The 3-Year Effect of the Mediterranean Diet Intervention on Inflammatory Biomarkers Related to Cardiovascular Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Plasma Inflammatory Biomarkers
2.3. Gene Expression Analysis
2.4. Other Clinical Measurement
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Participants and Compliance to Intervention
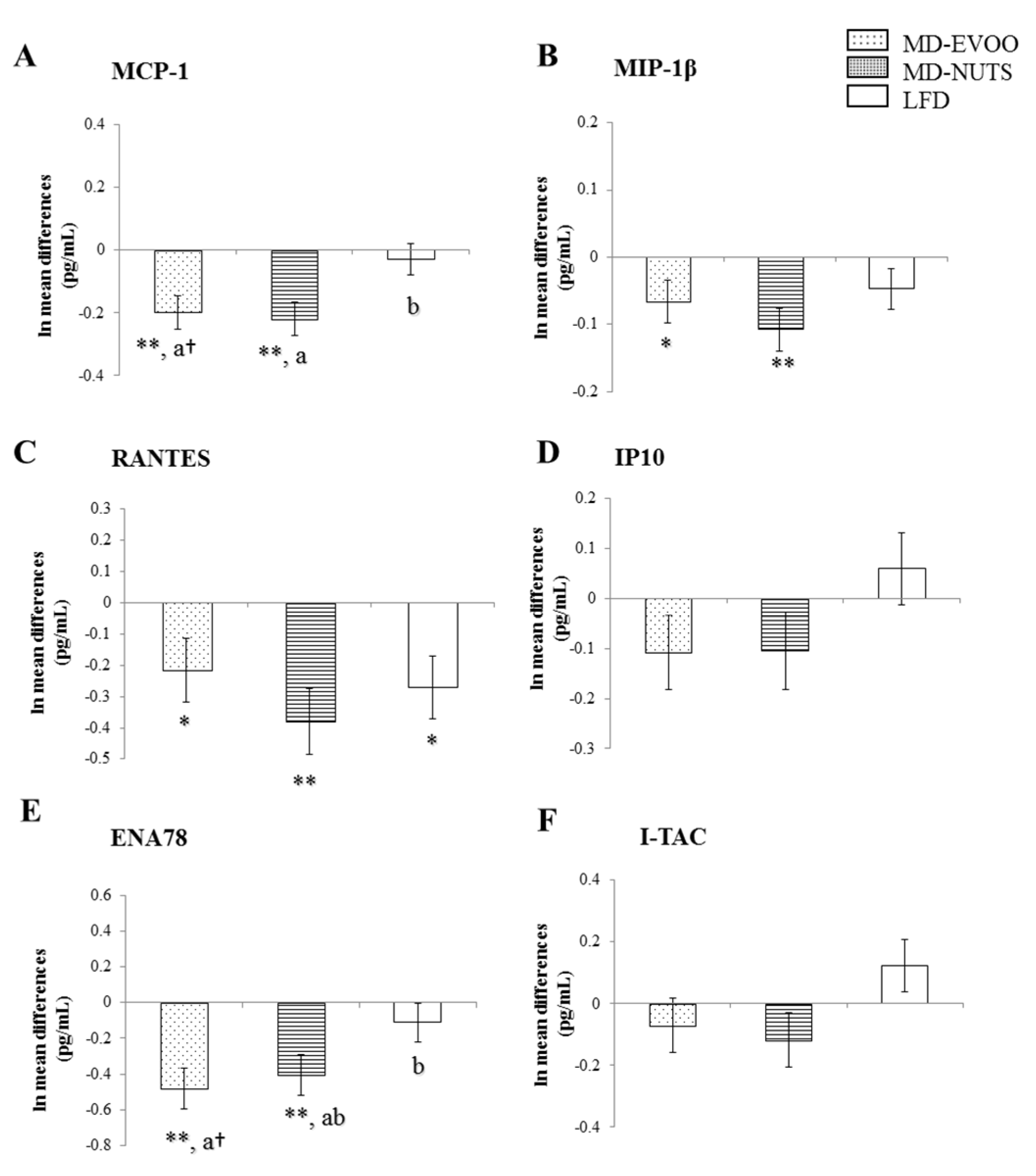
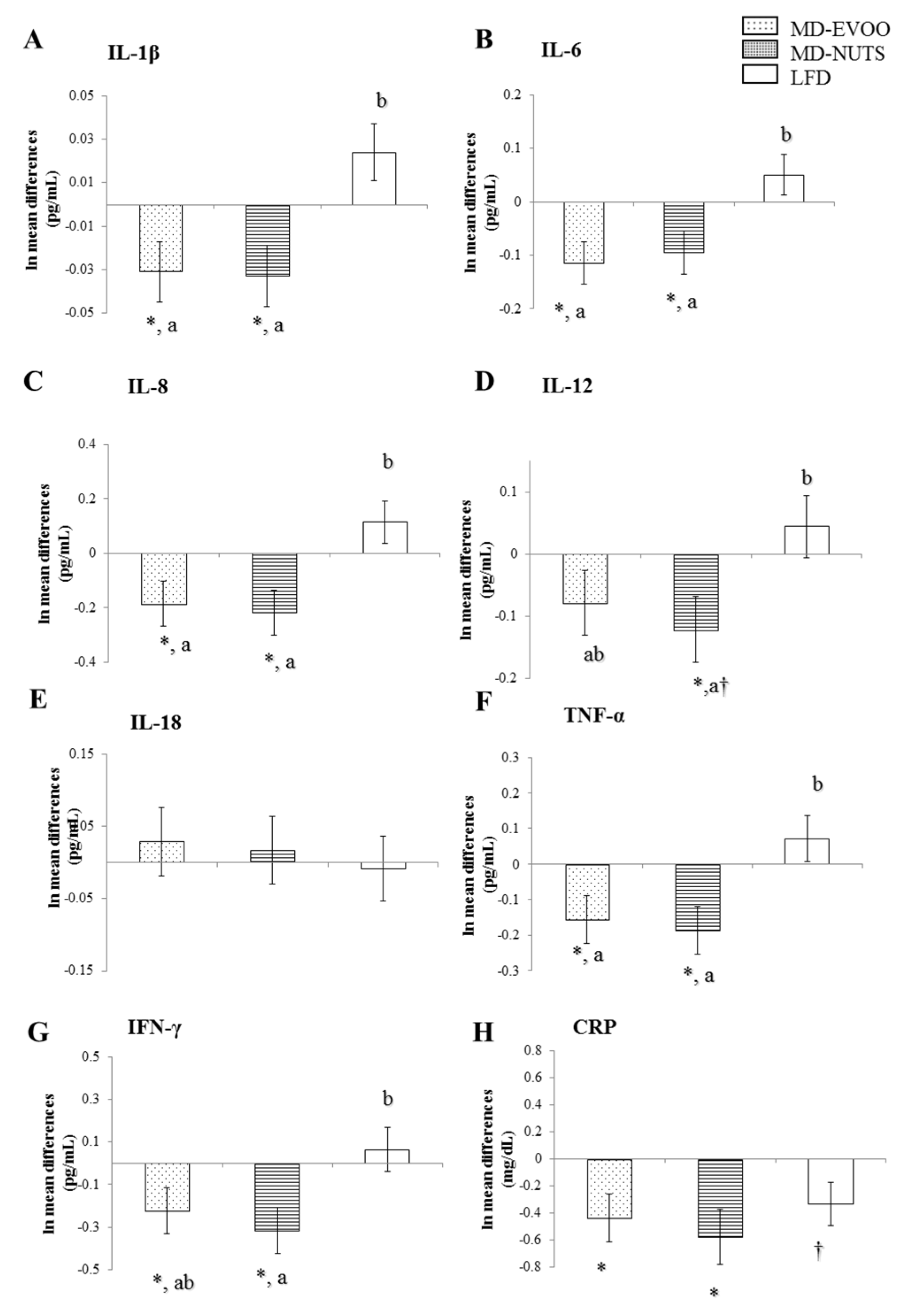
3.2. Changes in Chemokines and Cytokines between Baseline and 3 Years of Intervention
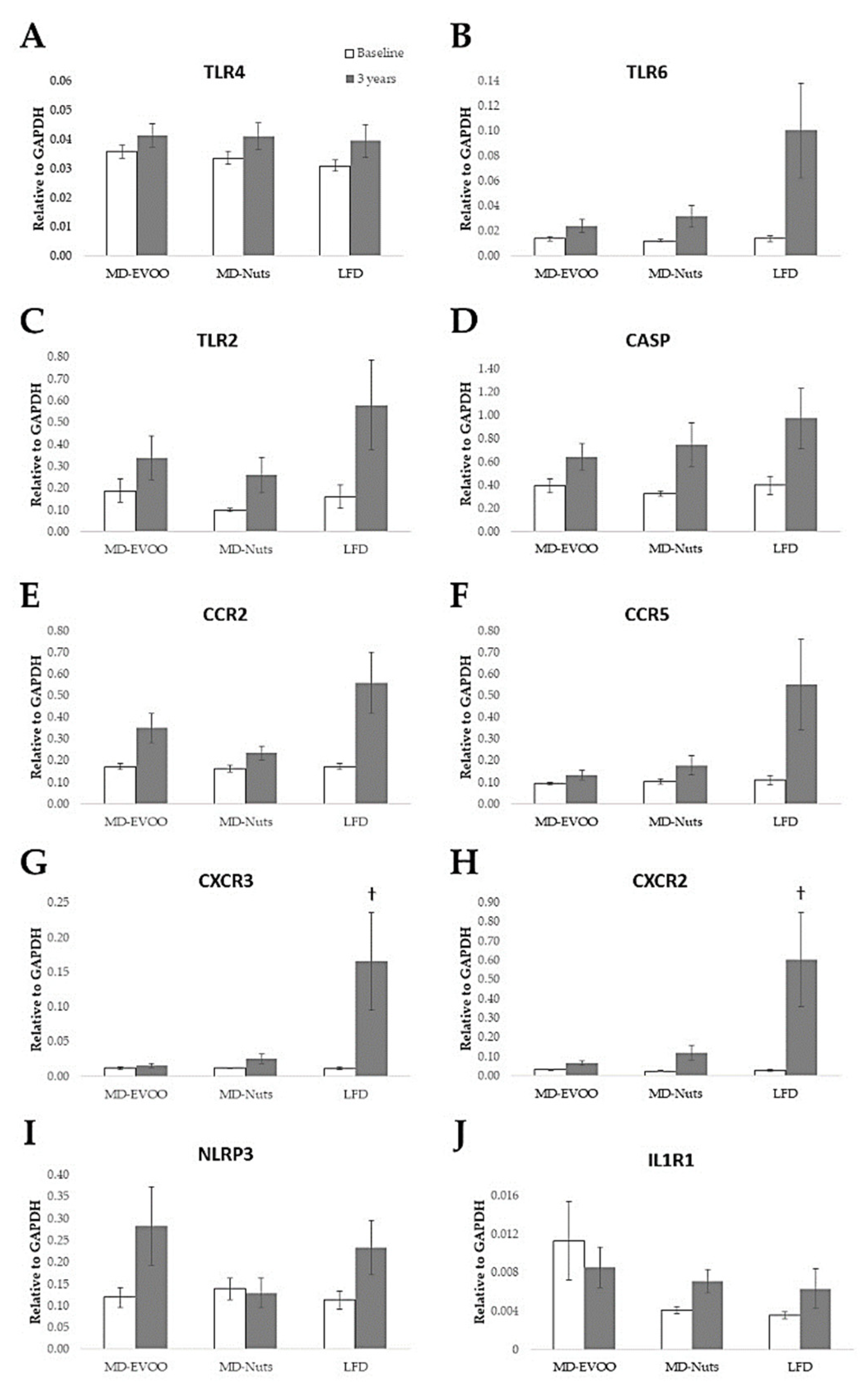
3.3. Pilot Study of Gene Expression Changes at Baseline and after 3 Years of Intervention
3.4. Correlation Analysis of Changes in Inflammatory Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Talaverón-Rey, M.; Suárez-Carrillo, A.; Munuera-Cabeza, M.; Sánchez-Alcázar, J.A. From Mitochondria to Atherosclerosis: The Inflammation Path. Biomedicines 2021, 9, 258. [Google Scholar] [CrossRef]
- Bäck, M.; Yurdagul, A.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.-K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell. Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef]
- Noels, H.; Weber, C.; Koenen, R.R. Chemokines as Therapeutic Targets in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 583–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikolajczyk, T.P.; Szczepaniak, P.; Vidler, F.; Maffia, P.; Graham, G.J.; Guzik, T.J. Role of inflammatory chemokines in hypertension. Pharmacol. Ther. 2021, 223, 107799. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Yang, Y.; Wang, Q.; Li, M.; Tian, C.; Liu, Y.; Aung, L.H.H.; Li, P.-F.; Yu, T.; Chu, X.-M. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020, 11, 776. [Google Scholar] [CrossRef]
- Karasawa, T.; Takahashi, M. Role of NLRP3 Inflammasomes in Atherosclerosis. J. Atheroscler. Thromb. 2017, 24, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Wen, H.; Ting, J.P.-Y.; O’Neill, L.A.J. A role for the NLRP3 inflammasome in metabolic diseases—Did Warburg miss inflammation? Nat. Immunol. 2012, 13, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Marchini, T.; Mitre, L.S.; Wolf, D. Inflammatory Cell Recruitment in Cardiovascular Disease. Front. Cell Dev. Biol. 2021, 9, 635527. [Google Scholar] [CrossRef] [PubMed]
- Byun, E.-H.; Omura, T.; Yamada, K.; Tachibana, H. Green tea polyphenol epigallocatechin-3-gallate inhibits TLR2 signaling induced by peptidoglycan through the polyphenol sensing molecule 67-kDa laminin receptor. FEBS Lett. 2011, 585, 814–820. [Google Scholar] [CrossRef] [Green Version]
- Ellis, L.Z.; Liu, W.; Luo, Y.; Okamoto, M.; Qu, D.; Dunn, J.H.; Fujita, M. Green tea polyphenol epigallocatechin-3-gallate suppresses melanoma growth by inhibiting inflammasome and IL-1β secretion. Biochem. Biophys. Res. Commun. 2011, 414, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Ghanim, H.; Sia, C.L.; Korzeniewski, K.; Lohano, T.; Abuaysheh, S.; Marumganti, A.; Chaudhuri, A.; Dandona, P. A Resveratrol and Polyphenol Preparation Suppresses Oxidative and Inflammatory Stress Response to a High-Fat, High-Carbohydrate Meal. J. Clin. Endocrinol. Metab. 2011, 96, 1409–1414. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Zhao, L.; Hwang, D.H. Modulation of pattern recognition receptor-mediated inflammation and risk of chronic diseases by dietary fatty acids. Nutr. Rev. 2010, 68, 38–61. [Google Scholar] [CrossRef] [PubMed]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Ravaut, G.; Légiot, A.; Bergeron, K.F.; Mounier, C. Monounsaturated fatty acids in obesity--related inflammation. Int. J. Mol. Sci. 2021, 22, 330. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, V.; Covas, M.I.; Sola, R.; Fitó, M. Up-to date knowledge on the in vivo transcriptomic effect of the Mediterranean diet in humans. Mol. Nutr. Food Res. 2013, 57, 772–783. [Google Scholar] [CrossRef]
- Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-Style Diet on Cardiovascular Risk Factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Retraction and Republication: Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2018, 368, 2441–2442. [Google Scholar] [CrossRef] [Green Version]
- Corbera-Bellalta, M.; Planas-Rigol, E.; Lozano, E.; Terrades-García, N.; Alba, M.A.; Prieto-González, S.; García-Martínez, A.; Albero, R.; Enjuanes, A.; Espígol-Frigolé, G.; et al. Blocking interferon γ reduces expression of chemokines CXCL9, CXCL10 and CXCL11 and decreases macrophage infiltration in ex vivo cultured arteries from patients with giant cell arteritis. Ann. Rheum. Dis. 2016, 75, 1177–1186. [Google Scholar] [CrossRef] [Green Version]
- Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and Vascular Effect of the Mediterranean Diet. Int. J. Mol. Sci. 2019, 20, 4716. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Marcos, L.V.; Lou-Bonafonte, J.M.; Arnal, C.; Navarro, M.A.; Osada, J. Transcriptomics and the mediterranean diet: A systematic review. Nutrients 2017, 9, 472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruytinx, P.; Proost, P.; Van Damme, J.; Struyf, S. Chemokine-Induced Macrophage Polarization in Inflammatory Conditions. Front. Immunol. 2018, 9, 1930. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Fu, J. Novel Insights Into the NLRP3 Inflammasome in Atherosclerosis. J. Am. Heart Assoc. 2019, 8, e012219. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream to Identify Novel Targets for Atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas, R.; Sacanella, E.; Urpí-Sardà, M.; Chiva-Blanch, G.; Ros, E.; Martínez-González, M.A.; Covas, M.I.; Lamuela-Raventos, R.M.; Salas-Salvadó, J.; Fiol, M.; et al. The effects of the Mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PLoS ONE 2014, 9, e100084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. J. Am. Med. Assoc. 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ukai, T.; Yumoto, H.; Davey, M.; Goswami, S.; Gibson, F.C.; Genco, C.A. Toll-like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis 2008, 196, 146–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Hundelshausen, P.; Schmitt, M.M.N. Platelets and their chemokines in atherosclerosis-clinical applications. Front. Physiol. 2014, 5, 924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraaijeveld, A.O.; De Jager, S.C.A.; De Jager, W.J.; Prakken, B.J.; McColl, S.R.; Haspels, I.; Putter, H.; Van Berkel, T.J.C.; Nagelkerken, L.; Jukema, J.W.; et al. CC chemokine ligand-5 (CCL5/RANTES) and CC chemokine ligand-18 (CCL18/PARC) are specific markers of refractory unstable angina pectoris and are transiently raised during severe ischemic symptoms. Circulation 2007, 116, 1931–1941. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, S.J.; Feskens, E.J.M.; Bos, M.B.; Hoelen, D.W.M.; Heijligenberg, R.; Bromhaar, M.G.; De Groot, L.C.P.G.M.; De Vries, J.H.M.; Müller, M.; Afman, L.A. A saturated fatty acid-rich diet induces an obesity-linked proinflammatory gene expression profile in adipose tissue of subjects at risk of metabolic syndrome. Am. J. Clin. Nutr. 2009, 90, 1656–1664. [Google Scholar] [CrossRef] [Green Version]
- Szentes, V.; Gazdag, M.; Szokodi, I.; Dézsi, C.A. The Role of CXCR3 and Associated Chemokines in the Development of Atherosclerosis and During Myocardial Infarction. Front. Immunol. 2018, 9, 1932. [Google Scholar] [CrossRef]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bastida, J.A.; González-Sarrías, A.; Larrosa, M.; Tomás-Barberán, F.; Espín, J.C.; García-Conesa, M.T. Ellagitannin metabolites, urolithin A glucuronide and its aglycone urolithin A, ameliorate TNF-α-induced inflammation and associated molecular markers in human aortic endothelial cells. Mol. Nutr. Food Res. 2012, 56, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Scotece, M.; Conde, J.; Abella, V.; López, V.; Francisco, V.; Ruiz, C.; Campos, V.; Lago, F.; Gomez, R.; Pino, J.; et al. Oleocanthal Inhibits Catabolic and Inflammatory Mediators in LPS-Activated Human Primary Osteoarthritis (OA) Chondrocytes Through MAPKs/NF-κB Pathways. Cell. Physiol. Biochem. 2018, 49, 2414–2426. [Google Scholar] [CrossRef]
- Bonaccio, M.; Pounis, G.; Cerletti, C.; Donati, M.B.; Iacoviello, L.; de Gaetano, G. Mediterranean diet, dietary polyphenols and low grade inflammation: Results from the MOLI-SANI study. Br. J. Clin. Pharmacol. 2017, 83, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urpi-Sarda, M.; Casas, R.; Chiva-Blanch, G.; Romero-Mamani, E.; Valderas-Martínez, P.; Arranz, S.; Andres-Lacueva, C.; Llorach, R.; Medina-Remón, A.; Lamuela-Raventós, R.; et al. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomakers related to atherosclerosis. Pharmacol. Res. 2012, 65, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Duchene, J.; Cayla, C.; Vessillier, S.; Scotland, R.; Yamashiro, K.; Lecomte, F.; Syed, I.; Vo, P.; Marrelli, A.; Pitzalis, C.; et al. Laminar shear stress regulates endothelial kinin B1 receptor expression and function: Potential implication in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1757–1763. [Google Scholar] [CrossRef] [Green Version]
- Rousselle, A.; Qadri, F.; Leukel, L.; Yilmaz, R.; Fontaine, J.F.; Sihn, G.; Bader, M.; Ahluwalia, A.; Duchene, J. CXCL5 limits macrophage foam cell formation in atherosclerosis. J. Clin. Investig. 2013, 123, 1343–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balamayooran, G.; Batra, S.; Cai, S.; Mei, J.; Worthen, G.S.; Penn, A.L.; Jeyaseelan, S. Role of CXCL5 in leukocyte recruitment to the lungs during secondhand smoke exposure. Am. J. Respir. Cell Mol. Biol. 2012, 47, 104–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björkbacka, H.; Kunjathoor, V.V.; Moore, K.J.; Koehn, S.; Ordija, C.M.; Lee, M.A.; Means, T.; Halmen, K.; Luster, A.D.; Golenbock, D.T.; et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat. Med. 2004, 10, 416–421. [Google Scholar] [CrossRef]
- Tecchio, C.; Cassatella, M.A. Neutrophil-derived chemokines on the road to immunity. Semin. Immunol. 2016, 28, 119–128. [Google Scholar] [CrossRef]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 2008, 47, 348–380. [Google Scholar] [CrossRef]
| Characteristics | MD-EVOO (n = 93) | MD-Nuts (n = 92) | LFD (n = 100) | p-Value 2 |
|---|---|---|---|---|
| Age, (years) | 68 ± 6 1 | 67 ± 5 | 67 ± 6 | 0.230 |
| Men, n (%) | 30 (32.3) 1 | 34 (37.0) | 32 (32.0) | 0.722 |
| Family history of CHD, n (%) | 34 (36.6) | 19 (20.7) | 23 (23.0) | 0.066 |
| Current smokers, n (%) | 7 (7.5) | 12 (13.0) | 14 (14.0) | 0.670 |
| BMI (kg/m2) | 30.9 ± 4.1 | 29.8 ± 4.0 | 30.5 ± 4.3 | 0.194 |
| Waist circumference (cm) | 103 ± 10.9 | 101 ± 10.2 | 102 ± 10.9 | 0.318 |
| Systolic blood pressure (mmHg) | 151 ± 21.1 | 154 ± 20.0 | 147 ± 18.2 | 0.111 |
| Diastolic blood pressure (mmHg) | 82.5 ± 9.9 | 86.1 ± 9.3 | 84.5 ± 10.1 | 0.127 |
| Type 2 diabetes mellitus, n (%) | 51 (54.8) | 54 (58.7) | 53 (53.0) | 0.723 |
| Hypertension, n (%) | 76 (81.7) | 76 (82.6) | 81 (81.0) | 0.959 |
| Dyslipidemia, n (%) | 62 (66.7) | 64 (69.6) | 75 (75.0) | 0.434 |
| Medication, n (%) | ||||
| ACE inhibitors | 17 (18.3) | 28 (30.4) | 28 (28.0) | 0.132 |
| Diuretics | 24 (25.8) | 19 (20.7) | 18 (18.0) | 0.408 |
| Other antihypertensive agents | 45 (57.6) | 36 (39.1) | 47 (47.0) | 0.395 |
| Statins | 34 (36.6) | 24 (26.1) | 48 (48.0) | 0.007 |
| Other-lipid-lowering agents | 8 (8.6) | 8 (8.7) | 4 (4.0) | 0.341 |
| Insulin | 6 (6.5) | 7 (7.6) | 10 (10.0) | 0.652 |
| Oral hypoglycemic drugs | 31 (33.3) | 25 (27.2) | 37 (37.0) | 0.344 |
| Aspirin or antiplatelet drugs | 10 (10.8) | 19 (20.7) | 16 (16) | 0.181 |
| NSAIDS | 11 (11.8) | 10 (10.9) | 13 (13.0) | 0.901 |
| 3-Year Changes | MIP-1β | RANTES | ENA78 |
|---|---|---|---|
| MD-EVOO group | |||
| MCP-1 | 0.636 ** | 0.187 † | 0.441 ** |
| MIP-1β | 0.247 * | 0.392 ** | |
| RANTES | 0.444 ** | ||
| MD-Nuts group | |||
| MCP-1 | 0.482 ** | 0.280 * | 0.282 * |
| MIP-1β | 0.513 ** | 0.322 * | |
| RANTES | 0.632 ** | ||
| LFD group | |||
| MCP-1 | 0.387 ** | 0.308 * | 0.124 |
| MIP-1β | 0.451 ** | 0.114 | |
| RANTES | 0.494 ** | ||
| 3-Year Changes | IFN-γ | TNF-α | IL-1β | IL-6 | IL-8 |
|---|---|---|---|---|---|
| MD-EVOO group | |||||
| MCP-1 | 0.270 * | 0.326 * | 0.340 * | 0.365 ** | 0.480 ** |
| MIP-1β | 0.175 † | 0.190 | 0.233 * | 0.310 * | 0.313 * |
| RANTES | 0.270 * | 0.122 | 0.074 | 0.295 * | 0.190 † |
| ENA78 | 0.528 ** | 0.393 ** | 0.316 * | 0.369 * | 0.471 ** |
| MD-Nuts group | |||||
| MCP-1 | 0.328 * | 0.403 ** | 0.397 ** | 0.398 ** | 0.353 * |
| MIP-1β | 0.217 * | 0.116 | 0.233 * | 0.301 * | 0.230 * |
| RANTES | 0.329 * | 0.181 | 0.191 † | 0.336 * | 0.301 * |
| ENA78 | 0.455 ** | 0.460 ** | 0.375 ** | 0.495 ** | 0.522 ** |
| LFD group | |||||
| MCP-1 | 0.323 * | 0.241 * | 0.218 * | 0.343 * | 0.403 ** |
| MIP-1β | 0.227 * | 0.024 | 0.012 | 0.298 * | 0.189 † |
| RANTES | 0.127 | −0.044 | 0.009 | 0.020 | 0.193 † |
| ENA78 | 0.280 * | 0.194 † | 0.089 | 0.127 | 0.319 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urpi-Sarda, M.; Casas, R.; Sacanella, E.; Corella, D.; Andrés-Lacueva, C.; Llorach, R.; Garrabou, G.; Cardellach, F.; Sala-Vila, A.; Ros, E.; et al. The 3-Year Effect of the Mediterranean Diet Intervention on Inflammatory Biomarkers Related to Cardiovascular Disease. Biomedicines 2021, 9, 862. https://doi.org/10.3390/biomedicines9080862
Urpi-Sarda M, Casas R, Sacanella E, Corella D, Andrés-Lacueva C, Llorach R, Garrabou G, Cardellach F, Sala-Vila A, Ros E, et al. The 3-Year Effect of the Mediterranean Diet Intervention on Inflammatory Biomarkers Related to Cardiovascular Disease. Biomedicines. 2021; 9(8):862. https://doi.org/10.3390/biomedicines9080862
Chicago/Turabian StyleUrpi-Sarda, Mireia, Rosa Casas, Emilio Sacanella, Dolores Corella, Cristina Andrés-Lacueva, Rafael Llorach, Gloria Garrabou, Francesc Cardellach, Aleix Sala-Vila, Emilio Ros, and et al. 2021. "The 3-Year Effect of the Mediterranean Diet Intervention on Inflammatory Biomarkers Related to Cardiovascular Disease" Biomedicines 9, no. 8: 862. https://doi.org/10.3390/biomedicines9080862
APA StyleUrpi-Sarda, M., Casas, R., Sacanella, E., Corella, D., Andrés-Lacueva, C., Llorach, R., Garrabou, G., Cardellach, F., Sala-Vila, A., Ros, E., Ruiz-Canela, M., Fitó, M., Salas-Salvadó, J., & Estruch, R. (2021). The 3-Year Effect of the Mediterranean Diet Intervention on Inflammatory Biomarkers Related to Cardiovascular Disease. Biomedicines, 9(8), 862. https://doi.org/10.3390/biomedicines9080862












