Abstract
High plasma level of low-density lipoprotein (LDL) is the main driver of the initiation and progression of cardiovascular disease (CVD). Nevertheless, high-density lipoprotein (HDL) is considered an anti-atherogenic lipoprotein due to its role in reverse cholesterol transport and its ability to receive cholesterol that effluxes from macrophages in the artery wall. The scavenger receptor B class type 1 (SR-B1) was identified as the high-affinity HDL receptor, which facilitates the selective uptake of cholesterol ester (CE) into the liver via HDL and is also implicated in the plasma clearance of LDL, very low-density lipoprotein (VLDL) and lipoprotein(a) (Lp(a)). Thus, SR-B1 is a multifunctional receptor that plays a main role in the metabolism of different lipoproteins. The aim of this review is to highlight the association between SR-B1 and CVD risk through mice and human genetic studies.
1. Introduction
Cardiovascular disease (CVD) remains the primary cause of mortality and morbidity worldwide [1]. The principal risk factor for developing CVD is relatively high plasma level of low-density lipoprotein cholesterol (LDLc) [2,3,4]. Numerous epidemiological and clinical investigations have revealed that plasma level of high-density lipoprotein cholesterol (HDLc) correlates inversely with the risk of CVD [5,6]. This association has been described by anti-atherogenic capacities of HDL, comprising its role in reverse cholesterol transport (RCT), in which cholesterol from peripheral tissues is transferred to the liver for excretion in bile and its ability to receive cholesterol from macrophages in the artery wall [7,8]. However, Mendelian randomization studies [9,10] and pharmacological interventional studies [11,12] do not support the concept that HDLc directly reduces the risk of CVD [8]. In addition, a retrospective analysis of large epidemiological studies showed that high HDLc concentration is associated with higher risk for CVD [13,14]. These results support the hypothesis that HDL metabolism and functionality is more important than HDLc levels for CVD risk prediction [14]. Acton et al. identified the scavenger receptor B class 1 (SR-B1) as a high-affinity HDL receptor, which facilitates the selective uptake of cholesterol esters (CE) in HDL into the liver [15]. This receptor is also implicated in the plasma clearance of LDL, very low-density lipoprotein (VLDL) and lipoprotein(a) (Lp(a)), lipoproteins with pro-atherogenic properties [16,17,18,19]. Therefore, SR-B1 is involved in cholesterol homeostasis, lipoprotein metabolism and atherosclerosis [4]. In addition, SR-B1 plays a relevant role in HDL-mediated cellular signaling [20], and might play a crucial role in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) [21], since this receptor is linked to dyslipidemia [22]. Given that SR-B1 is a multifunctional receptor involved in the metabolism of different lipoproteins, the purpose of this review is to highlight the association between SR-B1 and CVD risk through mice and human genetic studies.
2. SR-B1 in Lipoprotein Metabolism
SR-B1 is mainly identified for promoting selective uptake of CE from HDL or other lipoproteins to cells by a non-endocytic process [23,24,25,26,27,28]. Moreover, SR-B1 mediates selective hepatic uptake of HDL-CE, free cholesterol (FC), triglycerides (TG), and phospholipids by a three step mechanism [24,25,26,27]. First, cholesterol-rich donor lipoprotein particles could bind to the extracellular loop domain of the receptor. Then, SR-B1 could promote the transfer of CE from the lipoprotein particles to the plasma membrane, and finally, the cholesterol poor lipoprotein particles could release back into the circulation [3,23,24]. Furthermore, SR-B1 requires oligomerization to promote selective lipid uptake, but not HDL binding [26,27,28,29,30,31]. Although the mechanism by which SR-B1 facilitates the transfer of CE to the plasma membrane is not fully understood, a model has been proposed in which a hydrophobic tunnel is formed by the extracellular domain of the receptor between lipoprotein particles and the cell membrane through which CE diffuse in a concentration gradient manner [27,32]. The recent publication of the high-resolution crystal structure of the extracellular domain of LIMP-2, a homologue of SR-B1, supports the validity of this mechanism [33].
In addition to selective CE uptake, SR-B1 also facilitates the efflux of free cholesterol between cells and lipoproteins [34,35]. Briefly, this mechanism carried out by HDL is known as RCT and consists of the transport of cholesterol via HDL from peripheral tissues such as macrophages or endothelial cells to the liver for cholesterol excretion, bile acid production or steroid hormone synthesis in steroidogenic organs [26,36]. Apart from SR-B1, two more receptors are involved in this process: ATP-binding cassette A1 (ABCA1), that mediates unidirectional efflux of cholesterol and phospholipids to apolipoprotein (apo) A-I and apo E [27,37], and ATP-binding cassette G1 (ABCG1), that promotes unidirectional efflux of cholesterol to nascent HDL particles [27,38].
3. SR-B1, an Important Participant in the Development of Cardiovascular Disease
SR-B1 has been involved in the progression of atherosclerosis [4]. SR-B1, via HDL, contributes to the transport of cholesterol from macrophages through cholesterol efflux to the liver, and is also implicated in reducing inflammation and oxidation [3,4,27]. SR-B1 interaction with HDL modulates macrophage inflammation through activation of Akt and decreased activation of nuclear factor-κB (NF-κB), promoting the release of anti-inflammatory cytokines, including interleukin 10 and transforming growth factor-beta (TGF-ß) [26,39]. In the endothelial cells, SR-B1 inhibits inflammation via endothelial nitric oxide synthase (eNOS) activation and expression of the antioxidant enzyme, 3-beta-hydroxysteroid-delta 24-reductase (DHCR24) [4,40]. HDL and apo A-I also reduce oxidative modification of apo B containing lipoproteins [4]. Furthermore, SR-B1 in macrophages and endothelial cells could suppress the progression of atherosclerosis by modifying cholesterol trafficking and reducing atherosclerotic lesion through limiting foam cell formation [41,42]. Moreover, SR-B1 in macrophages and endothelial cells could also promote the uptake by HDL of modified lipoproteins that contribute to the development of early atherosclerotic lesions [43,44,45]. Hepatic SR-B1 mediates the clearance of VLDL, LDL, and Lp(a), whose accumulation in plasma facilitate the progression of atherosclerosis [16,17,18,19]. SR-B1 promotes the reduction of apoptosis, and mediates efferocytosis of apoptotic cells in macrophages of atherosclerotic lesions [46,47]. Platelet SR-B1 has been implicated as a negative controller in the development of thrombosis [48,49] (Figure 1) (Table 1).
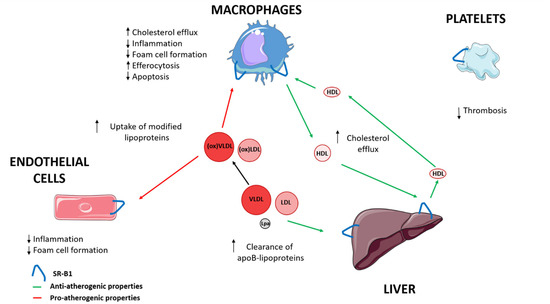
Figure 1.
The role of SR-B1 in progression of atherosclerosis. LDL, low-density lipoprotein; Lp(a), lipoprotein (a); VLDL, very low-density lipoprotein; ↑ increase; ↓ decrease.

Table 1.
The role of SR-B1 in atherosclerosis. LDL, low-density lipoprotein; Lp(a), lipoprotein (a); VLDL, very low-density lipoprotein; ↑ increase; ↓ decrease.
4. Studies in Gene-Targeted Mice Related to Lipoprotein Metabolism and Cardiovascular Disease
Studies using mice models contribute to elucidate the role of SR-B1 in cholesterol homeostasis, lipoprotein metabolism and atherosclerosis, as shown by global gene deletion or overexpression of SR-B1 [4,26]. Despite the fact that the tissue expression of SR-B1 in humans is similar to that in mice [17], the metabolism of lipids and lipoproteins is different between both species [50,51]. Mice transport the bulk of plasma cholesterol in HDL, while plasma cholesterol in humans is carried predominantly by apo B containing lipoproteins such as LDL and VLDL [52]. This discrepancy between mice and humans is caused by the lack of cholesteryl ester transfer protein (CETP) in mice. This protein mediates the exchange of cholesterol and TG between HDL and LDL/VLDL. Therefore, humans have an alternative pathway for cholesterol transported by HDL to reach the liver through transferring cholesterol to LDL and VLDL and then, these lipoproteins are uptaken to the liver by the LDL receptor [41,49].
The absence of SR-B1 has been shown to accelerate the onset of atherosclerosis although SR-B1 knockout mice displayed two-fold elevated HDLc levels [53,54,55] whereas mice overexpression of SR-B1 showed decreased atherosclerosis [56,57], exhibiting the potential important role of SR-B1 in the pathophysiology of CVD. The increased atherosclerosis observed in SR-B1 knockout mice could be a consequence of reduced cholesterol efflux from macrophages to HDL and an impaired delivery of HDL-CE to the liver [54]. However, the expression of human CETP in SR-B1 deficient mice reduced HDLc plasma levels, although this genetic manipulation was not able to protect mice from atherosclerosis, suggesting that SR-B1 could have protective properties in addition to its role in the RCT mediated by HDL particles [58].
Studies in which global SR-B1 deletion has been carried out have demonstrated its relevance in lipoprotein metabolism [53]. Total cholesterol levels were significantly increased in SR-B1 knockout mice as well as HDLc and HDL size. This fact was a result of enhancement of the core of the HDL particles with CE and was suggested to occur due to the failure of hepatic SR-B1 to selectively uptake this core of CE [53]. As a consequence, the biliary cholesterol and adrenal cholesterol were decreased in SR-B1 deficient mice [53,55,59] and showed elevated concentrations of LDLc, VLDL cholesterol, and Lp(a) after human Lp(a) infusion [18,53]. These mice also had a modified cholesterol distribution in platelets, that altered platelet aggregation [48]. Other proatherogenic effects have been associated with the blockage of SR-B1 function, including altered transport of HDLc through the endothelial cells [3,39] and impaired reendothelialization in mouse arteries [3,60]. Moreover, previous studies have shown that female SR-B1 knockout mice were more susceptible to accelerated atherosclerosis and infertility than male mice [55]. A similar phenotype as that exhibited by SR-B1 deficient mice was found in hypomorphic liver-specific SR-B1 knockout mice [53,55,61]. Besides, Huby et al. have exposed that hypomorphic SR-B1 knockout mice, that displayed a reduce expression of SR-B1 in multiple non-hepatic tissues, needed additionally specific deletion of hepatocyte SR-B1 to increase the predisposition for the development of atherosclerotic lesions [3,62].
SR-B1 deficient mice in the background of apo E or LDL receptor depletion have revealed accelerated atherosclerosis and increased LDLc without significant modifications in HDLc levels, proposing that reduced LDLc clearance could play a role in the increased atherosclerosis in these mice [63,64]. SR-B1 depletion in apo E knockout mice showed severe dyslipidemia, early occlusive atherosclerotic coronary artery disease (CAD), spontaneous myocardial infarctions, severe cardiac dysfunction, and mice died prematurely between six and eight weeks of age [28,65]. Consequently, these double knockout mice might be a suitable animal model to evaluate the mechanisms involved in the development of complex CAD, myocardial infarction and heart failure, as well as for preclinical testing of potential genetic or pharmacological treatments for coronary heart disease (CHD) [55]. Interestingly, in support of the anti-atherogenic properties of hepatocyte SR-B1, liver-specific transgenic or adenoviral overexpression of SR-B1 in hyperlipidemic apo E or LDL receptor knockout mice had demonstrated the reduction in atherosclerosis predisposition [3,56,66].
Regarding SR-B1 in macrophages, Van Eck and collaborators investigated the role of macrophage SR-B1 in atherosclerosis by employing the bone marrow transplantation technique to particularly modulate SR-B1 expression in leukocytes in atherosclerosis-susceptible LDL receptor knockout mice [3,43]. In agreement with a pro-atherogenic role of macrophage SR-B1, the early steps involved in the development of atherosclerosis in the LDL receptor knockout mice were prevented by the particular depletion of SR-B1 function in bone marrow-derived cells [3,43]. Moreover, in normolipidemic C57BL/6 mice fed with an atherogenic cholic acid-containing diet, the deficiency of bone marrow-specific SR-B1 reduced the development of atherosclerosis [3,43]. In contrast to small macrophage-rich lesions, a notable anti-atherogenic function for macrophage SR-B1 was found in advanced phases of the disease. Transplantation of deficient SR-B1 bone marrow into lethally irradiated LDL receptor knockout mice promoted the development of greater lesions in the context of a similar extent of hyperlipidemia [3,43]. In addition, SR-B1 inactivation in bone marrow derived cells induced apoptotic cell accumulation within atherosclerotic plaques, due to reduced efferocytosis by SR-B1-deficient macrophages, increasing necrotic core development [46].
Galle-Treger and collaborators have also demonstrated the SR-B1 anti-atherogenic properties. In their study, they generated mice deficient for SR-B1 in monocytes/macrophages and transplanted their bone marrow into LDL receptor knockout or in mice expressing CETP. These mice exhibited accelerated aortic atherosclerosis characterized by larger macrophage-rich areas and decreased macrophage apoptosis when were fed with a cholesterol-rich diet [67]. They found that expression of apoptosis inhibitor of macrophage was induced in SR-B1-deficient macrophages; therefore, they suggested that macrophage SR-B1 was involved in plaque growth by controlling macrophage apoptosis [67]. These results contrast with those obtained by Tao et al. reporting increased apoptotic cell accumulation using the same experimental approach [46]. These contradictions might show that macrophage-SR-B1 exerted various protective activities that depended on the lesion stage development [46,67]. In this sense, the shift from SR-B1 as a pro-atherogenic factor to an anti-atherogenic factor seemed to take place at a lesion size between 150.000 and 250.000 mm 2 [3]. Furthermore, LDL receptor knockout mice that received bone marrow from mice deficient in ABCA1 and SR-B1 exhibited a noticeable increase in the extent of atherosclerosis as compared to ABCA1 deficiency bone marrow in LDL receptor knockout recipient mice [3,68]. These studies showed that the role of SR-B1 in macrophages within atherosclerotic lesions appeared to be dependent on the lipid context and the stage on atherosclerosis development. Therefore, to better evaluate the function of macrophage SR-B1 in human atherosclerosis, mice models with a more human-like lipoprotein profile would be needed [3].
Opposite effects than those shown in SR-B1 deficient mice were found in hepatic overexpression of SR-B1 in mice. These mice displayed lower levels of circulating HDLc, increased HDL-CE clearance and transport of cholesterol from the liver into the bile, and increased biliary cholesterol content [56,57,59,60]. Hepatic overexpression of SR-B1 was associated with reduced concentrations of VLDL and LDL [57,61] as well as increased plasma clearance of Lp(a) [18]. Arai et al. have proposed that at least a part of the protection against atherosclerosis related to SR-B1 overexpression could be attributed to its role on apo B containing lipoproteins such as LDL and VLDL [66]. Studies developed by Vaisman and collaborators have presented that endothelial cell-specific overexpression of SR-B1 in high fat/high cholesterol diet-fed C57BL/6 mice and apo E knockout mice decreased atherosclerosis susceptibility [3,69].
In summary, mice studies have showed the relevant involvement of SR-B1 in the development of CVD. In addition, mice models are needed in order to evaluate the diverse roles of SR-B1 in the different cells and tissues to develop new SR-B1-target treatments for CVD. However, mice experiments have some limitations due to the differences in lipoprotein metabolism between mice and humans.
5. Human Genetic Variants of SCARB1 in Lipoprotein Metabolism and Cardiovascular Disease
The evaluation of gene variants in SCARB1 has contributed to elucidate the association of SR-B1 in the regulation of lipoprotein metabolism in humans (Table 2) (Figure 2). The first study to show the involvement of genetic variants at SCARB1 on SR-B1 in humans described three common polymorphisms, at exons 1 and 8 and intron 5 in Spanish Caucasians (Table 2) (Figure 2) [70]. The single nucleotide variant (SNV) in exon 1 at base pair (bp) 4 encoded a change from glycine to serine at the second aa position, c.4G>A, p.(Gly2Ser). At exon 8, the SNV was confirmed to compromise a change in bp 1050 encoding aa 350 (c.1050T > G, p.(Ala350 = )), but there was no aa change. Finally, SNV close to exon 5 was located in adjacent intron but was not in the canonical splice-site sequence (c.726 + 54C > T). Subsequent analyses demonstrated the involvement of SR-B1 variants in lipoprotein metabolism. In this sense, exon 1 variant (c.4G > A, p.(Gly2Ser)) was significantly related to an increase of HDLc and lower LDLc levels in men linked with a reduced atherogenic profile. Women carriers of exon 8 genetic variant exhibited reduced LDLc values. Intron 5 variant was related to higher body mass index in women and lower TG levels in men [70]. Further population-base studies of SCARB1 gene polymorphisms established the involvement of these genetic variants in plasma lipoprotein profile [71,72], although several studies resulted in conflicting results [73,74] or failed to find an association [75,76]. These studies focused on different populations, including women and estrogen therapy [71], diabetics [72,75], different ethnic groups [77,78,79,80] individuals with familial hypercholesterolemia [51,81], and CVD patients [73,74,75,76,77,78,79,80,81,82,83]. Moreover, additional factors related to lipoprotein metabolism were evaluated in association with these genetic variants including the response to different diets [84,85] and pharmacological interventions [86,87]. The differences found in lipoprotein metabolism between studies is possible to be explained by sample size, gender, ethnic groups, physical condition, and other variables [87]. The precise role of SCARB1 genetic variants in the alterations observed in lipoprotein metabolism is unknown [51]. The polymorphism located in exon 1, p.(Gly2Ser), results in an aa change in the protein. Further experiments showed that selective cholesterol uptake resided in the extracellular domain of SR-B1 receptor, but the aa change takes place in the intracellular N-terminus [51,71]. Although exon 8 polymorphism does not involve an aa change, it has been shown that it decreases protein expression by changing RNA secondary structure, therefore could alter the functionality of the receptor [88,89]. Intron 5 SNV has no recognized effect on splicing or gene expression [71]. Several studies have suggested that none of these three polymorphisms is functional and that the associations found are a consequence of linkage to other genetic variants at the SR-B1 locus or neighboring loci yet to be recognized [51,87].

Table 2.
Effects of SCARB1 gene variants on serum lipid profile. cDNA position was related to the SCARB1 gene (NM.005505.5; encoding SR-B1). † Nucleotide position 1 is the first nucleotide at the ATG initiation codon. * Acton et al., 1999 results are presented in this table since it was the first time that these variants were described, although several studies have shown different results. NA indicates not applicable. Apo, apolipoprotein; CE, cholesterol ester; CHD, coronary heart disease; CVD, cardiovascular disease; HDL, high-density lipoprotein; HDLc, high-density lipoprotein cholesterol; LDL, low-density lipoprotein; LDLc, low-density lipoprotein cholesterol; Lp(a), lipoprotein (a); SR-B1, scavenger receptor B class 1; VLDL, very low-density lipoprotein; ↓ decrease; ↑ increase.
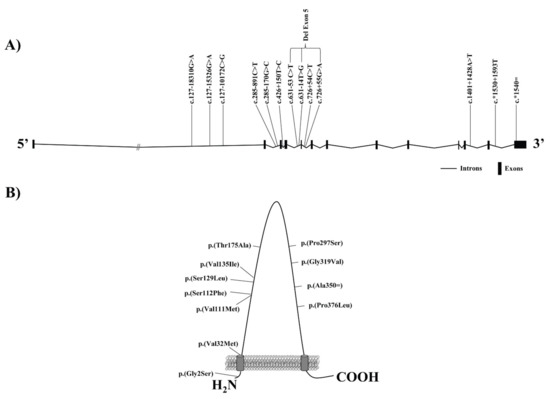
Figure 2.
Localization of intronic (A) and exonic (B) variants involved in lipoprotein metabolism described in this review are shown in a schematic SCARB1 gene (A) and SR-B1 protein (B).
In humans, more genetic variants in SCARB1 have been reported (Table 2) (Figure 2). A study developed in the Taiwanese Chinese population found two novel variants in the SCARB1 gene promoter region. One of them was an 11-bp CCCCGCCCCGT deletion from positions −140 to −150 (c.-140_-150del) from the transcription start site, corresponding to a Sp1 binding site, and the other one was a C→ to T substitution at position −142 (c.-142C>T). In vitro experiments showed that the promoter containing the −140 to −150del allele exhibited reduced activity and this finding was in accordance with the higher plasma HDLc levels presented in these subjects. However, no significant results were identified in the c.-142C>T [77]. Research developed in the Amish population showed that the missense variant in exon 3, c.403G>A, p.(Val135Ile) was associated with higher levels of HDLc in women [72]. This genetic variant was also related to higher apo B levels in a study developed in US non-hispanic white people with extreme values of HDLc [80]. The objective of the study carried out by Niemsiri et al. was to resequence the SCARB1 gene in selected US non-Hispanic white individuals with extreme HDLc levels to identify common and rare variants and then to evaluate the role of the identified variants with plasma HDLc, LDLc, TG, and apo B levels [80]. Single-locus analysis revealed three nominally significant associations with HDLc: c.127–18310G>A, c.*1530+1593T, and c.*1540=, that have been reported to be genome-wide significant [80,93]. In addition, three common SCARB1 polymorphisms (c.285–891C>T, c.285–170G>C, and c.426+150T>C) showed significant associations with apo B [80]. Notably, c.285–891C>T has been previously established to be related to carotid intima-media thickness and incidence of CHD [90]. The risk of allele c.285–891C>T has also been found to be associated with lower SR-B1 protein levels [91].
Naj et al. found that the c.127–15329G>A genetic variant was associated with higher common carotid intima-media thickness, one of the subclinical atherosclerosis features, in multiple ethnic groups [90]. Chiba-Falek et al. showed that c.1401+1428A>T was associated with endogenous estradiol levels, HDLc, TG, and the TG:HDLc ratio in postmenopausal Caucasian women. In addition, this study found that this variant was related to decreased level of liver SR-B1 in women under 45 years old, suggesting that this SNV could be associated with CHD [94]. However, no associations of these variants were found in the Han Chinese population [92]. Naj et al. also showed that the C allele of c.127–10172C>G variant was related to higher common carotid intima-media thickness in African American, Chinese, European American, and Hispanic subjects, although, this association was independent of lipid levels [90]. However, this genetic variant in the Han Chinese population seemed to increase CHD risk as well as the HDLc level [92].
Vergeer and collaborators sequenced the gene encoding SR-B1 in the Caucasian population with high HDLc levels and reported a family with a mutation in the nucleotide 889 producing a proline to serine substitution at aa position 297, c.889C>T, p.(Pro297Ser). This aa change was located at the extracellular loop, suggesting it to be functionally relevant [98,99]. These subjects displayed increased HDLc, reduced capacity to efflux cholesterol from macrophages, impaired platelet function, and decreased adrenal steroidogenesis. In addition, primary murine hepatocytes expressing SR-B1 p.(Pro297Ser) exhibited reduced cholesterol uptake from HDL. However, carotid intima-media thickness was similar in carriers and family controls. Vergeer et al. explained that the statistical power to distinguish a difference in carotid intima-media thickness was low, assumed the small number of carriers and their relatively young age [41]. Further experiments found that p.(Pro297Ser) mutation alters the protein composition of HDL and LDL/VLDL [99]. Applying the same strategy explained above, Brunham et al. identified two novel missense mutations, c.335C>T, p.(Ser112Phe), and c.523A>G, p.(Thr175Ala) by sequencing SCARB1 gene in the Caucasian ancestry population with elevated HDLc level. p.(Ser112Phe) and p.(Thr175Ala) mutations occurring in the large extracellular loop of the SR-B1 protein. None of those mutation carriers had a history of CVD [98]. In vitro studies showed that both mutant receptors exhibited altered HDL binding, selective uptake of HDL-CE, and delivery of FC from cells to HDL. These results suggest that increased plasma HDLc in these settings could not be associated with reduced risk of CVD [100].
As mentioned before, Yang and collaborators described, by experiments in vitro and in vivo, the role of Lp(a) as ligand of SR-B1, which mediates the selective uptake of Lp(a)-associated lipids [18]. To assess this new mechanism in humans, two cohorts, that included multi-ethnic populations, were examined for combined elevations of HDLc and Lp(a) to evaluate its interaction with SCARB1 genetic variants and with reduced function of SR-B1 protein [89]. Five novel missense or splice site variants in SCARB1 were identified. One SNV in exon 3 results in a missense serine to leucine substitution at aa 129 (c386C>T, p.(Ser129Leu)). Two mutations produce the deletion of exon 5: the splicing variant c.631–14T>G and a compound mutation at c.631–53C>T and c.726+55G>A. In the SR-B1 isoform 2, c.1495G>A induces a p.(Gly499Arg) substitution in the carboxy-terminal tail. Finally, a missense substitution at exon 3 results in aa change in the extracellular loop of SR-B1: p.(Glu130Gly) (c.386A>G). The function of p.(Gly499Arg) and p.(Glu130Gly) was not tested. In addition, they also distinguished two common polymorphisms described before, p.(Gly2Ser) and p.(Ala350A=). Posterior in vitro studies showed that the p.(Ser129Leu) polymorphism and the mutations that caused the deletion of exon 5 lower CE uptake from HDL and Lp(a) [89]. The consequence of high HDLc/high Lp(a) phenotype on human CVD is not established. In this sense, Lp(a) is a pro-atherogenic lipoprotein [19,89], whereas high HDLc could reduce CVD risk [5,6]; however, not all conditions that elevate HDL are protective in humans [95,96].
Recently, Zanoni et al. identified a missense mutation in SCARB1, which replaces proline at the 376 position by leucine (c.1127C>T, p.(Pro376Leu)), by targeted sequencing of coding regions of lipid-modifying genes in individuals of European ancestry with extremely elevated HDLc levels. This aa substitution occurs on the extracellular loop proximal to the C-terminal transmembrane domain. In the homozygous subject, p.(Pro376Leu) mutation altered posttranslational processing of SR-B1, abolished selective HDLc uptake in vitro and in vivo experiments and reduced the carotid intima-media thickness. In addition, a meta-analysis of 16 studies showed that p.(Pro376Leu) carriers had a significantly higher risk of CAD compared with non-carriers [95]. In agreement with these results, Samadi et al. concluded that carriers of p.(Pro376Leu) mutation were more susceptible to develop CVD, although no differences were found in HDLc levels;serum HDL lipid peroxidation, measured as dysfunctional HDL, was increased in the presence of p.(Pro376Leu) mutation [96]. This study was developed in CVD patients from the Mashhad Stroke and Heart Atherosclerotic Disorders (MASHAD) cohort. Some authors have suggested that given the extremely low and variable carrier rate of this genetic variant between study groups in the meta-analysis and taking into account that this study was relatively specific to Ashkenazi Jews, p.(Pro376Leu) mutation could be an indirect indicator for a substratum of the population [8,101]. Subsequent to the publication of Zanoni and collaborators’ results, Helgadottir et al. decided to study the hypothesis that alleles in SCARB1 gene related to higher levels of HDLc are also associated with increased risk of CAD in the relatively homogeneous population of Iceland. They identified three novel SCARB1 missense genetic variants, c.956G>T, p.(Gly319Val), c.331G>A, p.(Val111Met), and c.94G>A, p.(Val32Met), associated with increased HDLc levels. p.(Gly319Val) and p.(Val111Met) variants take place in the large extracellular loop of the SR-B1 protein. Moreover, they also identified a missense variant, p.(Val135Ile) described before in Amish population and in US non-Hispanic white people [72,80]. They studied the association between the described polymorphisms in Iceland population and CAD risk, although no linkage was found. The lack of relation between these polymorphisms producing high HDLc levels and the risk of CAD suggested no alteration in the hepatocellular trafficking of cholesterol to bile. However, they proposed that an enhancement of CETP-mediated exchange of CE from HDL to apo B containing lipoproteins could prevent the increase in HDLc in genetically impairment of SR-B1. Therefore, no effect was found on the hepatic cholesterol clearance in carriers of the Icelandic variants. To validate this hypothesis, they evaluated the formation of gallstone since it has been described as a manifestation of cholesterol hypersecretion to bile [8,102]. They found a significant increase of gallstone formation in p.(Val111Met) carriers, supporting the finding that an increase of HDLc levels in humans impairs cholesterol excretion through bile [8]. May et al. described a patient from the Lipid Genetics Clinic at the London Health Sciences Centre, University Hospital (London, ON, Canada) who had elevated levels of HDLc. This subject was found to carry a heterozygous variant of SCARB1 consisting in a missense arginine to cysteine substitution at aa 174 (p.Arg174Cys). This mutation showed reduced cholesterol transport, suggesting an impairment in the cholesterol clearance [97].
SCARB1 genetic variants research and the description of mutations in human SCARB1 have established the involvement of SR-B1 in regulating lipoprotein metabolism in humans [27]. However, based on the divergent results, more research is needed in order to elucidate the role of SR-B1 variants in the increment of HDLc levels and CVD risk.
6. Genome-Wide Association Studies of SCARB1
Genome-wide association studies (GWAS) have been used to distinguish disease susceptibility loci, proposing novel genetic variants at new loci related to CVD and serum HDLc concentrations [103]. GWAS for plasma lipids first associated SCARB1 common polymorphisms with HDLc in 2010 [93]. In this study, the principal SNV in SCARB1 implicated from this GWAS, c.*1540G>T, was related to increased HDLc [93]. However, the relationship of the haplotype to SCARB1 expression has not been confirmed yet at molecular level [104]. Recently, two studies identified significant associations for the SCARB1 locus with CAD risk [103,104]. Remarkably, these studies did not find that this CAD-associated haplotype is in linkage disequilibrium with the HDLc associated haplotype from GWAS for lipids. However, Webb and collaborators showed that the lead variant, c.127–4800C>T, has a strong association with plasma LDLc and TG levels, and this genetic variant has also been related to increased lipoprotein-associated phospholipase A2 activity [103]. In addition, c.127–14490G>A is related to expression of SCARB1 in the intestine [103]. The causal mechanisms explaining the apparent lack of association between HDLc, CAD traits and SCARB1 significant signals continue to be unknown, suggesting that further functional genomic investigations to better comprehend the function of these regulatory variants are needed [105].
7. Conclusions
SR-B1 not only plays a role in the metabolism of HDL but also is involved in the clearance of LDL, VLDL, and Lp(a), proposing an important participation in the development of CVD [16,17,18]. Therefore, mice and human genetic studies have shown genetic variants in SCARB1 gene-induced higher levels of these mentioned lipoproteins and can alter their composition. In addition, SR-B1 variants can modify cholesterol efflux of macrophages and hepatic reverse cholesterol transport, reduce adrenal steroidogenesis, alter platelet functions, as well as decrease the selective uptake of CE.
The available data propose that plasma HDLc levels are not an optimal therapeutic target and that the quality of the HDL particles circulating in plasma could be more critical for therapeutic interventions than HDLc concentration [14], since high HDLc levels may not always be protective against CVD [95,106]. In fact, European Society of Cardiology and European Atherosclerosis Society Guidelines for the Management of Dyslipidaemias [107] do not recommend HDLc as a target for treatment. The differential HDLc uptake by SR-B1 among subjects may be one factor associated with modified HDL quality and functionality associated with CVD risk without substantial modification of HDLc concentration.
The contradictory results in human SR-B1 variants in the relationship between increased HDLc levels and CVD risk, as well as GWAS of SCARB1, suggest the involvement of other pathways. Furthermore, human SR-B1 variants provide evidence that modulating different functions of SR-B1 might improve the development of CVD [8]. It is important to mention that the role of SR-B1 in CVD is cell/tissue type-specific [3,4,44], highlighting the complexities of potential therapeutic development with SR-B1 modulating agents [8]. Therefore, further research is needed in order to develop a proper SR-B1-based therapeutic approach.
Author Contributions
I.G.-R. wrote the manuscript. C.M., F.C., and A.C. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from Gobierno de Aragón, B14–7R, Spain, and the Spanish Ministry of Science and Innovation PI18/01777, PI19/00694 and CIBERCV. These projects are co-financed by Instituto de Salud Carlos III and the European Regional Development Fund (ERDF) of the European Union ‘‘A way to make Europe’’. I.G.-R. was funded by Sara Borrell fellowship CD19/00245.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Aa: amino acid; ABCA1, ATP-binding cassette A1; apo, apolipoprotein; ATP-binding cassette G1 (ABCG1); bp, base pair; CAD, coronary artery disease; CE, cholesterol ester; CETP, cholesteryl ester transfer protein; CHD, Coronary heart disease; CVD, cardiovascular disease; DHCR24, 3-beta-hydroxysteroid-delta 24-reductase; eNOS, endothelial nitric oxide synthase; FC, free cholesterol; GWAS, Genome-wide association studies; HDL, high-density lipoprotein; HDLc, high-density lipoprotein cholesterol; LDL, low-density lipoprotein; LDLc, low-density lipoprotein cholesterol; LIMP-2, lysosomal integral membrane protein 2; Lp(a), Lipoprotein (a); MASHAD, Mashhad-Stroke and Heart-Atherosclerotic-Disorders; NAFLD, non-alcoholic fatty liver disease; NF-κB, nuclear factor-κB; PDZK1, PDZ Domain Containing 1; RCT, reverse cholesterol transport; SNV, single nucleotide variant; SR-B1, scavenger receptor B class 1; SR-B2, scavenger receptor B class 2; TG, triglycerides; TGF-ß, transforming growth factor-beta; VLDL, very low-density lipoprotein.
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics’2017 Update: A Report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar]
- Castelli, W.P.; Garrison, R.J.; Wilson, P.W.F.; Abbott, R.D.; Kalousdian, S.; Kannel, W.B. Incidence of Coronary Heart Disease and Lipoprotein Cholesterol Levels: The Framingham Study. JAMA J. Am. Med. Assoc. 1986, 256, 2835–2838. [Google Scholar] [CrossRef]
- Hoekstra, M. SR-BI as target in atherosclerosis and cardiovascular disease—A comprehensive appraisal of the cellular functions of SR-BI in physiology and disease. Atherosclerosis 2017, 258, 153–161. [Google Scholar] [CrossRef]
- Linton, M.F.; Tao, H.; Linton, E.F.; Yancey, P.G. SR-BI: A Multifunctional Receptor in Cholesterol Homeostasis and Atherosclerosis. Trends Endocrinol. Metab. 2017, 28, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; Packard, C.J.; et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA J. Am. Med. Assoc. 2009, 302, 1993–2000. [Google Scholar] [CrossRef]
- Assmann, G.; Cullen, P.; Schulte, H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Münster (PROCAM) study. Circulation 2002, 105, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Brunham, L.R.; Hayden, M.R. Human genetics of HDL: Insight into particle metabolism and function. Prog. Lipid Res. 2015, 58, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Helgadottir, A.; Sulem, P.; Thorgeirsson, G.; Gretarsdottir, S.; Thorleifsson, G.; Jensson, B.Ö.; Arnadottir, G.A.; Olafsson, I.; Eyjolfsson, G.I.; Sigurdardottir, O.; et al. Rare SCARB1 mutations associate with high-density lipoprotein cholesterol but not with coronary artery disease. Eur. Heart J. 2018, 39, 2172–2178. [Google Scholar] [CrossRef]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Hólm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef]
- Holmes, M.V.; Ala-Korpela, M.; Smith, G.D. Mendelian randomization in cardiometabolic disease: Challenges in evaluating causality. Nat. Rev. Cardiol. 2017, 14, 577–599. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Olsson, A.G.; Abt, M.; Ballantyne, C.M.; Barter, P.J.; Brumm, J.; Chaitman, B.R.; Holme, I.M.; Kallend, D.; Leiter, L.A.; et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 2012, 367, 2089–2099. [Google Scholar] [CrossRef]
- Barter, P.J.; Caulfield, M.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.P.; Komajda, M.; Lopez-Sendon, J.; Mosca, L.; Tardif, J.C.; Waters, D.D.; et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 2007, 357, 2109–2122. [Google Scholar] [CrossRef]
- Van der Steeg, W.A.; Holme, I.; Boekholdt, S.M.; Larsen, M.L.; Lindahl, C.; Stroes, E.S.G.; Tikkanen, M.J.; Wareham, N.J.; Faergeman, O.; Olsson, A.G.; et al. High-Density Lipoprotein Cholesterol, High-Density Lipoprotein Particle Size, and Apolipoprotein A-I: Significance for Cardiovascular Risk. The IDEAL and EPIC-Norfolk Studies. J. Am. Coll. Cardiol. 2008, 51, 634–642. [Google Scholar] [CrossRef]
- Chroni, A.; Kardassis, D. HDL Dysfunction Caused by Mutations in apoA-I and Other Genes that are Critical for HDL Biogenesis and Remodeling. Curr. Med. Chem. 2018, 26, 1544–1575. [Google Scholar] [CrossRef]
- Acton, S.; Rigotti, A.; Landschulz, K.T.; Xu, S.; Hobbs, H.H.; Kriegert, M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science (80-.) 1996, 271, 518–520. [Google Scholar] [CrossRef]
- Acton, S.L.; Scherer, P.E.; Lodish, H.F.; Krieger, M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J. Biol. Chem. 1994, 269, 21003–21009. [Google Scholar] [CrossRef]
- Calvo, D.; Gómez-Coronado, D.; Lasunción, M.A.; Vega, M.A. CLA-1 is an 85-kD plasma membrane glycoprotein that acts as a high- affinity receptor for both native (HDL, LDL, and VLDL) and modified (OxLDL and AcLDL) lipoproteins. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2341–2349. [Google Scholar] [CrossRef]
- Yang, X.P.; Amar, M.J.; Vaisman, B.; Bocharov, A.V.; Vishnyakova, T.G.; Freeman, L.A.; Kurlander, R.J.; Patterson, A.P.; Becker, L.C.; Remaley, A.T. Scavenger receptor-BI is a receptor for lipoprotein(a). J. Lipid Res. 2013, 54, 2450–2457. [Google Scholar] [CrossRef]
- Utermann, G. The mysteries of lipoprotein(a). Science (80-.) 1989, 246, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Al-Jarallah, A.; Trigatti, B.L. A role for the scavenger receptor, class B type I in high density lipoprotein dependent activation of cellular signaling pathways. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Citro, V.; Capone, D. Nonalcoholic Fatty Liver Disease: A Challenge from Mechanisms to Therapy. J. Clin. Med. 2019, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Liu, S.; Chen, H.T.; Yu, C.H.; Teng, X.D.; Yao, H.T.; Xu, G.Q. Upregulation of caveolin-1 and SR-B1 in mice with non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2013, 12, 630–636. [Google Scholar] [CrossRef]
- Krieger, M. Charting the fate of the “good cholesterol”: Identification and characterization of the high-density lipoprotein receptor SR-BI. Annu. Rev. Biochem. 1999, 68, 523–558. [Google Scholar] [CrossRef] [PubMed]
- Connelly, M.A.; Williams, D.L. Scavenger receptor BI: A scavenger receptor with a mission to transport high density lipoprotein lipids. Curr. Opin. Lipidol. 2004, 15, 287–295. [Google Scholar] [CrossRef]
- Rigotti, A.; Miettinen, H.E.; Krieger, M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr. Rev. 2003, 24, 357–387. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-J.; Azhar, S.; Kraemer, F.B. SR-B1: A Unique Multifunctional Receptor for Cholesterol Influx and Efflux. Annu. Rev. Physiol. 2018, 80, 95–116. [Google Scholar] [CrossRef]
- Shen, W.J.; Asthana, S.; Kraemer, F.B.; Azhar, S. Scavenger receptor B type 1: Expression, molecular regulation, and cholesterol transport function. J. Lipid Res. 2018, 59, 1114–1131. [Google Scholar] [CrossRef]
- Shen, W.J.; Hu, J.; Hu, Z.; Kraemer, F.B.; Azhar, S. Scavenger Receptor class B type i (SR-BI): A versatile receptor with multiple functions and actions. Metabolism 2014, 63, 875–886. [Google Scholar] [CrossRef]
- Gaidukov, L.; Nager, A.R.; Xu, S.; Penman, M.; Krieger, M. Glycine dimerization motif in the N-terminal transmembrane domain of the high density lipoprotein receptor SR-BI required for normal receptor oligomerization and lipid transport. J. Biol. Chem. 2011, 286, 18452–18464. [Google Scholar] [CrossRef]
- Sahoo, D.; Darlington, Y.F.; Pop, D.; Williams, D.L.; Connelly, M.A. Scavenger receptor class B Type I (SR-BI) assembles into detergent-sensitive dimers and tetramers. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2007, 1771, 807–817. [Google Scholar] [CrossRef]
- Marques, P.E.; Nyegaard, S.; Collins, R.F.; Troise, F.; Freeman, S.A.; Trimble, W.S.; Grinstein, S. Multimerization and Retention of the Scavenger Receptor SR-B1 in the Plasma Membrane. Dev. Cell 2019, 50, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Rodrigueza, W.V.; Thuahnai, S.T.; Temel, R.E.; Lund-Katz, S.; Phillips, M.C.; Williams, D.L. Mechanism of scavenger receptor class B type I-mediated selective uptake of cholesteryl esters from high density lipoprotein to adrenal cells. J. Biol. Chem. 1999, 274, 20344–20350. [Google Scholar] [CrossRef]
- Neculai, D.; Schwake, M.; Ravichandran, M.; Zunke, F.; Collins, R.F.; Peters, J.; Neculai, M.; Plumb, J.; Loppnau, P.; Pizarro, J.C.; et al. Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature 2013, 504, 172–176. [Google Scholar] [CrossRef]
- Yancey, P.G.; De La Llera-Moya, M.; Swarnakar, S.; Monzo, P.; Klein, S.M.; Connelly, M.A.; Johnson, W.J.; Williams, D.L.; Rothblat, G.H. High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J. Biol. Chem. 2000, 275, 36596–36604. [Google Scholar] [CrossRef] [PubMed]
- Mineo, C. Lipoprotein Receptor Signaling in Atherosclerosis. Cardiovasc. Res. 2020, 116, 1254–1274. [Google Scholar] [CrossRef] [PubMed]
- Reaven, E.; Chen, Y.D.I.; Spicher, M.; Azhar, S. Morphological evidence that high density lipoproteins are not internalized by steroid-producing cells during in situ organ perfusion. J. Clin. Investig. 1984, 74, 1384–1397. [Google Scholar] [CrossRef] [PubMed]
- Oram, J.F.; Lawn, R.M.; Garvin, M.R.; Wade, D.P. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J. Biol. Chem. 2000, 275, 34508–34511. [Google Scholar] [CrossRef]
- Sankaramarayanan, S.; Oram, J.F.; Asztalos, B.F.; Vaughan, A.M.; Lund-Katz, S.; Adorni, M.P.; Phillips, M.C.; Rothblat, G.H. Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J. Lipid Res. 2009, 50, 275–284. [Google Scholar] [CrossRef]
- Lim, H.Y.; Thiam, C.H.; Yeo, K.P.; Bisoendial, R.; Hii, C.S.; McGrath, K.C.Y.; Tan, K.W.; Heather, A.; Alexander, J.S.J.; Angeli, V. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-Mediated transport of HDL. Cell Metab. 2013, 17, 671–684. [Google Scholar] [CrossRef]
- McGrath, K.C.Y.; Li, X.H.; Puranik, R.; Liong, E.C.; Tan, J.T.M.; Dy, V.M.; Dibartolo, B.A.; Barter, P.J.; Rye, K.A.; Heather, A.K. Role of 3β-hydroxysteroid-Δ24 reductase in mediating antiinflammatory effects of high-density lipoproteins in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 877–882. [Google Scholar] [CrossRef]
- Vergeer, M.; Korporaal, S.J.A.; Franssen, R.; Meurs, I.; Out, R.; Hovingh, G.K.; Hoekstra, M.; Sierts, J.A.; Dallinga-Thie, G.M.; Motazacker, M.M.; et al. Genetic Variant of the Scavenger Receptor BI in Humans. N. Engl. J. Med. 2011, 364, 136–145. [Google Scholar] [CrossRef]
- Yancey, P.G.; Jerome, W.G.; Yu, H.; Griffin, E.E.; Cox, B.E.; Babaev, V.R.; Fazio, S.; Linton, M.F. Severely altered cholesterol homeostasis in macrophages lacking apoE and SR-BI. J. Lipid Res. 2007, 48, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Van Eck, M.; Bos, I.S.T.; Hildebrand, R.B.; Van Rij, B.T.; Van Berkel, T.J.C. Dual role for scavenger receptor class B, type I on bone marrow-derived cells in atherosclerotic lesion development. Am. J. Pathol. 2004, 165, 785–794. [Google Scholar] [CrossRef]
- Huang, L.; Chambliss, K.L.; Gao, X.; Yuhanna, I.S.; Behling-Kelly, E.; Bergaya, S.; Ahmed, M.; Michaely, P.; Luby-Phelps, K.; Darehshouri, A.; et al. SR-B1 drives endothelial cell LDL transcytosis via DOCK4 to promote atherosclerosis. Nature 2019, 569, 565–569. [Google Scholar] [CrossRef]
- Yu, L.; Dai, Y.; Mineo, C. Novel Functions of Endothelial Scavenger Receptor Class B Type I. Curr. Atheroscler. Rep. 2021, 23. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Yancey, P.G.; Babaev, V.R.; Blakemore, J.L.; Zhang, Y.; Ding, L.; Fazio, S.; Linton, M.F. Macrophage SR-BI mediates efferocytosis via Src/PI3K/Rac1 signaling and reduces atherosclerotic lesion necrosis. J. Lipid Res. 2015, 56, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- Mineo, C.; Shaul, P.W. Novel biological functions of high-density lipoprotein cholesterol. Circ. Res. 2012, 111, 1079–1090. [Google Scholar] [CrossRef]
- Dole, V.S.; Matuskova, J.; Vasile, E.; Yesilaltay, A.; Bergmeier, W.; Bernimoulin, M.; Wagner, D.D.; Krieger, M. Thrombocytopenia and platelet abnormalities in high-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1111–1116. [Google Scholar] [CrossRef]
- Korporaal, S.J.A.; Meurs, I.; Hauer, A.D.; Hildebrand, R.B.; Hoekstra, M.; Ten Cate, H.; Praticò, D.; Akkerman, J.W.N.; Van Berkel, T.J.C.; Kuiper, J.; et al. Deletion of the high-density lipoprotein receptor scavenger receptor BI in mice modulates thrombosis susceptibility and indirectly affects platelet function by elevation of plasma free cholesterol. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 34–42. [Google Scholar] [CrossRef]
- Barter, P.J.; Brewer, H.B.; Chapman, M.J.; Hennekens, C.H.; Rader, D.J.; Tall, A.R. Cholesteryl ester transfer protein: A novel target for raising HDL and inhibiting atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 160–167. [Google Scholar] [CrossRef]
- Tai, E.S.; Ordovas, J.M.; Carmena-Ramon, R.; Real, J.; Corella, D.; Ascaso, J.; Carmena, R. Polymorphisms at the SRBI locus are associated with lipoprotein levels in subjects with heterozygous familial hypercholesterolemia. Clin. Genet. 2003, 63, 53–58. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Hagi, A.; Inoue, Y. The relationship between plasma high density lipoprotein cholesterol levels and cholesteryl ester transfer protein activity in six species of healthy experimental animals. Biol. Pharm. Bull. 2001, 24, 579–581. [Google Scholar] [CrossRef]
- Rigotti, A.; Trigatti, B.L.; Penman, M.; Rayburn, H.; Herz, J.; Krieger, M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc. Natl. Acad. Sci. USA 1997, 94, 12610–12615. [Google Scholar] [CrossRef] [PubMed]
- Van Eck, M.; Twisk, J.; Hoekstra, M.; Van Rij, B.T.; Van der Lans, C.A.C.; Bos, I.S.T.; Kruijt, J.K.; Kuipers, F.; Van Berkel, T.J.C. Differential effects of scavenger receptor BI deficiency on lipid metabolism in cells of the arterial wall and in the liver. J. Biol. Chem. 2003, 278, 23699–23705. [Google Scholar] [CrossRef]
- Trigatti, B.L.; Krieger, M.; Rigotti, A. Influence of the HDL receptor SR-BI on lipoprotein metabolism and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Kozarsky, K.F.; Donahee, M.H.; Glick, J.M.; Krieger, M.; Rader, D.J. Gene transfer and hepatic overexpression of the HDL receptor SR-BI reduces atherosclerosis in the cholesterol-fed LDL receptor-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Royer, L.; Gong, E.; Zhang, J.; Cooper, P.N.; Francone, O.; Rubin, E.M. Lower plasma levels and accelerated clearance of high density lipoprotein (HDL) and non-HDL cholesterol in scavenger receptor class B type I transgenic mice. J. Biol. Chem. 1999, 274, 7165–7171. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, R.B.; Lammers, B.; Meurs, I.; Korporaal, S.J.A.; De Haan, W.; Zhao, Y.; Kruijt, J.K.; Praticò, D.; Schimmel, A.W.M.; Holleboom, A.G.; et al. Restoration of high-density lipoprotein levels by cholesteryl ester transfer protein expression in scavenger receptor class B Type i (SR-BI) knockout mice does not normalize pathologies associated with SR-BI deficiency. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1439–1445. [Google Scholar] [CrossRef][Green Version]
- Ji, Y.; Wang, N.; Ramakrishnan, R.; Sehayek, E.; Huszar, D.; Breslow, J.L.; Tall, A.R. Hepatic scavenger receptor BI promotes rapid clearance of high density lipoprotein free cholesterol and its transport into bile. J. Biol. Chem. 1999, 274, 33398–33402. [Google Scholar] [CrossRef]
- Mineo, C.; Shaul, P.W. Role of High-Density Lipoprotein and Scavenger Receptor B Type I in the Promotion of Endothelial Repair. Trends Cardiovasc. Med. 2007, 17, 156–161. [Google Scholar] [CrossRef]
- Varban, M.L.; Rinninger, F.; Wang, N.; Fairchild-Huntress, V.; Dunmore, J.H.; Fang, Q.; Gosselin, M.L.; Dixon, K.L.; Deeds, J.D.; Acton, S.L.; et al. Targeted mutation reveals a central role for SR-BI in hepatic selective uptake of high density lipoprotein cholesterol. Proc. Natl. Acad. Sci. USA 1998, 95, 4619–4624. [Google Scholar] [CrossRef]
- Huby, T.; Doucet, C.; Dachet, C.; Ouzilleau, B.; Ueda, Y.; Afzal, V.; Rubin, E.; Chapman, M.J.; Lesnik, P. Knockdown expression and hepatic deficiency reveal an atheroprotective role for SR-BI in liver and peripheral tissues. J. Clin. Investig. 2006, 116, 2767–2776. [Google Scholar] [CrossRef]
- Huszar, D.; Varban, M.L.; Rinninger, F.; Feeley, R.; Arai, T.; Fairchild-Huntress, V.; Donovan, M.J.; Tall, A.R. Increased LDL cholesterol and atherosclerosis in LDL receptor-deficient mice with attenuated expression of scavenger receptor B1. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Covey, S.D.; Krieger, M.; Wang, W.; Penman, M.; Trigatti, B.L. Scavenger receptor class B type I-mediated protection against atherosclerosis in LDL receptor-negative mice involves its expression in bone marrow-derived cells. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Trigatti, B.L.; Post, M.J.; Sato, K.; Simons, M.; Edelberg, J.M.; Rosenberg, R.D.; Schrenzel, M.; Krieger, M. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ. Res. 2002, 90, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Wang, N.; Bezouevski, M.; Welch, C.; Tall, A.R. Decreased atherosclerosis in heterozygous low density lipoprotein receptor-deficient mice expressing the scavenger receptor BI transgene. J. Biol. Chem. 1999, 274, 2366–2371. [Google Scholar] [CrossRef] [PubMed]
- Galle-Treger, L.; Moreau, M.; Ballaire, R.; Poupel, L.; Huby, T.; Sasso, E.; Troise, F.; Poti, F.; Lesnik, P.; Le Goff, W.; et al. Targeted invalidation of SR-B1 in macrophages reduces macrophage apoptosis and accelerates atherosclerosis. Cardiovasc. Res. 2020, 116, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pennings, M.; Hildebrand, R.B.; Ye, D.; Calpe-Berdiel, L.; Out, R.; Kjerrulf, M.; Hurt-Camejo, E.; Groen, A.K.; Hoekstra, M.; et al. Enhanced foam cell formation, atherosclerotic lesion development, and inflammation by combined deletion of ABCA1 and SR-BI in bone marrow-derived cells in LDL receptor knockout mice on western-type diet. Circ. Res. 2010, 107, e20–e31. [Google Scholar] [CrossRef]
- Vaisman, B.L.; Vishnyakova, T.G.; Freeman, L.A.; Amar, M.J.; Demosky, S.J.; Liu, C.; Stonik, J.A.; Sampson, M.L.; Pryor, M.; Bocharov, A.V.; et al. Endothelial Expression of Scavenger Receptor Class B, Type I Protects against Development of Atherosclerosis in Mice. Biomed Res. Int. 2015, 2015, 607120. [Google Scholar] [CrossRef]
- Acton, S.; Osgood, D.; Donoghue, M.; Corella, D.; Pocovi, M.; Cenarro, A.; Mozas, P.; Keilty, J.; Squazzo, S.; Woolf, E.A.; et al. Association of polymorphisms at the SR-BI gene locus with plasma lipid levels and body mass index in a white population. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1734–1743. [Google Scholar] [CrossRef]
- Richard, E.; Von Muhlen, D.; Barrett-Connor, E.; Alcaraz, J.; Davis, R.; McCarthy, J.J. Modification of the effects of estrogen therapy on HDL cholesterol levels by polymorphisms of the HDL-C receptor, SR-BI: The Rancho Bernardo Study. Atherosclerosis 2005, 180, 255–262. [Google Scholar] [CrossRef]
- Roberts, C.G.P.; Shen, H.; Mitchell, B.D.; Damcott, C.M.; Shuldiner, A.R.; Rodriguez, A. Variants in scavenger receptor class B type I gene are associated with HDL cholesterol levels in younger women. Hum. Hered. 2007, 64, 107–113. [Google Scholar] [CrossRef]
- Rodríguez-Esparragón, F.; Rodríguez-Pérez, J.C.; Hernández-Trujillo, Y.; Macías-Reyes, A.; Medina, A.; Caballero, A.; Ferrario, C.M. Allelic variants of the human scavenger receptor class B type 1 and paraoxonase 1 on coronary heart disease: Genotype-phenotype correlations. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 854–860. [Google Scholar] [CrossRef]
- De Andrade, F.M.; Fiegenbaum, M.; De Almeida, S.; Hutz, M.H. Influência de combinações genéticas nos níveis de HDL-C em uma população do sul do Brasil. Arq. Bras. Cardiol. 2010, 95, 430–435. [Google Scholar] [CrossRef]
- Osgood, D.; Corella, D.; Demissie, S.; Cupples, L.A.; Wilson, P.W.F.; Meigs, J.B.; Schaefer, E.J.; Coltell, O.; Ordovas, J.M. Genetic variation at the scavenger receptor class B type I gene locus determines plasma lipoprotein concentrations and particle size and interacts with type 2 diabetes: The Framingham study. J. Clin. Endocrinol. Metab. 2003, 88, 2869–2879. [Google Scholar] [CrossRef]
- Morabia, A.; Ross, B.M.; Costanza, M.C.; Cayanis, E.; Flaherty, M.S.; Alvin, G.B.; Das, K.; James, R.; Yang, A.S.; Evagrafov, O.; et al. Population-based study of SR-BI genetic variation and lipid profile. Atherosclerosis 2004, 175, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.A.; Ko, Y.L.; Wu, S.; Teng, M.S.; Peng, T.Y.; Chen, C.F.; Chen, C.F.; Lee, Y.S. Association between a novel 11-base pair deletion mutation in the promoter region of the scavenger receptor class B type I gene and plasma HDL cholesterol levels in Taiwanese Chinese. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1869–1874. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, J.; Omezzine, A.; Boumaiza, I.; Rebhi, L.; Kacem, S.; Rejeb, N.B.; Nabli, N.; Abdelaziz, A.B.; Boughzala, E.; Bouslama, A. Association of three polymorphisms of scavenger receptor class BI gene (exon8, exon1, intron5) with coronary stenosis in a coronary Tunisian population. Gene 2012, 511, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Stanislovaitiene, D.; Lesauskaite, V.; Zaliuniene, D.; Smalinskiene, A.; Gustiene, O.; Zaliaduonyte-Peksiene, D.; Tamosiunas, A.; Luksiene, D.; Petkeviciene, J.; Zaliunas, R. SCARB1 single nucleotide polymorphism (rs5888) is associated with serum lipid profile and myocardial infarction in an age- and gender-dependent manner. Lipids Health Dis. 2013, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Niemsiri, V.; Wang, X.; Pirim, D.; Radwan, Z.H.; Hokanson, J.E.; Hamman, R.F.; Barmada, M.M.; Demirci, F.Y.; Kamboh, M.I. Impact of genetic variants in human scavenger receptor class B type i (SCARB1) on plasma lipid traits. Circ. Cardiovasc. Genet. 2014, 7, 838–847. [Google Scholar] [CrossRef][Green Version]
- Durst, R.; Colombo, R.; Shpitzen, S.; Avi, L.B.; Friedlander, Y.; Wexler, R.; Raal, F.J.; Marais, D.A.; Defesche, J.C.; Mandelshtam, M.Y.; et al. Recent origin and spread of a common lithuanian mutation, G197del LDLR, causing familial hypercholesterolemia: Positive selection is not always necessary to account for disease incidence among Ashkenazi Jews. Am. J. Hum. Genet. 2001, 68, 1172–1188. [Google Scholar] [CrossRef][Green Version]
- Goodarzynejad, H.; Boroumand, M.; Behmanesh, M.; Ziaee, S.; Jalali, A. The rs5888 single nucleotide polymorphism in scavenger receptor class B type 1 (SCARB1) gene and the risk of premature coronary artery disease: A case-control study. Lipids Health Dis. 2016, 15, 7. [Google Scholar] [CrossRef]
- Manichaikul, A.; Naj, A.C.; Herrington, D.; Post, W.; Rich, S.S.; Rodriguez, A. Association of SCARB1 variants with subclinical atherosclerosis and incident cardiovascular disease: The multi-ethnic study of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1991–1999. [Google Scholar] [CrossRef]
- Pérez-Martínez, P.; López-Miranda, J.; Ordovás, J.M.; Bellido, C.; Marín, C.; Gómez, P.; Paniagua, J.A.; Moreno, J.A.; Fuentes, F.; Pérez-Jiménez, F. Postprandial lipemia is modified by the presence of the polymorphism present in the exon 1 variant at the SR-BI gene locus. J. Mol. Endocrinol. 2004, 32, 237–245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tanaka, T.; Delgado-Lista, J.; Lopez-Miranda, J.; Perez-Jimenez, F.; Marin, C.; Perez-Martinez, P.; Gomez, P.; Ordovas, J.M. Scavenger Receptor Class B Type I (SCARB1) c.1119C>T Polymorphism Affects Postprandial Triglyceride Metabolism in Men. J. Nutr. 2007, 137, 578–582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Y.; Ordovas, J.M.; Gao, G.; Province, M.; Straka, R.J.; Tsai, M.Y.; Lai, C.Q.; Zhang, K.; Borecki, I.; Hixson, J.E.; et al. The SCARB1 gene is associated with lipid response to dietary and pharmacological interventions. J. Hum. Genet. 2008, 53, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Cerda, Á.; Genvigir, F.D.V.; Arazi, S.S.; Hirata, M.H.; Dorea, E.L.; Bernik, M.M.S.; Bertolami, M.C.; Faludi, A.A.; Hirata, R.D.C. Influence of SCARB1 polymorphisms on serum lipids of hypercholesterolemic individuals treated with atorvastatin. Clin. Chim. Acta 2010, 411, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Constantineau, J.; Greason, E.; West, M.; Filbin, M.; Kieft, J.S.; Carletti, M.Z.; Christenson, L.K.; Rodriguez, A. A synonymous variant in scavenger receptor, class B, type I gene is associated with lower SR-BI protein expression and function. Atherosclerosis 2010, 210, 177–182. [Google Scholar] [CrossRef]
- Yang, X.; Sethi, A.; Yanek, L.R.; Knapper, C.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Becker, D.M.; Mathias, R.A.; Remaley, A.T.; Becker, L.C. SCARB1 Gene Variants Are Associated with the Phenotype of Combined High High-Density Lipoprotein Cholesterol and High Lipoprotein (a). Circ. Cardiovasc. Genet. 2016, 9, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Naj, A.C.; West, M.; Rich, S.S.; Post, W.; Linda Kao, W.H.; Wasserman, B.A.; Herrington, D.M.; Rodriguez, A. Association of scavenger receptor class B type I polymorphisms with subclinical atherosclerosis: The multi-ethnic study of atherosclerosis. Circ. Cardiovasc. Genet. 2010, 3, 47–52. [Google Scholar] [CrossRef]
- West, M.; Greason, E.; Kolmakova, A.; Jahangiri, A.; Asztalos, B.; Pollin, T.I.; Rodriguez, A. Scavenger receptor class B type i protein as an independent predictor of high-density lipoprotein cholesterol levels in subjects with hyperalphalipoproteinemia. J. Clin. Endocrinol. Metab. 2009, 94, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.T.; Tang, D.J.; Ye, Y.X.; Su, J.; Jiang, H. Influence of SCARB1 gene SNPs on serum lipid levels and susceptibility to coronary heart disease and cerebral infarction in a Chinese population. Gene 2017, 626, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Teslovich, T.M.; Musunuru, K.; Smith, A.V.; Edmondson, A.C.; Stylianou, I.M.; Koseki, M.; Pirruccello, J.P.; Ripatti, S.; Chasman, D.I.; Willer, C.J.; et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 2010, 466, 707–713. [Google Scholar] [CrossRef]
- Chiba-Falek, O.; Nichols, M.; Suchindran, S.; Guyton, J.; Ginsburg, G.S.; Barrett-Connor, E.; McCarthy, J.J. Impact of gene variants on sex-specific regulation of human scavenger receptor class B type 1 (SR-BI) expression in liver and association with lipid levels in a population-based study. BMC Med. Genet. 2010, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, P.; Khetarpal, S.A.; Larach, D.B.; Hancock-Cerutti, W.F.; Millar, J.S.; Cuchel, M.; DerOhannessian, S.; Kontush, A.; Surendran, P.; Saleheen, D.; et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science (80-.) 2016, 351, 1166–1171. [Google Scholar] [CrossRef]
- Samadi, S.; Farjami, Z.; Hosseini, Z.S.; Ferns, G.A.; Mohammadpour, A.H.; Tayefi, M.; Fal-Soleiman, H.; Moohebati, M.; Ghayour-Mobarhan, M.; Esmaily, H.; et al. Rare P376L variant in the SR-BI gene associates with HDL dysfunction and risk of cardiovascular disease. Clin. Biochem. 2019, 73, 44–49. [Google Scholar] [CrossRef]
- May, S.C.; Dron, J.S.; Hegele, R.A.; Sahoo, D. Human Variant of Scavenger Receptor BI (R174C) Exhibits Impaired Cholesterol Transport Functions. J. Lipid Res. 2021, 62, 100045. [Google Scholar] [CrossRef] [PubMed]
- Brunham, L.R.; Tietjen, I.; Bochem, A.E.; Singaraja, R.R.; Franchini, P.L.; Radomski, C.; Mattice, M.; Legendre, A.; Hovingh, G.K.; Kastelein, J.J.P.; et al. Novel mutations in scavenger receptor BI associated with high HDL cholesterol in humans. Clin. Genet. 2011, 79, 575–581. [Google Scholar] [CrossRef]
- Ljunggren, S.A.; Levels, J.H.M.; Hovingh, K.; Holleboom, A.G.; Vergeer, M.; Argyri, L.; Gkolfinopoulou, C.; Chroni, A.; Sierts, J.A.; Kastelein, J.J.; et al. Lipoprotein profiles in human heterozygote carriers of a functional mutation P297S in scavenger receptor class B1. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, A.C.; Sahoo, D. Functional Characterization of Newly-Discovered Mutations in Human SR-BI. PLoS ONE 2012, 7, e45660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trigatti, B.L.; Hegele, R.A. Rare genetic variants and high-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 2016, 36, e53–e55. [Google Scholar] [CrossRef][Green Version]
- Fuchs, M.; Ivandic, B.; Müller, O.; Schalla, C.; Scheibner, J.; Bartsch, P.; Stange, E.F. Biliary cholesterol hypersecretion in gallstone-susceptible mice is associated with hepatic up-regulation of the high-density lipoprotein receptor SRBI. Hepatology 2001, 33, 1451–1459. [Google Scholar] [CrossRef]
- Webb, T.R.; Erdmann, J.; Stirrups, K.E.; Stitziel, N.O.; Masca, N.G.D.; Jansen, H.; Kanoni, S.; Nelson, C.P.; Ferrario, P.G.; König, I.R.; et al. Systematic Evaluation of Pleiotropy Identifies 6 Further Loci Associated With Coronary Artery Disease. J. Am. Coll. Cardiol. 2017, 69, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Howson, J.M.M.; Zhao, W.; Barnes, D.R.; Ho, W.K.; Young, R.; Paul, D.S.; Waite, L.L.; Freitag, D.F.; Fauman, E.B.; Salfati, E.L.; et al. Fifteen new risk loci for coronary artery disease highlight arterial-wall-specific mechanisms. Nat. Genet. 2017, 49, 1113–1119. [Google Scholar] [CrossRef]
- Vitali, C.; Khetarpal, S.A.; Rader, D.J. HDL Cholesterol Metabolism and the Risk of CHD: New Insights from Human Genetics. Curr. Cardiol. Rep. 2017, 19, 132. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A. High HDL-Cholesterol Paradox: SCARB1-LAG3-HDL Axis. Curr. Atheroscler. Rep. 2021, 23, 5. [Google Scholar] [CrossRef] [PubMed]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; John Chapman, M.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. 2016, 37, 2999–3058l. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).