 ± 14.04 | 0.904 | 265.92 ± 14.79 | 0.794 | 265.22 ± 18.72 | 0.655 | 253.50 ± 11.67 | 0.268 | 255.00 ± 18.17 | 0.253 |
| 271.04 ± 13.48 | 267.50 ± 10.68 | 268.80 ± 17.72 | 261.60 ± 9.91 | 265.00 ± 22.45 | ||||||
| inner inferior | 256.24 ± 0.14 | 0.904 | 256.17 ± 9.18 | 0.385 | 254.83 ± 9.19 | 0.478 | 248.50 ± 10.66 | 0.902 | 245.00 ± 12.91 | 0.668 |
| 256.54 ± 7.39 | 253.42 ± 8.59 | 250.60 ± 8.50 | 245.40 ± 10.13 | 247.50 ± 14.48 | ||||||
| outer inferior | 247.48 ± 6.75 | 0.520 | 246.58 ± 10.57 | 0.977 | 251.06 ± 11.36 | 0.036 | 242.25 ± 10.50 | 0.221 | 237.43 ± 11.74 | 0.568 |
| 246.33 ± 5.94 | 246.42 ± 10.71 | 239.20 ± 6.09 | 234.20 ± 2.58 | 234.33 ± 10.25 | ||||||
| inner superior | 250.12 ± 8.09 | 0.787 | 255.00 ± 24.93 | 0.750 | 253.11 ± 23.62 | 0.179 | 240.25 ± 3.50 | 0.539 | 240.86 ± 10.18 | 0.317 |
| 251.08 ± 7.70 | 253.75 ± 25.36 | 242.60 ± 15.50 | 238.20 ± 6.68 | 246.67 ± 16.23 | ||||||
| outer superior | 250.88 ± 6.39 | 0.367 | 257.67 ± 18.63 | 0.543 | 260.44 ± 22.89 | 0.048 | 246.25 ± 8.99 | 0.268 | 245.57 ± 15.61 | 0.775 |
| 250.21 ± 6.37 | 255.33 ± 20.02 | 244.40 ± 7.43 | 238.20 ± 6.18 | 249.00 ± 10.67 | ||||||
| inner nasal | 253.32 ± 6.44 | 0.880 | 256.00 ± 14.07 | 0.728 | 254.39 ± 17.03 | 0.191 | 251.75 ± 7.67 | 0.176 | 247.71 ± 17.28 | 0.431 |
| 253.21 ± 6.93 | 253.83 ± 15.81 | 245.40 ± 9.39 | 244.60 ± 7.63 | 244.83 ± 15.80 | ||||||
| outer nasal | 249.00 ± 6.29 | 0.521 | 253.50 ± 15.90 | 0.907 | 255.33 ± 18.95 | 0.080 | 246.75 ± 6.13 | 0.059 | 250.00 ± 8.22 | 0.520 |
| 248.00 ± 6.03 | 252.67 ± 16.90 | 242.20 ± 9.09 | 238.00 ± 5.61 | 242.00 ± 10.90 | ||||||
| inner temporal | 253.20 ± 10.02 | 0.976 | 256.58 ± 29.11 | 0.750 | 253.61 ± 22.14 | 0.232 | 243.50 ± 6.13 | 0.327 | 248.29 ± 13.62 | 0.250 |
| 253.04 ± 10.20 | 252.67 ± 16.90 | 244.20 ± 8.10 | 239.40 ± 5.45 | 247.33 ± 14.41 | ||||||
| outer temporal | 249.96 ± 8.57 | 0.560 | 258.58 ± 26.94 | 0.729 | 259.78 ± 24.89 | 0.117 | 249.25 ± 5.37 | 0.036 | 252.00 ± 16.41 | 0.943 |
| 248.38 ± 7.42 | 259.42 ± 26.63 | 246.20 ± 10.08 | 242.20 ± 2.86 | 250.67 ± 12.43 | ||||||
| total volume | 1.97 ± 0.07 | <0.001# | 1.81 ± 0.11 | 0.064 | 1.81 ± 0.11 | 0.156 | 1.74 ± 0.03 | 0.138 | 1.71 ± 0.16 | 0.720 |
| 1.71 ± 0.36 | 1.73 ± 0.05 | 1.74 ± 0.06 | 1.71 ± 0.36 | 1.75 ± 0.08 | ||||||
| RNFL Thickness | ||||||||||
| global | 46.16 ± 4.36 | 0.880 | 41.45 ± 3.53 | 0.974 | 43.15 ± 9.53 | 0.414 | 41.75 ± 5.12 | 0.532 | 40.86 ± 4.22 | 0.474 |
| 46.00 ± 4.40 | 42.36 ± 6.26 | 44.20 ± 3.27 | 39.80 ± 2.77 | 40.17 ± 8.65 | ||||||
| inferior temporal | 48.72 ± 11.03 | 0.764 | 40.36 ± 7.24 | 0.666 | 41.80 ± 12.46 | 0.563 | 35.75 ± 11.26 | 0.219 | 38.29 ± 4.46 | 0.283 |
| 46.75 ± 6.52 | 42.73 ± 7.86 | 45.00 ± 6.96 | 36.80 ± 4.65 | 44.67 ± 12.12 | ||||||
| inferior nasal | 48.40 ± 8.23 | 0.666 | 45.45 ± 5.57 | 0.691 | 45.10 ± 9.33 | 0.134 | 41.00 ± 13.54 | 0.537 | 42.43 ± 8.77 | 0.774 |
| 46.96 ± 6.19 | 44.82 ± 5.87 | 51.40 ± 6.80 | 40.20 ± 5.58 | 46.17 ± 10.77 | ||||||
| superior temporal | 48.32 ± 8.82 | 0.645 | 43.73 ± 11.85 | 0.598 | 43.60 ± 22.02 | 0.454 | 40.50 ± 7.32 | 0.461 | 40.00 ± 12.66 | 0.721 |
| 49.38 ± 8.15 | 40.64 ± 11.73 | 44.00 ± 9.43 | 37.40 ± 4.09 | 41.00 ± 12.61 | ||||||
| superior nasal | 38.56 ± 9.40 | 0.952 | 36.00 ± 14.58 | 0.307 | 33.55 ± 22.62 | 0.324 | 34.00 ± 2.16 | 0.624 | 35.57 ± 8.65 | 0.317 |
| 39.00 ± 8.20 | 32.64 ± 16.72 | 35.40 ± 4.03 | 36.80 ± 7.53 | 29.50 ± 9.99 | ||||||
| nasal | 43.68 ± 7.12 | 0.968 | 41.82 ± 3.40 | 0.894 | 43.15 ± 8.52 | 0.973 | 45.25 ± 6.29 | 0.221 | 43.29 ± 3.54 | 0.719 |
| 43.54 ± 6.15 | 42.82 ± 6.33 | 41.80 ± 6.90 | 40.60 ± 5.94 | 38.67 ± 12.06 | ||||||
| temporal | 48.80 ± 8.41 | 0.833 | 41.18 ± 7.11 | 0.156 | 47.40 ± 12.04 | 0.539 | 45.75 ± 10.24 | 0.806 | 42.43 ± 8.03 | 0.943 |
| 49.21 ± 8.21 | 46.18 ± 9.71 | 47.20 ± 6.09 | 42.60 ± 5.89 | 41.83 ± 8.32 | ||||||
| GCL Thickness | ||||||||||
| central | 22.32 ± 2.30 | 0.951 | 18.92 ± 3.47 | 0.663 | 16.78 ± 3.59 | 0.107 | 15.25 ± 1.50 | 0.080 | 15.57 ± 3.78 | 0.195 |
| 22.46 ± 2.10 | 19.42 ± 3.23 | 19.40 ± 2.70 | 18.20 ± 2.49 | 17.67 ± 3.55 | ||||||
| inner inferior | 27.88 ± 2.10 | 0.610 | 26.25 ± 2.05 | 0.741 | 23.50 ± 4.79 | 0.650 | 24.00 ± 1.41 | 0.167 | 21.57 ± 5.59 | 0.109 |
| 28.29 ± 1.57 | 26.33 ± 2.10 | 25.20 ± 1.64 | 25.40 ± 1.51 | 24.17 ± 3.25 | ||||||
| outer inferior | 26.84 ± 1.77 | 0.575 | 26.25 ± 3.59 | 0.497 | 24.44 ± 4.09 | 0.218 | 23.25 ± 2.98 | 0.366 | 22.14 ± 6.28 | 0.560 |
| 27.25 ± 1.35 | 26.58 ± 3.50 | 24.40 ± 1.67 | 24.80 ± 0.83 | 22.17 ± 4.79 | ||||||
| inner superior | 26.40 ± 1.58 | 0.324 | 22.42 ± 4.18 | 0.680 | 21.50 ± 4.50 | 0.142 | 18.00 ± 2.44 | 0.044 | 17.57 ± 4.92 | 0.150 |
| 26.96 ± 1.92 | 22.08 ± 3.82 | 23.60 ± 3.64 | 21.20 ± 1.09 | 21.17 ± 3.97 | ||||||
| outer superior | 25.40 ± 2.50 | 0.647 | 23.50 ± 3.89 | 0.520 | 22.44 ± 3.60 | 0.228 | 24.75 ± 2.21 | 0.900 | 20.14 ± 5.64 | 0.387 |
| 25.71 ± 2.57 | 22.83 ± 3.71 | 24.20 ± 2.77 | 24.80 ± 0.83 | 22.00 ± 5.21 | ||||||
| inner nasal | 26.16 ± 2.35 | 0.723 | 23.50 ± 1.78 | 0.379 | 21.56 ± 5.05 | 0.410 | 19.00 ± 4.08 | 0.082 | 19.57 ± 5.31 | 0.221 |
| 26.38 ± 2.33 | 22.83 ± 2.03 | 23.80 ± 2.38 | 22.60 ± 1.81 | 21.67 ± 5.78 | ||||||
| outer nasal | 26.68 ± 1.72 | 0.569 | 25.50 ± 1.62 | 0.479 | 22.00 ± 6.29 | 0.499 | 22.25 ± 4.99 | 0.530 | 22.00 ± 1.78 | 0.685 |
| 26.96 ± 1.75 | 24.92 ± 2.02 | 24.40 ± 2.07 | 24.80 ± 0.83 | 21.50 ± 3.93 | ||||||
| inner temporal | 25.56 ± 3.34 | 0.520 | 23.50 ± 4.70 | 0.748 | 20.89 ± 5.31 | 0.032 | 20.25 ± 1.70 | 0.268 | 18.71 ± 4.92 | 0.425 |
| 26.33 ± 2.18 | 23.08 ± 4.83 | 23.80 ± 1.92 | 21.60 ± 4.61 | 21.50 ± 4.18 | ||||||
| outer temporal | 26.72 ± 2.57 | 0.349 | 25.42 ± 3.47 | 0.605 | 25.00 ± 5.16 | 0.733 | 25.00 ± 1.41 | 0.898 | 20.43 ± 5.28 | 0.278 |
| 27.46 ± 1.56 | 25.17 ± 3.38 | 24.80 ± 2.86 | 24.00 ± 2.82 | 22.50 ± 3.14 | ||||||
| total volume | 0.18 ± 0.01 | 0.668 | 0.16 ± 0.00 | 0.914 | 0.16 ± 0.02 | 0.259 | 0.15 ± 0.01 | 0.093 | 0.13 ± 0.03 | 0.343 |
| 0.19 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.15 ± 0.03 | ||||||
| All-Sector Average Loss Rate (nm)/mmHg/day | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right Eye | Left Eye | |||||||||||
| Time | RETINA | RNFL | GCL | RETINA | RNFL | GCL | ||||||
| EPIm | Ms20/10 | EPIm | Ms20/10 | EPIm | Ms20/10 | EPIm | Ms20/10 | EPIm | Ms20/10 | EPIm | Ms20/10 | |
| 8 w | 2 | 4 | −5 | −8 | −2 | −5 | −7 | −31 | −4 | −18 | −2 | −12 |
| 12 w | 4 | −6 | −5 | −2 | −5 | −3 | −5 | −6 | −10 | −1 | −8 | −4 |
| 18 w | −8 | −11 | −7 | −8 | −6 | −4 | −12 | −14 | −9 | −9 | −8 | −3 |
| 24 w | −3 | −2 | −3 | −2 | −3 | −2 | −3 | −2 | −3 | −2 | −2 | −2 |
| Average | −1 | −4 | −5 | −5 | −4 | −3 | −7 | −14 | −7 | −7 | −5 | −5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigo, M.J.; Garcia-Herranz, D.; Subias, M.; Martinez-Rincón, T.; Mendez-Martínez, S.; Bravo-Osuna, I.; Carretero, A.; Ruberte, J.; Garcia-Feijoo, J.; Pablo, L.E.; et al. Chronic Glaucoma Using Biodegradable Microspheres to Induce Intraocular Pressure Elevation. Six-Month Follow-Up. Biomedicines 2021, 9, 682. https://doi.org/10.3390/biomedicines9060682
Rodrigo MJ, Garcia-Herranz D, Subias M, Martinez-Rincón T, Mendez-Martínez S, Bravo-Osuna I, Carretero A, Ruberte J, Garcia-Feijoo J, Pablo LE, et al. Chronic Glaucoma Using Biodegradable Microspheres to Induce Intraocular Pressure Elevation. Six-Month Follow-Up. Biomedicines. 2021; 9(6):682. https://doi.org/10.3390/biomedicines9060682
Chicago/Turabian StyleRodrigo, Maria Jesus, David Garcia-Herranz, Manuel Subias, Teresa Martinez-Rincón, Silvia Mendez-Martínez, Irene Bravo-Osuna, Ana Carretero, Jesús Ruberte, Julián Garcia-Feijoo, Luis Emilio Pablo, and et al. 2021. "Chronic Glaucoma Using Biodegradable Microspheres to Induce Intraocular Pressure Elevation. Six-Month Follow-Up" Biomedicines 9, no. 6: 682. https://doi.org/10.3390/biomedicines9060682
APA StyleRodrigo, M. J., Garcia-Herranz, D., Subias, M., Martinez-Rincón, T., Mendez-Martínez, S., Bravo-Osuna, I., Carretero, A., Ruberte, J., Garcia-Feijoo, J., Pablo, L. E., Herrero-Vanrell, R., & Garcia-Martin, E. (2021). Chronic Glaucoma Using Biodegradable Microspheres to Induce Intraocular Pressure Elevation. Six-Month Follow-Up. Biomedicines, 9(6), 682. https://doi.org/10.3390/biomedicines9060682