Aging Additively Influences Insulin- and Insulin-Like Growth Factor-1-Mediated Endothelial Dysfunction and Antioxidant Deficiency in Spontaneously Hypertensive Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Determination of Blood Glucose and Insulin Resistance
2.3. Measurement of Vasorelaxation
2.4. Serum Levels of Nitrate/Nitrite (NO), Malondialdehyde (MDA), and Antioxidant Activities
2.5. Statistical Analysis
3. Results
3.1. General Characteristics
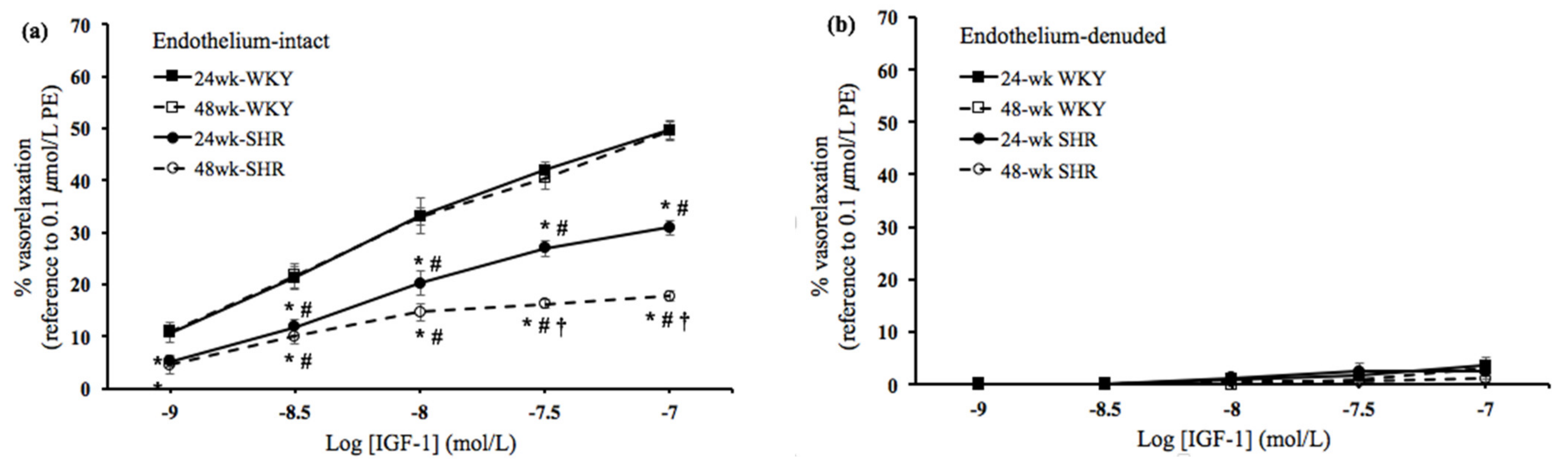
3.2. Insulin- and IGF-1-Mediated Vasorelaxation
3.3. Roles of NOS and PI3K in the Vasorelaxation Mediated by Insulin and IGF-1
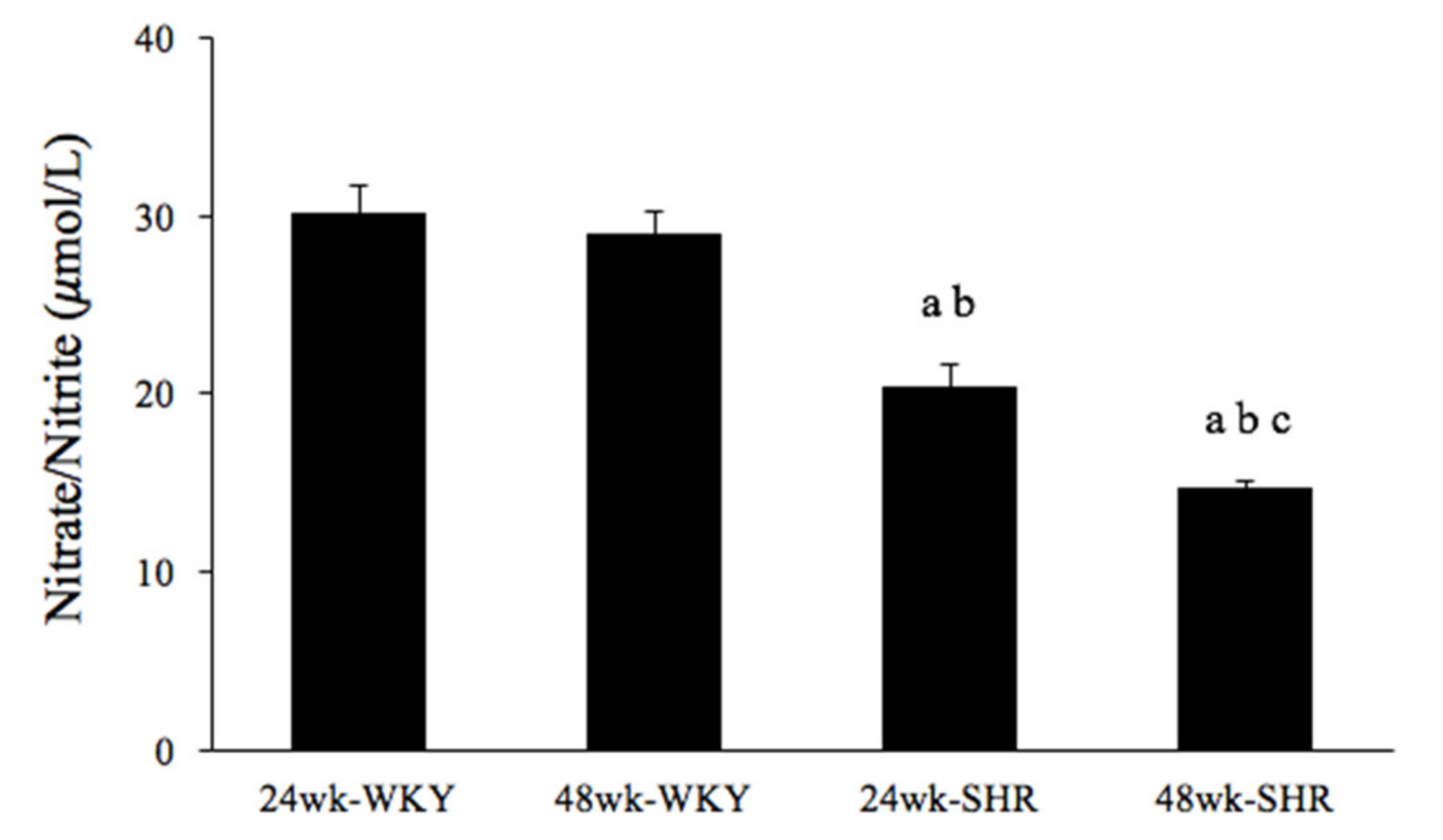
3.4. Serum Nitrate/Nitrite Concentration
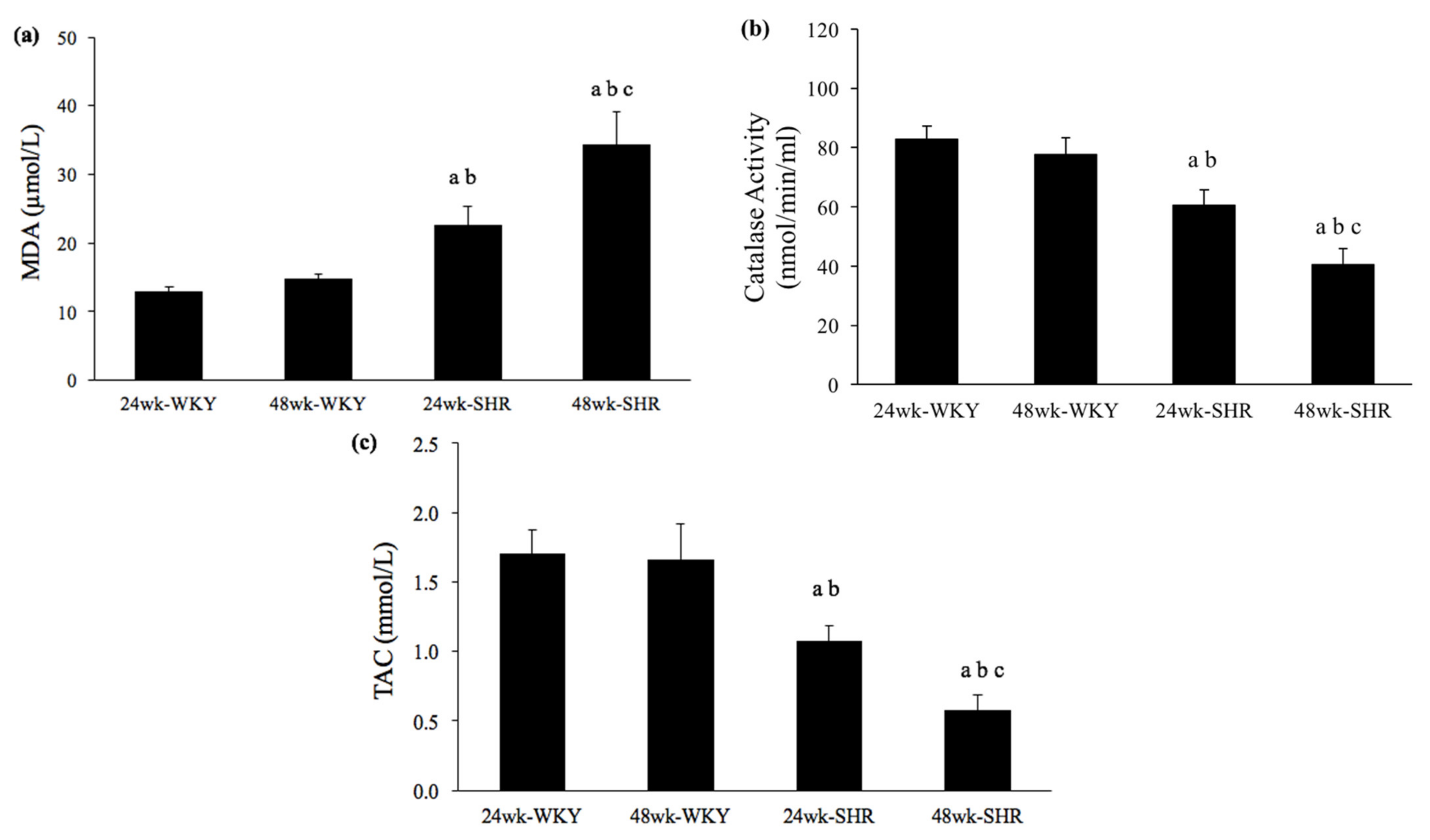
3.5. Serum Levels of MDA, Catalase Activity, and TAC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forrester, S.J.; Dolmatova, E.V.; Griendling, K.K. An acceleration in hypertension-related mortality for middle-aged and older Americans, 1999–2016: An observational study. PLoS ONE 2020, 15, e0225207. [Google Scholar] [CrossRef]
- Ferrari, A.U.; Radaelli, A.; Centola, M. Invited review: Aging and the cardiovascular system. J. Appl. Physiol. 2003, 95, 2591–2597. [Google Scholar] [CrossRef] [PubMed]
- Bolton, E.; Rajkumar, C. The ageing cardiovascular system. Rev. Clin. Gerontol. 2011, 21, 99–109. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [CrossRef]
- Buford, T.W. Hypertension and aging. Ageing Res. Rev. 2016, 26, 96–111. [Google Scholar] [CrossRef]
- Dinh, Q.N.; Drummond, G.R.; Sobey, C.G.; Chrissobolis, S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed. Res. Int. 2014, 2014, 406960. [Google Scholar] [CrossRef]
- Hirase, T.; Node, K. Endothelial dysfunction as a cellular mechanism for vascular failure. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H499–H505. [Google Scholar] [CrossRef]
- Park, K.H.; Park, W.J. Endothelial dysfunction: Clinical implications in cardiovascular disease and therapeutic approaches. J. Korean Med. Sci. 2015, 30, 1213–1225. [Google Scholar] [CrossRef]
- Montezano, A.C.; Dulak-Lis, M.; Tsiropoulou, S.; Harvey, A.; Briones, A.M.; Touyz, R.M. Oxidative stress and human hypertension: Vascular mechanisms, biomarkers, and novel therapies. Can. J. Cardiol. 2015, 31, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci. 2011, 120, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Touyz, R.M. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Rahman, H.S. Antioxidant and oxidative Stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Masodsai, K.; Lin, Y.Y.; Lee, S.D.; Yang, A.L. Exercise and endothelial dysfunction in hypertension. Adapt. Med. 2017, 9, 1–14. [Google Scholar] [CrossRef]
- Abbas, A.; Grant, P.J.; Kearney, M.T. Role of IGF-1 in glucose regulation and cardiovascular disease. Expert Rev. Cardiovasc. Ther. 2008, 6, 1135–1149. [Google Scholar] [CrossRef]
- Muniyappa, R.; Montagnani, M.; Koh, K.K.; Quon, M.J. Cardiovascular actions of insulin. Endocr. Rev. 2007, 28, 463–491. [Google Scholar] [CrossRef] [PubMed]
- Vecchione, C.; Colella, S.; Fratta, L.; Gentile, M.T.; Selvetella, G.; Frati, G.; Trimarco, B.; Lembo, G. Impaired insulin-like growth factor I vasorelaxant effects in hypertension. Hypertension 2001, 37, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.L.; Chao, J.I.; Lee, S.D. Altered insulin-mediated and insulin-like growth factor-1-mediated vasorelaxation in aortas of obese Zucker rats. Int. J. Obes. 2007, 31, 72–77. [Google Scholar] [CrossRef]
- Yang, A.L.; Yeh, C.K.; Su, C.T.; Lo, C.W.; Lin, K.L.; Lee, S.D. Aerobic exercise acutely improves insulin- and insulin-like growth factor-1-mediated vasorelaxation in hypertensive rats. Exp. Physiol. 2010, 95, 622–629. [Google Scholar] [CrossRef]
- Leong, X.F.; Ng, C.Y.; Jaarin, K. Animal models in cardiovascular research: Hypertension and atherosclerosis. Biomed. Res. Int. 2015, 2015, 528757. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, H.; Fan, W.; Jin, Q.; Chao, T.; Wu, Y.; Huang, J.; Hao, L.; Yang, X. Leucine supplementation differently modulates branched-chain amino acid catabolism, mitochondrial function and metabolic profiles at the different stage of insulin resistance in rats on high-fat diet. Nutrients 2017, 9, 565. [Google Scholar] [CrossRef]
- Sowers, J.R. Insulin and insulin-like growth factor in normal and pathological cardiovascular physiology. Hypertension 1997, 29, 691–699. [Google Scholar] [CrossRef]
- Goke, B.; Fehmann, H.C. Insulin and insulin-like growth factor-I: Their role as risk factors in the development of diabetic cardiovascular disease. Diabetes Res. Clin. Pract. 1996, 30, 93–106. [Google Scholar] [CrossRef]
- Conti, E.; Carrozza, C.; Capoluongo, E.; Volpe, M.; Crea, F.; Zuppi, C.; Andreotti, F. Insulin-like growth factor-1 as a vascular protective factor. Circulation 2004, 110, 2260–2265. [Google Scholar] [CrossRef]
- Zeng, G.; Nystrom, F.H.; Ravichandran, L.V.; Cong, L.N.; Kirby, M.; Mostowski, H.; Quon, M.J. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 2000, 101, 1539–1545. [Google Scholar] [CrossRef]
- Masodsai, K.; Lin, Y.Y.; Chaunchaiyakul, R.; Su, C.T.; Lee, S.D.; Yang, A.L. Twelve-week protocatechuic acid administration improves insulin-induced and insulin-like growth factor-1-induced vasorelaxation and antioxidant activities in aging spontaneously hypertensive rats. Nutrients 2019, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.X.; Xiong, Z.Y.; Hu, B.P.; Tian, Z.J.; Zhang, H.F.; Gou, W.Y.; Wang, H.C.; Gao, F.; Zhang, Q.J. Aging-associated insulin resistance predisposes to hypertension and its reversal by exercise: The role of vascular vasorelaxation to insulin. Basic Res. Cardiol. 2009, 104, 269–284. [Google Scholar] [CrossRef]
- Mateos-Caceres, P.J.; Zamorano-Leon, J.J.; Rodriguez-Sierra, P.; Macaya, C.; Lopez-Farre, A.J. New and old mechanisms associated with hypertension in the elderly. Int. J. Hypertens. 2012, 2012, 150107. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Youn, J.Y.; Cai, H. Mechanisms and consequences of endothelial nitric oxide synthase dysfunction in hypertension. J. Hypertens. 2015, 33, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Schlaich, M.P.; Parnell, M.M.; Ahlers, B.A.; Finch, S.; Marshall, T.; Zhang, W.Z.; Kaye, D.M. Impaired L-arginine transport and endothelial function in hypertensive and genetically predisposed normotensive subjects. Circulation 2004, 110, 3680–3686. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, D.E.; White, R.; Li, D.; Minhas, K.M.; Cernetich, A.; Kim, S.; Burke, S.; Shoukas, A.A.; Nyhan, D.; Champion, H.C.; et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 2003, 108, 2000–2006. [Google Scholar] [CrossRef]
- Tschudi, M.R.; Barton, M.; Bersinger, N.A.; Moreau, P.; Cosentino, F.; Noll, G.; Malinski, T.; Luscher, T.F. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. J. Clin. Investig. 1996, 98, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, T.; Maeda, M.; Hayashi, T. Administration of L-arginine plus L-citrulline or L-citrulline alone successfully retarded endothelial senescence. PLoS ONE 2018, 13, e0192252. [Google Scholar] [CrossRef] [PubMed]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part II: The aging heart in health: Links to heart disease. Circulation 2003, 107, 346–354. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Lee, S.D.; Su, C.T.; Cheng, T.L.; Yang, A.L. Long-term treadmill training ameliorates endothelium-dependent vasorelaxation mediated by insulin and insulin-like growth factor-1 in hypertension. J. Appl. Physiol. 2015, 119, 663–669. [Google Scholar] [CrossRef]
- Ling, W.C.; Murugan, D.D.; Lau, Y.S.; Vanhoutte, P.M.; Mustafa, M.R. Sodium nitrite exerts an antihypertensive effect and improves endothelial function through activation of eNOS in the SHR. Sci. Rep. 2016, 6, 33048. [Google Scholar] [CrossRef]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Salvetti, G.; Bernini, G.; Magagna, A.; Salvetti, A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 2001, 38, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.M.; Kong, Y.X.; Matata, B.M. Oxidative stress as a mediator of cardiovascular disease. Oxidative Med. Cell. Longev. 2009, 2, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.; Castillo, G.; Gonzalez, J.; Valls, N.; Rodrigo, R. Oxidative stress in hypertension: Mechanisms and therapeutic opportunities. Exp. Clin. Endocrinol. Diabetes 2015, 123, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Gori, T.; Munzel, T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011, 34, 665–673. [Google Scholar] [CrossRef]
- Subash, P.; Premagurumurthy, K.; Sarasabharathi, A.; Cherian, K.M. Total antioxidant status and oxidative DNA damage in a South Indian population of essential hypertensives. J. Hum. Hypertens. 2010, 24, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, A.; Hajian-Tilaki, K.; Omidvar, S.; Amiri, F.N. Association of lipid peroxidation and antioxidant status with metabolic syndrome in Iranian healthy elderly women. Biomed. Rep. 2017, 7, 331–336. [Google Scholar] [CrossRef]
- Gil, P.; Fariñas, F.; Casado, A.; López-Fernández, E. Malondialdehyde: A possible marker of ageing. Gerontology 2002, 48, 209–214. [Google Scholar] [CrossRef]
- Mao, C.; Yuan, J.-Q.; Lv, Y.-B.; Gao, X.; Yin, Z.-X.; Kraus, V.B.; Luo, J.-S.; Chei, C.-L.; Matchar, D.B.; Zeng, Y.; et al. Associations between superoxide dismutase, malondialdehyde and all-cause mortality in older adults: A community-based cohort study. BMC Geriatr. 2019, 19, 104. [Google Scholar] [CrossRef]
- Hensley, K.; Robinson, K.A.; Gabbita, S.P.; Salsman, S.; Floyd, R.A. Reactive oxygen species, cell signaling, and cell injury. Free Radic. Biol. Med. 2000, 28, 1456–1462. [Google Scholar] [CrossRef]
- Paneni, F.; Canestro, C.D.; Libby, P.; Luscher, T.F.; Camici, G.G. The aging cardiovascular system: Understanding it at the cellular and clinical levels. J. Am. Coll. Cardiol. 2017, 69, 1952–1967. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Nazarewicz, R.R. Angiotensin II-induced production of mitochondrial reactive oxygen species: Potential mechanisms and relevance for cardiovascular disease. Antioxid. Redox Signal. 2013, 19, 1085–1094. [Google Scholar] [CrossRef]
- Favero, G.; Franceschetti, L.; Rodella, L.F.; Rezzani, R. Sirtuins, aging, and cardiovascular risks. Age 2015, 37, 9804. [Google Scholar] [CrossRef]
- Poudel, S.B.; Dixit, M.; Neginskaya, M.; Nagaraj, K.; Pavlov, E.; Werner, H.; Yakar, S. Effects of GH/IGF on the aging mitochondria. Cells 2020, 9, 1384. [Google Scholar] [CrossRef] [PubMed]



| 24wk-WKY | 48wk-WKY | 24wk-SHR | 48wk-SHR | |
|---|---|---|---|---|
| Body weight (g) | 378.13 ± 1.89 | 401.00 ± 2.90 * | 369.63 ± 5.47 # | 393.63 ± 5.10 *,¶ |
| Heart rate (bpm) | 302.00 ± 3.86 | 301.75 ± 4.41 | 380.50 ± 4.46 *,# | 393.69 ± 10.53 *,# |
| SBP (mmHg) | 121.56 ± 0.85 | 121.25 ± 1.09 | 194.81 ± 2.42 *,# | 195.88 ± 0.99 *,# |
| DBP (mmHg) | 96.63 ± 0.58 | 95.56 ± 1.10 | 151.25 ± 1.38 *,# | 154.25 ± 1.50 *,# |
| MAP (mmHg) | 109.75 ± 1.15 | 108.56 ± 0.93 | 168.63 ± 1.04 *,# | 168.44 ± 1.12 *,# |
| Glucose (mg/dL) | 91.75 ± 2.05 | 99.00 ± 3.16 | 123.00 ± 3.51 *,# | 119.63 ± 5.84 *,# |
| Insulin (μg/L) | 0.33 ± 0.05 | 0.35 ± 0.07 | 0.43 ± 0.05 | 0.65 ± 0.08 *,#,¶ |
| HOMA-IR | 1.86 ± 0.29 | 2.06 ± 0.39 | 3.21 ± 0.34 *,# | 4.73 ± 0.51 *,#,¶ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masodsai, K.; Lin, Y.-Y.; Lin, S.-Y.; Su, C.-T.; Lee, S.-D.; Yang, A.-L. Aging Additively Influences Insulin- and Insulin-Like Growth Factor-1-Mediated Endothelial Dysfunction and Antioxidant Deficiency in Spontaneously Hypertensive Rats. Biomedicines 2021, 9, 676. https://doi.org/10.3390/biomedicines9060676
Masodsai K, Lin Y-Y, Lin S-Y, Su C-T, Lee S-D, Yang A-L. Aging Additively Influences Insulin- and Insulin-Like Growth Factor-1-Mediated Endothelial Dysfunction and Antioxidant Deficiency in Spontaneously Hypertensive Rats. Biomedicines. 2021; 9(6):676. https://doi.org/10.3390/biomedicines9060676
Chicago/Turabian StyleMasodsai, Kunanya, Yi-Yuan Lin, Sih-Yin Lin, Chia-Ting Su, Shin-Da Lee, and Ai-Lun Yang. 2021. "Aging Additively Influences Insulin- and Insulin-Like Growth Factor-1-Mediated Endothelial Dysfunction and Antioxidant Deficiency in Spontaneously Hypertensive Rats" Biomedicines 9, no. 6: 676. https://doi.org/10.3390/biomedicines9060676
APA StyleMasodsai, K., Lin, Y.-Y., Lin, S.-Y., Su, C.-T., Lee, S.-D., & Yang, A.-L. (2021). Aging Additively Influences Insulin- and Insulin-Like Growth Factor-1-Mediated Endothelial Dysfunction and Antioxidant Deficiency in Spontaneously Hypertensive Rats. Biomedicines, 9(6), 676. https://doi.org/10.3390/biomedicines9060676









