Cellular Senescence in Human Aldosterone-Producing Adrenocortical Cells and Related Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Adrenals
2.2. Immunohistochemistry (IHC) and Semi-Quantitative Analysis
2.3. Microdissection and DNA Extraction from FFPE Blocks
2.4. Statistical Analysis
3. Results
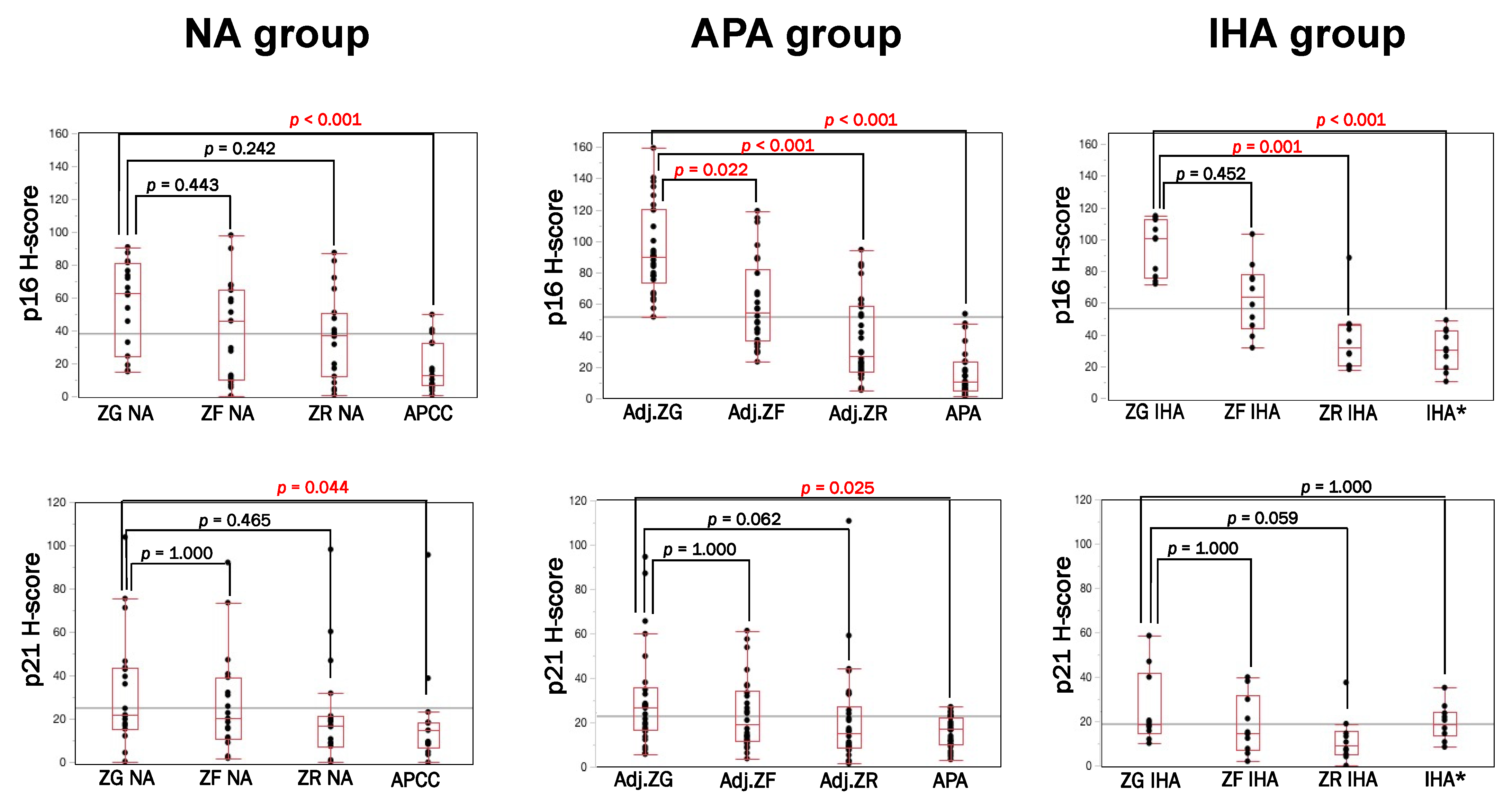
3.1. Immunolocalization of Cell Senescence Markers in NA, APA and IHA
3.2. p16–p21 in CYP11B2-Positive (Aldosterone-Producing) and Negative Cells
3.3. γ. H2AX Immunolocalization in NA, APA and IHA
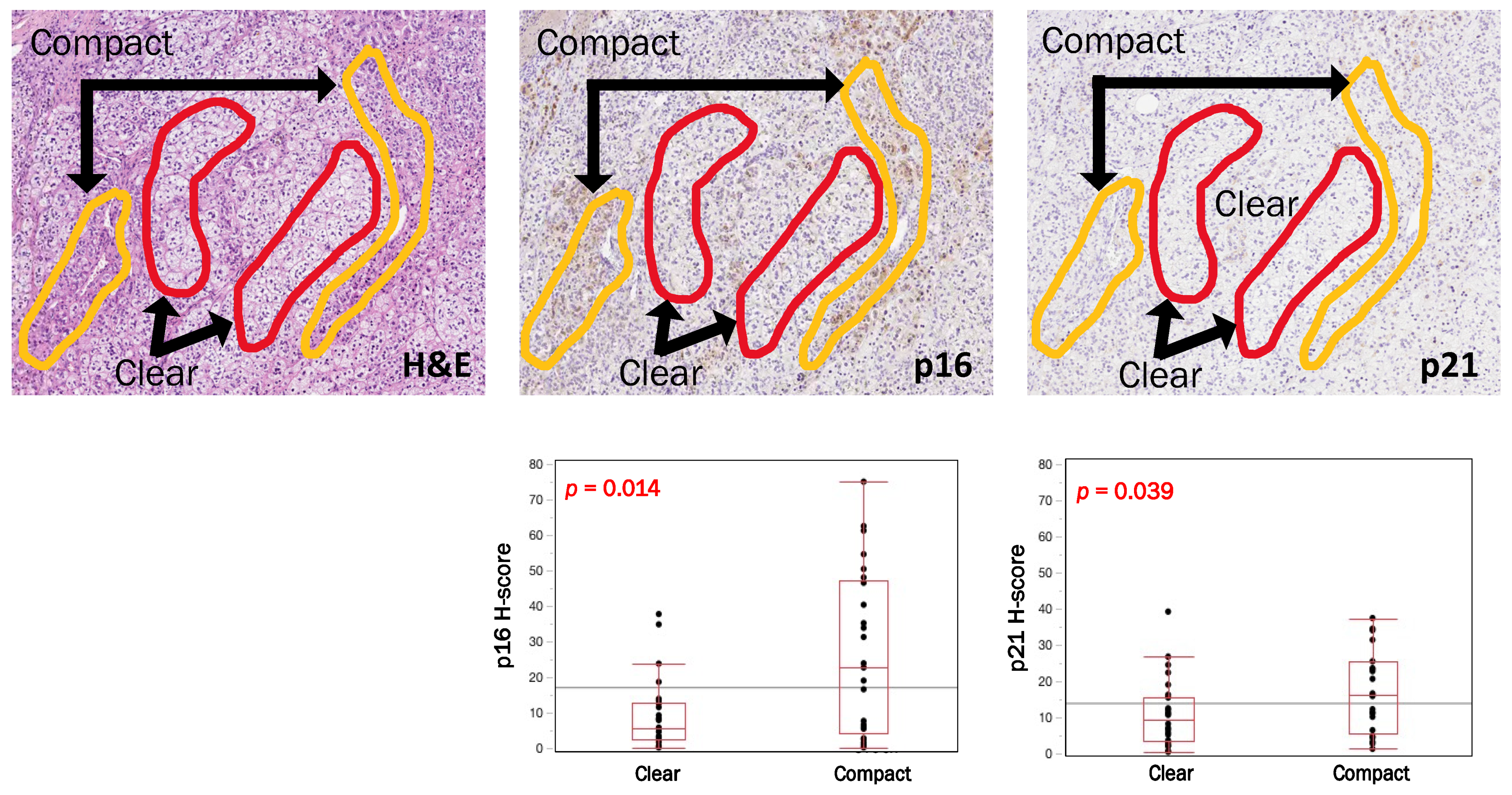
3.4. Intratumoral Heterogeneity of p16, p21 and CYP11B2 According to Cell Types in APAs
3.5. Comparative Analysis of p16 and p21 Immunoreactivity According to KCNJ5 Genotype (KCNJ5-Mutated vs. KCNJ5-Wild Type) in APAs
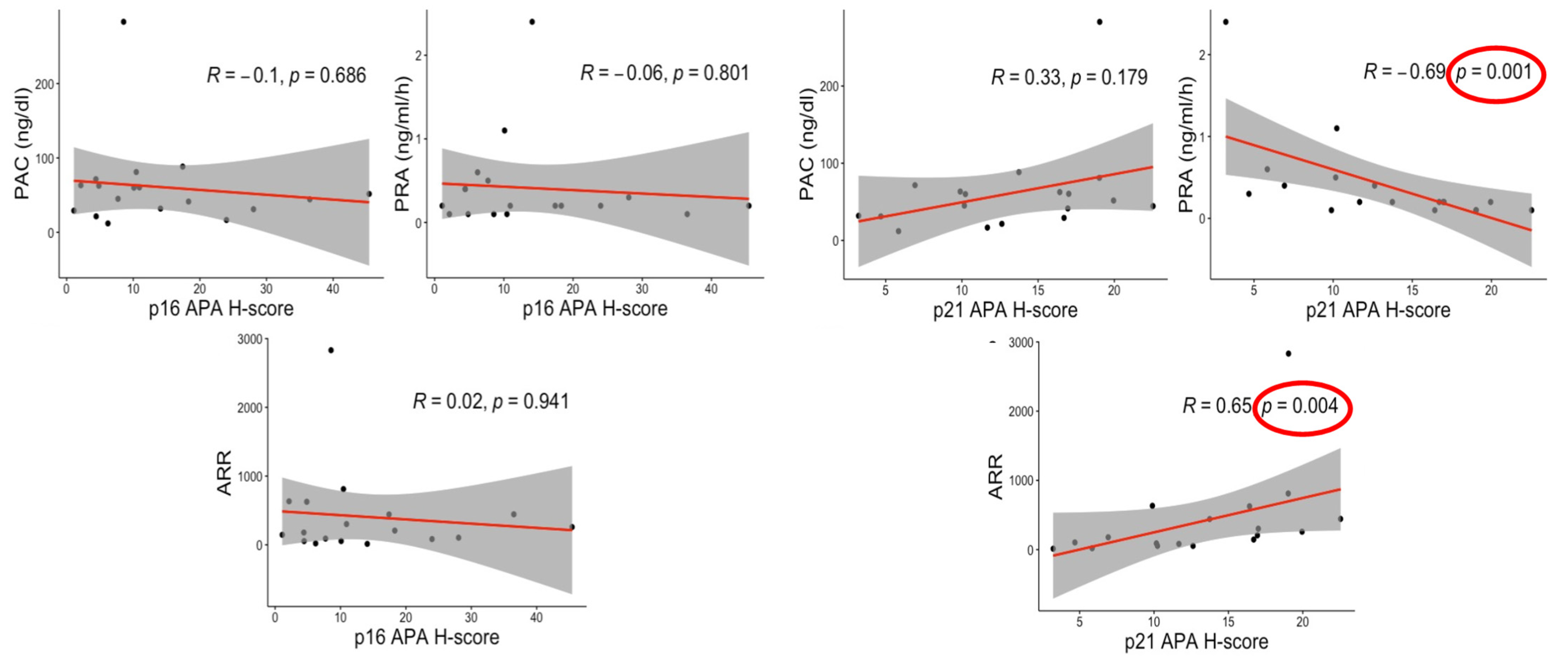
3.6. Correlations between Senescence Markers and Clinical Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharpless, N.E.; Sherr, C.J. Forging a signature of in vivo senescence. Nat. Rev. Cancer 2015, 15, 397–408. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Lujambio, A. To clear, or not to clear (senescent cells)? That is the question. Inside Cell 2016, 1, 87–95. [Google Scholar] [CrossRef]
- Anderson, R.; Richardson, G.D.; Passos, J.F. Mechanisms driving the ageing heart. Exp. Gerontol. 2018, 109, 5–15. [Google Scholar] [CrossRef]
- Di Micco, R.; Fumagalli, M.; Cicalese, A.; Piccinin, S.; Gasparini, P.; Luise, C.; Schurra, C.; Garre’, M.; Nuciforo, P.G.; Bensimon, A.; et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 2006, 444, 638–642. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Nonaka, K.; Aida, J.; Takubo, K.; Yamazaki, Y.; Takakuma, S.; Kakizaki, M.; Matsuda, Y.; Ishikawa, N.; Ishiwata, T.; Chong, J.-M.; et al. Correlation between Differentiation of Adrenocortical Zones and Telomere Lengths Measured by Q-FISH. J. Clin. Endocrinol. Metab. 2019, 104, 5642–5650. [Google Scholar] [CrossRef]
- Yiallouris, A.; Tsioutis, C.; Agapidaki, E.; Zafeiri, M.; Agouridis, A.P.; Ntourakis, D.; Johnson, E.O. Adrenal aging and its implications on stress responsiveness in humans. Front. Endocrinol. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Seccia, T.M.; Caroccia, B.; Gomez-Sanchez, E.P.; Gomez-Sanchez, C.E.; Rossi, G.P. The Biology of Normal Zona Glomerulosa and Aldosterone-Producing Adenoma: Pathological Implications. Endocr. Rev. 2018, 39, 1029–1056. [Google Scholar] [CrossRef]
- Hammer, G.D.; Basham, K.J. Stem cell function and plasticity in the normal physiology of the adrenal cortex. Mol. Cell. Endocrinol. 2020, 519. [Google Scholar] [CrossRef]
- Monticone, S.; D’Ascenzo, F.; Moretti, C.; Williams, T.A.; Veglio, F.; Gaita, F.; Mulatero, P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018, 6, 41–50. [Google Scholar] [CrossRef]
- Williams, T.A.; Dietz, A.S.; Theodoropoulou, M.; Riester, A.; Fischer, E.; Burrello, J.; Treitl, M.; Geyer, L.; Veglio, F.; Bidlingmaier, M.; et al. Coexisting prolactinoma and primary aldosteronism: Is there a pathophysiological link? J. Clin. Endocrinol. Metab. 2015, 100, E1262–E1269. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wotus, C.; Levay-Young, B.K.; Rogers, L.M.; Gomez-Sanchez, C.E.; Engeland, W.C. Development of adrenal zonation in fetal rats defined by expression of aldosterone synthase and 11β-hydroxylase. Endocrinology 1998, 139, 4397–4403. [Google Scholar] [CrossRef] [PubMed]
- Omata, K.; Anand, S.K.; Hovelson, D.H.; Liu, C.J.; Yamazaki, Y.; Nakamura, Y.; Ito, S.; Satoh, F.; Sasano, H.; Rainey, W.E.; et al. Aldosterone-producing cell clusters frequently harbor somatic mutations and accumulate with age in normal adrenals. J. Endocr. Soc. 2017, 1, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Nanba, K.; Vaidya, A.; Rainey, W.E. Aging and adrenal aldosterone production. Hypertension 2018, 71, 218–223. [Google Scholar] [CrossRef]
- Bassett, M.H.; White, P.C.; Rainey, W.E. The regulation of aldosterone synthase expression. Mol. Cell. Endocrinol. 2004, 217, 67–74. [Google Scholar] [CrossRef]
- Kitawaki, Y.; Nakamura, Y.; Kubota-Nakayama, F.; Yamazaki, Y.; Miki, Y.; Hata, S.; Ise, K.; Kikuchi, K.; Morimoto, R.; Satoh, F.; et al. Tumor microenvironment in functional adrenocortical adenomas: Immune cell infiltration in cortisol-producing adrenocortical adenoma. Hum. Pathol. 2018, 77, 88–97. [Google Scholar] [CrossRef]
- Gao, X.; Yamazaki, Y.; Tezuka, Y.; Pieroni, J.; Ishii, K.; Atsumi, N.; Morimoto, R.; Nakamura, Y.; Satoh, F.; Sasano, H.; et al. Intratumoral heterogeneity of the tumor cells based on in situ cortisol excess in cortisol-producing adenomas; ∼An association among morphometry, genotype and cellular senescence∼. J. Steroid Biochem. Mol. Biol. 2020, 204. [Google Scholar] [CrossRef]
- Nishikawa, T.; Omura, M.; Satoh, F.; Shibata, H.; Takahashi, K.; Tamura, N.; Tanabe, A. Guidelines for the diagnosis and treatment of primary aldosteronism-The Japan Endocrine Society 2009. Endocr. J. 2011, 58, 711–721. [Google Scholar] [CrossRef]
- Weiss, L.M. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am. J. Surg. Pathol. 1984, 8, 163–169. [Google Scholar] [CrossRef]
- Gomez-Sanchez, C.E.; Qi, X.; Velarde-Miranda, C.; Plonczynski, M.W.; Parker, C.R.; Rainey, W.; Maekawa, T.; Nakamura, Y.; Sasano, H.; Gomez-Sanchez, E.P.; et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol. Cell. Endocrinol. 2014, 383, 111–117. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Omata, K.; Tezuka, Y.; Ono, Y.; Morimoto, R.; Adachi, Y.; Ise, K.; Nakamura, Y.; Gomez-Sanchez, C.E.; Shibahara, Y.; et al. Tumor cell subtypes based on the intracellular hormonal activity in KCNJ5-mutated aldosterone-producing adenoma. Hypertension 2018, 72, 632–640. [Google Scholar] [CrossRef]
- Konosu-Fukaya, S.; Nakamura, Y.; Satoh, F.; Felizola, S.J.; Maekawa, T.; Ono, Y.; Morimoto, R.; Ise, K.; Takeda, K.; Katsu, K.; et al. 3Β-Hydroxysteroid Dehydrogenase Isoforms in Human Aldosterone-Producing Adenoma. Mol. Cell. Endocrinol. 2015, 408, 205–212. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Nakamura, Y.; Omata, K.; Ise, K.; Tezuka, Y.; Ono, Y.; Morimoto, R.; Nozawa, Y.; Gomez-Sanchez, C.E.; Tomlins, S.A.; et al. Histopathological classification of cross-sectional image-negative hyperaldosteronism. J. Clin. Endocrinol. Metab. 2017, 102, 1182–1192. [Google Scholar]
- Nishimoto, K.; Tomlins, S.A.; Kuick, R.; Cani, A.K.; Giordano, T.J.; Hovelson, D.H.; Liu, C.-J.; Sanjanwala, A.R.; Edwards, M.A.; Gomez-Sanchez, C.E.; et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc. Natl. Acad. Sci. USA 2015, 112, E4591–E4599. [Google Scholar] [CrossRef]
- Tezuka, Y.; Yamazaki, Y.; Kitada, M.; Morimoto, R.; Kudo, M.; Seiji, K.; Takase, K.; Kawasaki, Y.; Mitsuzuka, K.; Ito, A.; et al. 18-Oxocortisol Synthesis in Aldosterone-Producing Adrenocortical Adenoma and Significance of KCNJ5 Mutation Status. Hypertension 2019, 73, 1283–1290. [Google Scholar] [CrossRef]
- Choi, M.; Scholl, U.I.; Yue, P.; Björklund, P.; Zhao, B.; Nelson-Williams, C.; Ji, W.; Cho, Y.; Patel, A.; Men, C.J.; et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 2011, 331, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J. Ein Beitrag zu der feineren Structur und dem Chemismus der Nebennieren. Virchows Arch. 1866, 35, 64–107. [Google Scholar] [CrossRef]
- Freedman, B.D.; Kempna, P.B.; Carlone, D.L.; Shah, M.S.; Guagliardo, N.A.; Barrett, P.Q.; Gomez-Sanchez, C.E.; Majzoub, J.A.; Breault, D.T. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev. Cell 2013, 26, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Zwemer, R.L. A Study of Adrenal Cortex Morphology. J. Nerv. Ment. Dis. 1936, 84, 107. [Google Scholar] [CrossRef]
- Salmon, T.N.; Zwemer, R.L. A study of the life history of cortico-adrenal gland cells of the rat by means of trypan blue injections. Anat. Rec. 1941, 80, 421–429. [Google Scholar] [CrossRef]
- Jones, I.C. Variation in the Mouse Adrenal Cortex with Special Reference to the Zona Reticularis and to Brown Degeneration, Together with a Discussion of the ‘Cell Migration’ Theory. Q. J. Microsc. Sci. 1948, 3, 53–73. [Google Scholar]
- Swann, H.G. The Pituitary-Adrenocortical Relationship. Physiol. Rev. 1940, 20, 493–521. [Google Scholar] [CrossRef]
- Pihlajoki, M.; Dörner, J.; Cochran, R.S.; Heikinheimo, M.; Wilson, D.B. Adrenocortical zonation, renewal, and remodeling. Front. Endocrinol. 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Nakamura, Y.; Maekawa, T.; Felizola, S.J.; Satoh, F.; Qi, X.; Velarde-Miranda, C.; Plonczynski, M.W.; Ise, K.; Kikuchi, K.; Rainey, W.E.; et al. Adrenal CYP11B1/2 expression in primary aldosteronism: Immunohistochemical analysis using novel monoclonal antibodies. Mol. Cell. Endocrinol. 2014, 392, 73–79. [Google Scholar] [CrossRef]
- Kopp, B.; Khoury, L.; Audebert, M. Validation of the γH2AX biomarker for genotoxicity assessment: A review. Arch. Toxicol. 2019, 93, 2103–2111. [Google Scholar] [CrossRef]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021, 288, 56–80. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Murakami, M.; Yoshimoto, T.; Nakabayashi, K.; Nakano, Y.; Fukaishi, T.; Tsuchiya, K.; Minami, I.; Bouchi, R.; Okamura, K.; Fujii, Y. Molecular characteristics of the KCNJ5 mutated aldosterone-producing adenomas. Endocr. Reated Cancer 2017, 24, 531–541. [Google Scholar] [CrossRef]
- Zheng, F.F.; Zhu, L.M.; Nie, A.F.; Li, X.Y.; Lin, J.R.; Zhang, K.; Chen, J.; Zhou, W.-L.; Shen, Z.-J.; Zhu, Y.-C.; et al. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension 2015, 65, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Monticone, S.; Castellano, I.; Versace, K.; Lucatello, B.; Veglio, F.; Gomez-Sanchez, C.E.; Williams, T.A.; Mulatero, P. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol. Cell. Endocrinol. 2015, 411, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Rosa, F.L.; Williams, T.A.; Riester, A.; Steichen, O.; Beuschlein, F.; Boulkroun, S.; Strom, T.M.; Monticone, S.; Amar, L.; Meatchi, T.; et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension 2014, 64, 354–361. [Google Scholar] [CrossRef]
- Hung, C.S.; Sung, S.H.; Liao, C.W.; Pan, C.T.; Chang, C.C.; Chen, Z.W.; Wu, V.-C.; Chen-Huan Chen, C.H.; Cheng, H.M.; Lin, Y.H.; et al. Aldosterone induces vascular damage: A wave reflection analysis study. Hypertension 2019, 74, 623–629. [Google Scholar] [CrossRef]
- Chen, Z.W.; Hung, C.S.; Wu, V.C.; Lin, Y.H.; TAIPAI Study Group. Primary Aldosteronism and Cerebrovascular Diseases. Endocrinol. Metab. 2018, 33, 429–434. [Google Scholar] [CrossRef]
- Mulatero, P.; Monticone, S.; Bertello, C.; Viola, A.; Tizzani, D.; Iannaccone, A.; Crudo, V.; Burrello, J.; Milan, A.; Rabbia, F.; et al. Long-Term cardio- and cerebrovascular events in patients with primary aldosteronism. J. Clin. Endocrinol. Metab. 2013, 98, 4826–4833. [Google Scholar] [CrossRef]
- Kitada, K.; Nakano, D.; Hitomi, H.; Kobori, H.; Deguchi, K.; Mori, H.; Masaki, T.; Nishiyama, A. Aldosterone induces p21-regulated apoptosis via increased synthesis and secretion of tumour necrosis factor-α in human proximal tubular cells. Clin. Exp. Pharmacol. Physiol. 2012, 39, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Kohno, M.; Hitomi, H.; Kitada, K.; Fujisawa, Y.; Yatabe, J.; Yatabe, M.; Felder, R.A.; Ohsaki, H.; Rafiq, K.; et al. Aldosterone/Mineralocorticoid Receptor Stimulation Induces Cellular Senescence in the Kidney. Endocrinology 2011, 152, 680–688. [Google Scholar] [CrossRef]

| Variable | KCNJ5 (N = 18) | WT (N = 12) | p-Value |
|---|---|---|---|
| p16 APA | 10.27 (4.74; 19.72) | 11.74 (5.62; 23.46) | 0.688 |
| p16 Clear Cells | 6.52 (3.70; 14.15) | 4.18 (2.62; 12.35) | 0.467 |
| p16 Compact Cells | 31.16 (3.60; 47.44) | 21.14 (5.94; 38.67) | 0.718 |
| p21 APA | 13.18 (9.15; 17.51) | 22.47 (13.25; 24.71) | 0.008 |
| p21 Clear Cells | 6.60 (2.80; 12.17) | 12.03 (7.00; 24.90) | 0.029 |
| p21 Compact Cells | 10.44 (4.84; 30.98) | 19.47 (12.25; 23.92) | 0.305 |
| CYP11B2 APA | 15.90 (2.69; 35.12) | 44.87 (13.42; 99.17) | 0.060 |
| CYP11B2 Clear Cells | 15.45 (9.11; 33.48) | 10.48 (3.39; 24.44) | 0.764 |
| CYP11B2 Compact Cells | 44.54 (9.03; 52.43) | 56.56 (25.98; 91.88) | 0.349 |
| Tumor Size (cm) | 14.50 (9.75; 21.00) | 7.50 (6.00; 10.00) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieroni, J.; Yamazaki, Y.; Gao, X.; Tezuka, Y.; Ogata, H.; Omata, K.; Ono, Y.; Morimoto, R.; Nakamura, Y.; Satoh, F.; et al. Cellular Senescence in Human Aldosterone-Producing Adrenocortical Cells and Related Disorders. Biomedicines 2021, 9, 567. https://doi.org/10.3390/biomedicines9050567
Pieroni J, Yamazaki Y, Gao X, Tezuka Y, Ogata H, Omata K, Ono Y, Morimoto R, Nakamura Y, Satoh F, et al. Cellular Senescence in Human Aldosterone-Producing Adrenocortical Cells and Related Disorders. Biomedicines. 2021; 9(5):567. https://doi.org/10.3390/biomedicines9050567
Chicago/Turabian StylePieroni, Jacopo, Yuto Yamazaki, Xin Gao, Yuta Tezuka, Hiroko Ogata, Kei Omata, Yoshikiyo Ono, Ryo Morimoto, Yasuhiro Nakamura, Fumitoshi Satoh, and et al. 2021. "Cellular Senescence in Human Aldosterone-Producing Adrenocortical Cells and Related Disorders" Biomedicines 9, no. 5: 567. https://doi.org/10.3390/biomedicines9050567
APA StylePieroni, J., Yamazaki, Y., Gao, X., Tezuka, Y., Ogata, H., Omata, K., Ono, Y., Morimoto, R., Nakamura, Y., Satoh, F., & Sasano, H. (2021). Cellular Senescence in Human Aldosterone-Producing Adrenocortical Cells and Related Disorders. Biomedicines, 9(5), 567. https://doi.org/10.3390/biomedicines9050567







