Optoacoustic Imaging in Inflammation
Abstract
1. Introduction
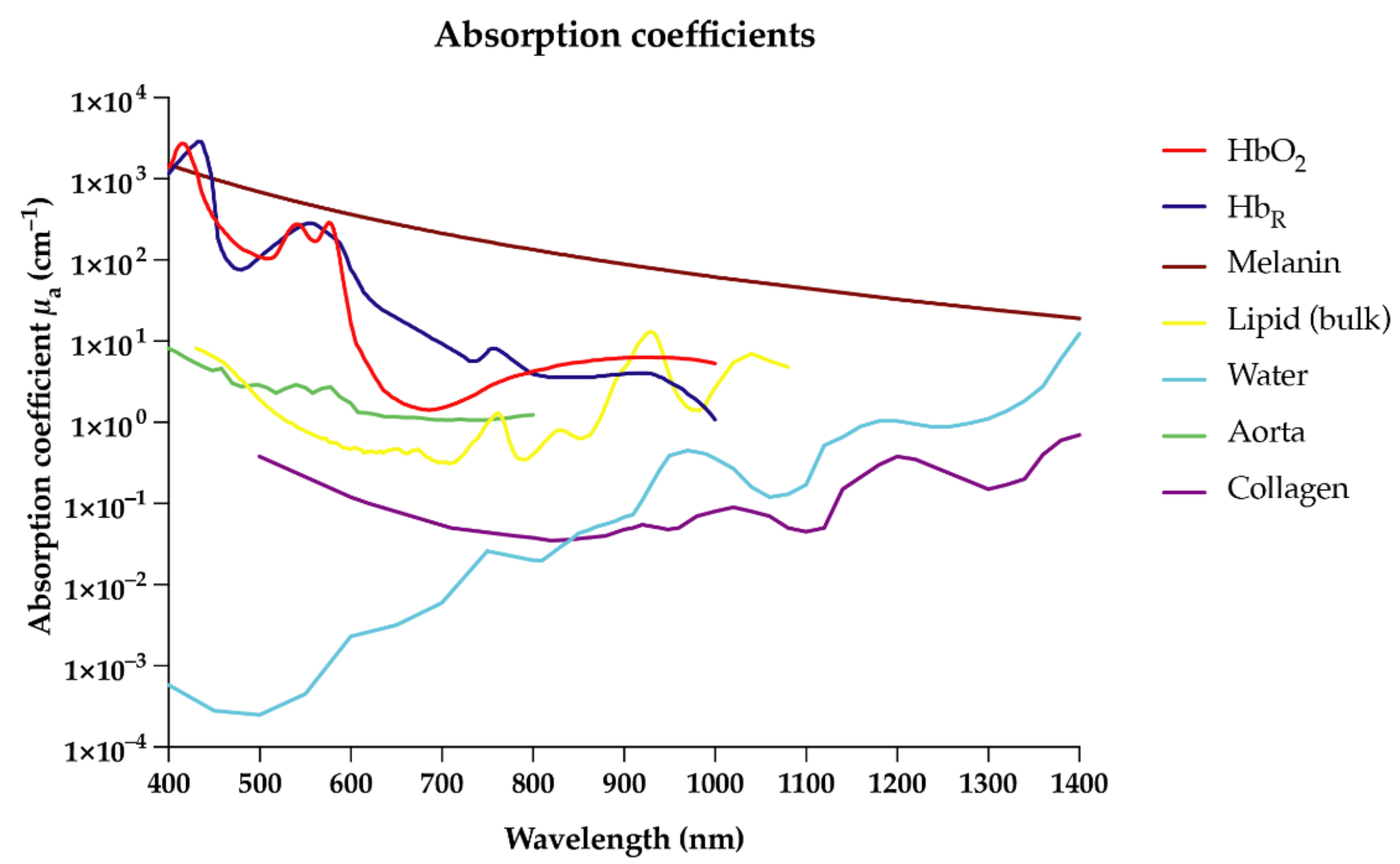
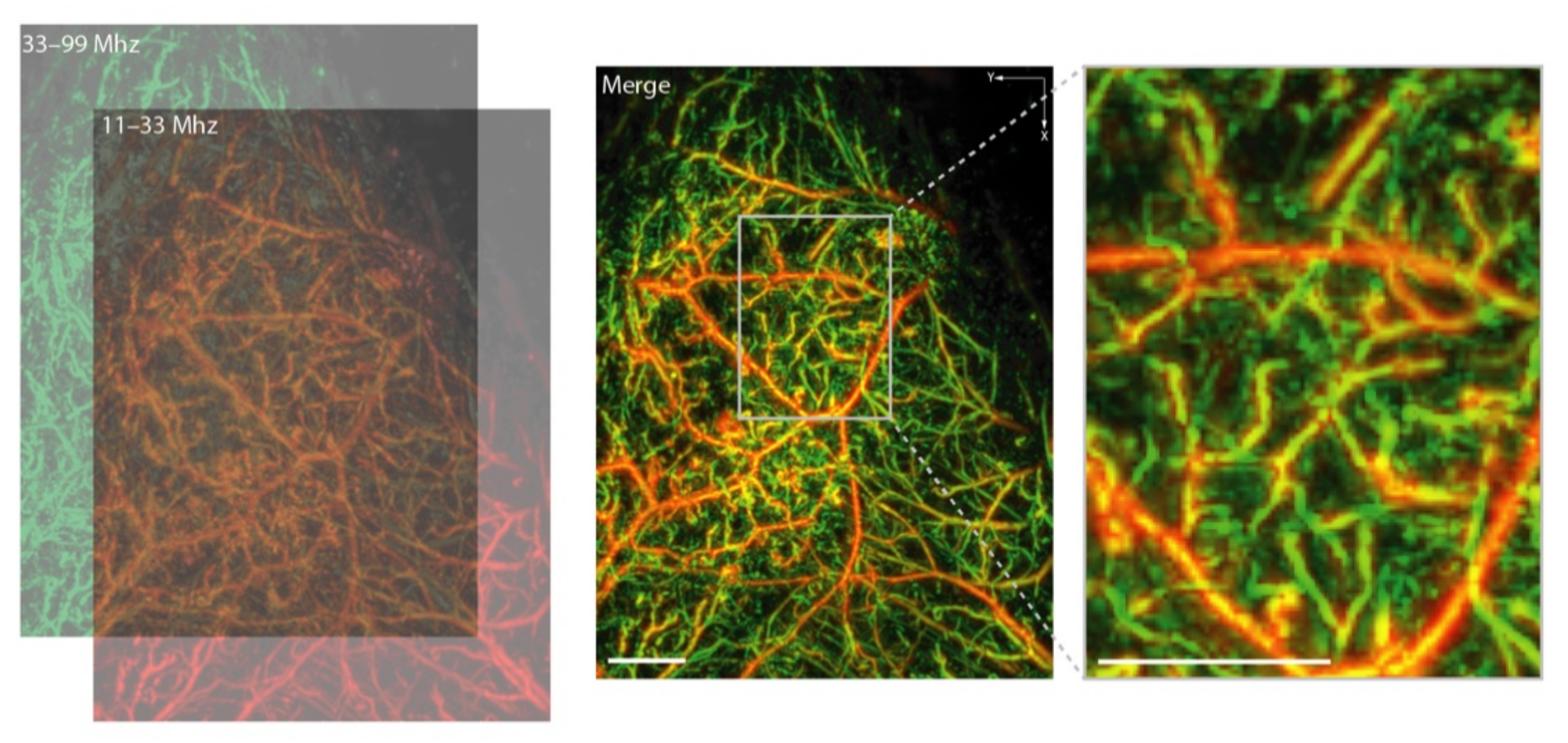
2. Optoacoustic Imaging across Scales
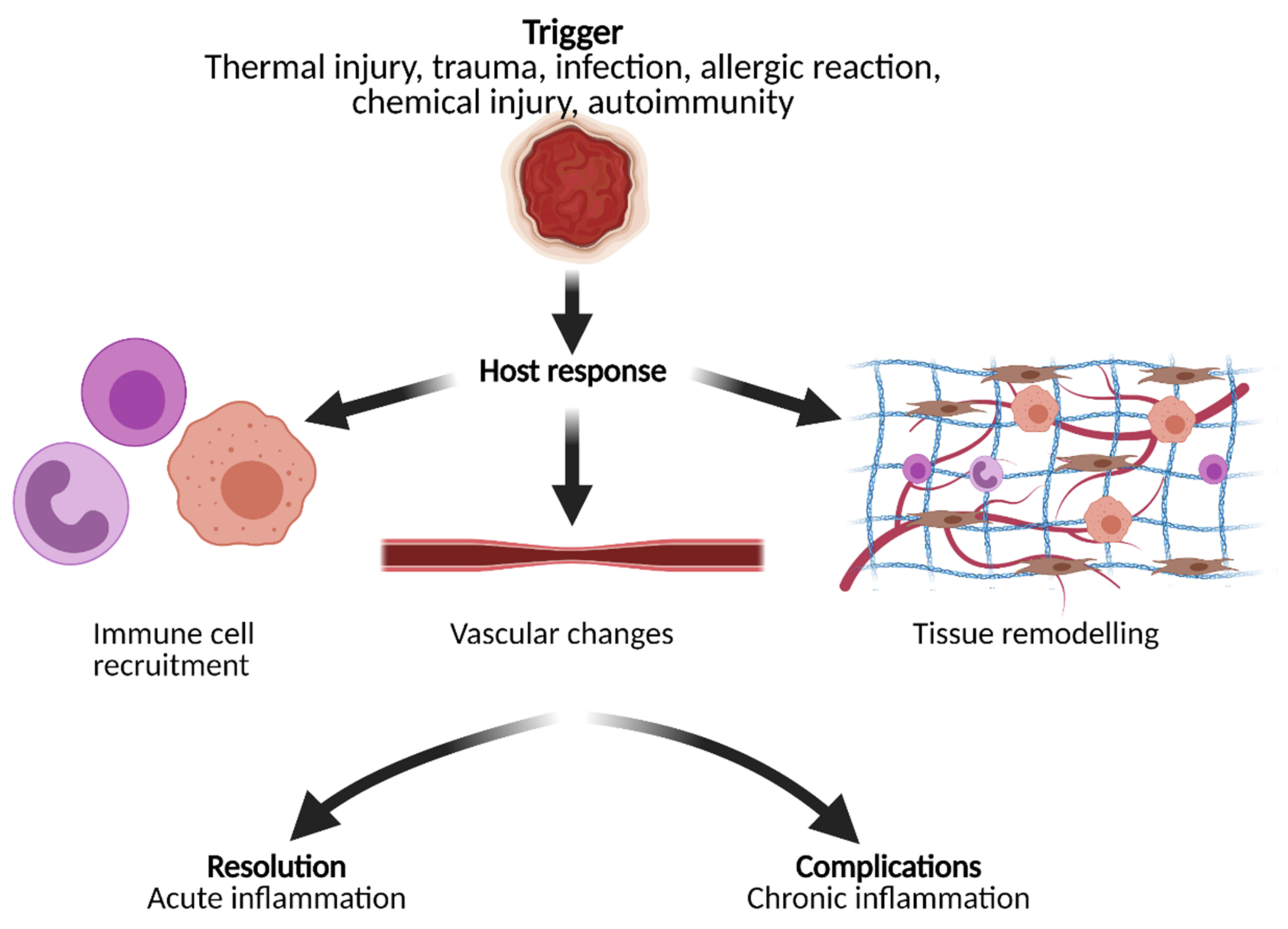
3. Acute and Chronic Inflammation
4. Optoacoustic Imaging of Inflammation: Applications
4.1. Cardiovascular Diseases
4.2. Dermatologic Diseases, Wound and Infection
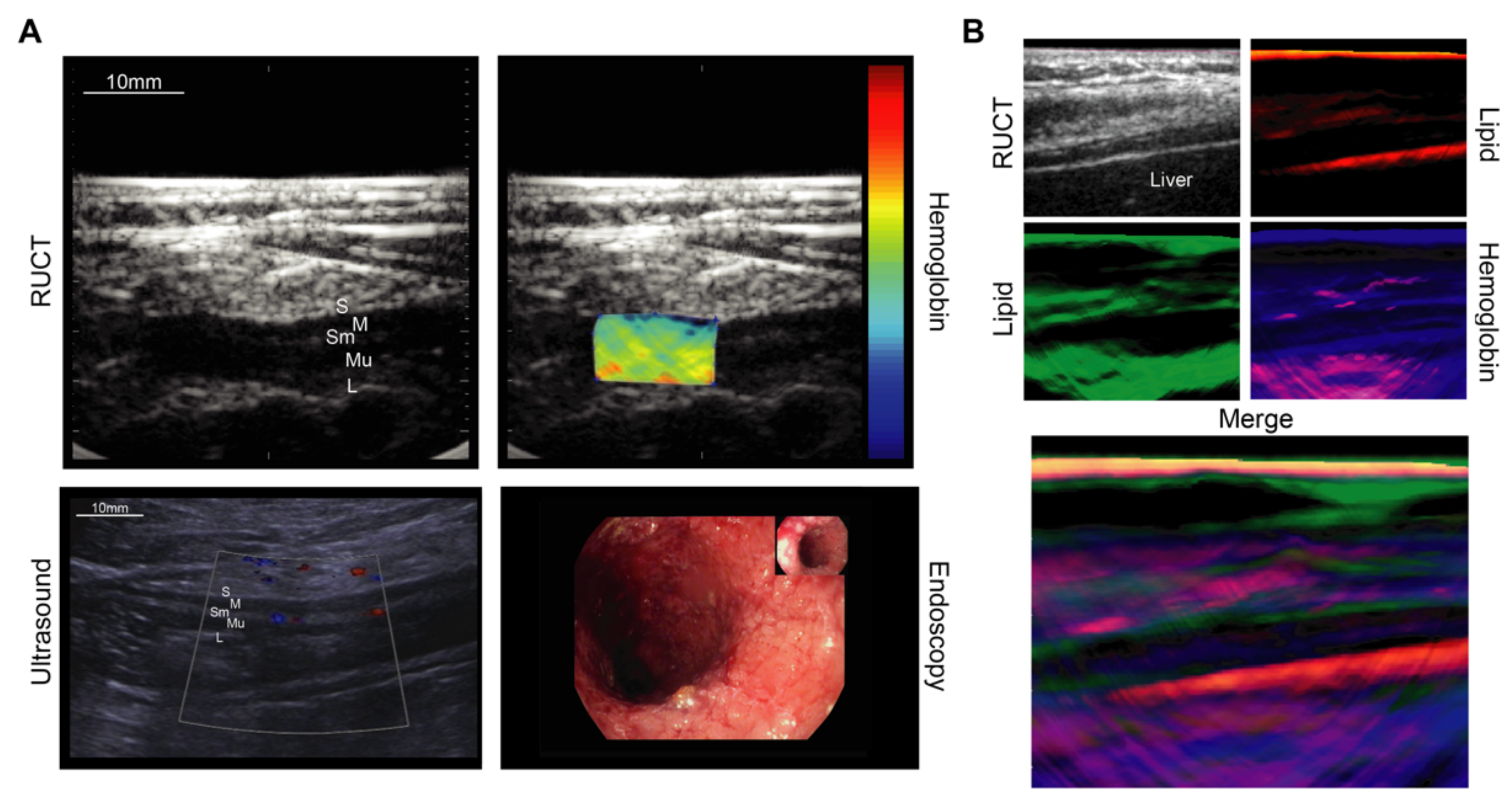
4.3. Gastrointenstinal Diseases
4.4. Musculoskeletal Diseases
4.5. (Neuro-)Degenerative Diseases
4.6. Kidney Diseases
4.7. Gynaecologic Imaging
5. Limitations of OAI
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilting, J.; Becker, J.; Buttler, K.; Weich, H.A. Lymphatics and inflammation. Curr. Med. Chem. 2009, 16, 4581–4592. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Kunnumakkara, A.B.; Aggarwal, S.; Aggarwal, B.B. Inflammation, a Double-Edge Sword for Cancer and Other Age-Related Diseases. Front. Immunol. 2018, 9, 2160. [Google Scholar] [CrossRef]
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, D.A. Molecular Imaging of Inflammation: Current Status. J. Nucl. Med. 2016, 57, 1161–1165. [Google Scholar] [CrossRef]
- Willmann, J.K.; van Bruggen, N.; Dinkelborg, L.M.; Gambhir, S.S. Molecular imaging in drug development. Nat. Rev. Drug Discov. 2008, 7, 591–607. [Google Scholar] [CrossRef]
- Manohar, S.; Razansky, D. Photoacoustics: A historical review. Adv. Opt. Photon. 2016, 8, 586–617. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, V.; Razansky, D. Molecular imaging by means of multispectral optoacoustic tomography (MSOT). Chem. Rev. 2010, 110, 2783–2794. [Google Scholar] [CrossRef]
- Tzoumas, S.; Deliolanis, N.; Morscher, S.; Ntziachristos, V. Unmixing Molecular Agents From Absorbing Tissue in Multispectral Optoacoustic Tomography. IEEE Trans. Med. Imaging 2014, 33, 48–60. [Google Scholar] [CrossRef]
- Zeng, L.; Ma, G.; Lin, J.; Huang, P. Photoacoustic Probes for Molecular Detection: Recent Advances and Perspectives. Small 2018, 14, e1800782. [Google Scholar] [CrossRef]
- Bayer, C.L.; Joshi, P.P.; Emelianov, S.Y. Photoacoustic imaging: A potential tool to detect early indicators of metastasis. Expert Rev. Med. Devices 2013, 10, 125–134. [Google Scholar] [CrossRef][Green Version]
- Zhang, C.; Kimura, R.; Abou-Elkacem, L.; Levi, J.; Xu, L.; Gambhir, S.S. A Cystine Knot Peptide Targeting Integrin alphavbeta6 for Photoacoustic and Fluorescence Imaging of Tumors in Living Subjects. J. Nucl. Med. 2016, 57, 1629–1634. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De la Zerda, A.; Bodapati, S.; Teed, R.; May, S.Y.; Tabakman, S.M.; Liu, Z.; Khuri-Yakub, B.T.; Chen, X.; Dai, H.; Gambhir, S.S. Family of enhanced photoacoustic imaging agents for high-sensitivity and multiplexing studies in living mice. ACS Nano 2012, 6, 4694–4701. [Google Scholar] [CrossRef]
- Zackrisson, S.; van de Ven, S.; Gambhir, S.S. Light in and sound out: Emerging translational strategies for photoacoustic imaging. Cancer Res. 2014, 74, 979–1004. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L.; McAuliffe, D.J. The melanosome: Threshold temperature for explosive vaporization and internal absorption coefficient during pulsed laser irradiation. Photochem. Photobiol. 1991, 53, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.; Glickman, R.; Schwartz, J. Internal Absorption Coefficient and Threshold for Pulsed Laser Disruption of Melanosomes Isolated from Retinal Pigment Epithelium; SPIE: Bellingham, WA, USA, 1996; Volume 2681. [Google Scholar]
- Sliney, D.H.; Palmisano, W.A. The evaluation of laser hazards. Am. Ind. Hyg. Assoc. J. 1968, 29, 425–431. [Google Scholar] [CrossRef]
- Goldman, L. The skin. Arch. Environ. Health 1969, 18, 434–436. [Google Scholar] [CrossRef]
- Van Veen, R.L.P.; Sterenborg, H.J.C.M.; Pifferi, A.; Torricelli, A.; Cubeddu, R. Determination of VIS-NIR absorption coefficients of mammalian fat, with time- and spatially resolved diffuse reflectance and transmission spectroscopy. In Proceedings of the Biomedical Topical Meeting, Miami Beach, FL, USA, 14 April 2004; p. SF4. [Google Scholar]
- Hale, G.M.; Querry, M.R. Optical Constants of Water in the 200-nm to 200-microm Wavelength Region. Appl. Opt. 1973, 12, 555–563. [Google Scholar] [CrossRef]
- Oraevsky, A.A.; Jacques, S.L.; Pettit, G.H.; Saidi, I.S.; Tittel, F.K.; Henry, P.D. XeCl laser ablation of atherosclerotic aorta: Optical properties and energy pathways. Lasers Surg. Med. 1992, 12, 585–597. [Google Scholar] [CrossRef]
- Sekar, S.K.; Bargigia, I.; Mora, A.D.; Taroni, P.; Ruggeri, A.; Tosi, A.; Pifferi, A.; Farina, A. Diffuse optical characterization of collagen absorption from 500 to 1700 nm. J. Biomed. Opt. 2017, 22, 15006. [Google Scholar] [CrossRef]
- Oraevsky, A.; Jacques, S.; Esenaliev, R.; Tittel, F. Laser-Based Optoacoustic Imaging in Biological Tissues; SPIE: Bellingham, WA, USA, 1994; Volume 2134. [Google Scholar]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, H.E.; Tummers, W.S.; Gambhir, S.S. Photoacoustic clinical imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef]
- Thomas, R.J.; Rockwell, B.A.; Marshall, W.J.; Aldrich, R.C.; Gorschboth, M.F.; Zimmerman, S.A.; Rockwell, R.J. A procedure for the estimation of intrabeam hazard distances and optical density requirements under the ANSI Z136.1-2000 Standard. J. Laser Appl. 2004, 16, 167–177. [Google Scholar] [CrossRef]
- Wang, X.; Xie, X.; Ku, G.; Wang, L.V.; Stoica, G. Noninvasive imaging of hemoglobin concentration and oxygenation in the rat brain using high-resolution photoacoustic tomography. J. Biomed. Opt. 2006, 11, 024015. [Google Scholar] [CrossRef] [PubMed]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.S.; Bi, R.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef]
- Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L.V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat. Biotechnol. 2006, 24, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, C.; Kim, J.; Kim, C. Acoustic resolution photoacoustic microscopy. Biomed. Eng. Lett. 2014, 4, 213–222. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L.V. Photoacoustic imaging and characterization of the microvasculature. J. Biomed. Opt. 2010, 15, 011101. [Google Scholar] [CrossRef]
- Zhang, H.F.; Maslov, K.; Wang, L.V. In vivo imaging of subcutaneous structures using functional photoacoustic microscopy. Nat. Protoc. 2007, 2, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.; Aguirre, J.; Ntziachristos, V. Optoacoustic mesoscopy for biomedicine. Nat. Biomed. Eng. 2019. [Google Scholar] [CrossRef]
- Razansky, D.; Buehler, A.; Ntziachristos, V. Volumetric real-time multispectral optoacoustic tomography of biomarkers. Nat. Protoc. 2011, 6, 1121–1129. [Google Scholar] [CrossRef]
- Brecht, H.P.; Su, R.; Fronheiser, M.; Ermilov, S.A.; Conjusteau, A.; Oraevsky, A.A. Whole-body three-dimensional optoacoustic tomography system for small animals. J. Biomed. Opt. 2009, 14, 064007. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, V.; Razansky, D. Optical and opto-acoustic imaging. Mol. Imaging Oncol. 2013, 187, 133–150. [Google Scholar] [CrossRef]
- Schellenberg, M.W.; Hunt, H.K. Hand-held optoacoustic imaging: A review. Photoacoustics 2018, 11, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.G.; Beart, P.M. Inflammation: Maladies, models, mechanisms and molecules. Br. J. Pharm. 2016, 173, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.; Laufer, J.G.; Arridge, S.R.; Beard, P.C. Quantitative spectroscopic photoacoustic imaging: A review. J. Biomed. Opt. 2012, 17, 061202. [Google Scholar] [CrossRef]
- Wang, L.; Lei, P.; Wen, X.; Zhang, P.; Yang, S. Tapered fiber-based intravascular photoacoustic endoscopy for high-resolution and deep-penetration imaging of lipid-rich plaque. Opt. Express 2019, 27, 12832–12840. [Google Scholar] [CrossRef]
- Iskander-Rizk, S.; Wu, M.; Springeling, G.; van Beusekom, H.M.M.; Mastik, F.; Te Lintel Hekkert, M.; Beurskens, R.; Hoogendoorn, A.; Hartman, E.M.J.; van der Steen, A.F.W.; et al. In vivo intravascular photoacoustic imaging of plaque lipid in coronary atherosclerosis. EuroIntervention 2019, 15, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Hui, J.; Cao, Y.; Zhang, Y.; Kole, A.; Wang, P.; Yu, G.; Eakins, G.; Sturek, M.; Chen, W.; Cheng, J.X. Real-time intravascular photoacoustic-ultrasound imaging of lipid-laden plaque in human coronary artery at 16 frames per second. Sci. Rep. 2017, 7, 1417. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Hui, J.; Kole, A.; Wang, P.; Yu, Q.; Chen, W.; Sturek, M.; Cheng, J.X. High-sensitivity intravascular photoacoustic imaging of lipid-laden plaque with a collinear catheter design. Sci. Rep. 2016, 6, 25236. [Google Scholar] [CrossRef] [PubMed]
- Sangha, G.S.; Phillips, E.H.; Goergen, C.J. In vivo photoacoustic lipid imaging in mice using the second near-infrared window. Biomed. Opt. Express 2017, 8, 736–742. [Google Scholar] [CrossRef]
- Sangha, G.S.; Goergen, C.J. Label-free photoacoustic and ultrasound imaging for murine atherosclerosis characterization. APL Bioeng. 2020, 4, 026102. [Google Scholar] [CrossRef]
- Sethuraman, S.; Amirian, J.H.; Litovsky, S.H.; Smalling, R.W.; Emelianov, S.Y. Spectroscopic intravascular photoacoustic imaging to differentiate atherosclerotic plaques. Opt. Express 2008, 16, 3362–3367. [Google Scholar] [CrossRef]
- Wang, B.; Karpiouk, A.; Yeager, D.; Amirian, J.; Litovsky, S.; Smalling, R.; Emelianov, S. Intravascular photoacoustic imaging of lipid in atherosclerotic plaques in the presence of luminal blood. Opt. Lett. 2012, 37, 1244–1246. [Google Scholar] [CrossRef]
- Wang, B.; Karpiouk, A.; Yeager, D.; Amirian, J.; Litovsky, S.; Smalling, R.; Emelianov, S. In vivo intravascular ultrasound-guided photoacoustic imaging of lipid in plaques using an animal model of atherosclerosis. Ultrasound Med. Biol. 2012, 38, 2098–2103. [Google Scholar] [CrossRef]
- Xie, Z.; Yang, Y.; He, Y.; Shu, C.; Chen, D.; Zhang, J.; Chen, J.; Liu, C.; Sheng, Z.; Liu, H.; et al. In vivo assessment of inflammation in carotid atherosclerosis by noninvasive photoacoustic imaging. Theranostics 2020, 10, 4694–4704. [Google Scholar] [CrossRef]
- Liu, Y.; Hanley, T.; Chen, H.; Long, S.R.; Gambhir, S.S.; Cheng, Z.; Wu, J.C.; Fakhri, G.E.; Anvari, B.; Zaman, R.T. Non-Invasive Photoacoustic Imaging of In Vivo Mice with Erythrocyte Derived Optical Nanoparticles to Detect CAD/MI. Sci. Rep. 2020, 10, 5983. [Google Scholar] [CrossRef]
- Wang, B.; Yantsen, E.; Larson, T.; Karpiouk, A.B.; Sethuraman, S.; Su, J.L.; Sokolov, K.; Emelianov, S.Y. Plasmonic intravascular photoacoustic imaging for detection of macrophages in atherosclerotic plaques. Nano Lett. 2009, 9, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Taruttis, A.; Timmermans, A.C.; Wouters, P.C.; Kacprowicz, M.; van Dam, G.M.; Ntziachristos, V. Optoacoustic Imaging of Human Vasculature: Feasibility by Using a Handheld Probe. Radiology 2016, 281, 256–263. [Google Scholar] [CrossRef]
- Masthoff, M.; Helfen, A.; Claussen, J.; Roll, W.; Karlas, A.; Becker, H.; Gabriels, G.; Riess, J.; Heindel, W.; Schafers, M.; et al. Multispectral optoacoustic tomography of systemic sclerosis. J. Biophotonics 2018, 11, e201800155. [Google Scholar] [CrossRef] [PubMed]
- Ivankovic, I.; Mercep, E.; Schmedt, C.G.; Dean-Ben, X.L.; Razansky, D. Real-time Volumetric Assessment of the Human Carotid Artery: Handheld Multispectral Optoacoustic Tomography. Radiology 2019, 291, 181325. [Google Scholar] [CrossRef]
- Karlas, A.; Fasoula, N.A.; Paul-Yuan, K.; Reber, J.; Kallmayer, M.; Bozhko, D.; Seeger, M.; Eckstein, H.H.; Wildgruber, M.; Ntziachristos, V. Cardiovascular optoacoustics: From mice to men—A review. Photoacoustics 2019, 14, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Ida, T.; Iwazaki, H.; Kawaguchi, Y.; Kawauchi, S.; Ohkura, T.; Iwaya, K.; Tsuda, H.; Saitoh, D.; Sato, S.; Iwai, T. Burn depth assessments by photoacoustic imaging and laser Doppler imaging. Wound Repair Regen. 2016, 24, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Vionnet, L.; Gateau, J.; Schwarz, M.; Buehler, A.; Ermolayev, V.; Ntziachristos, V. 24-MHz scanner for optoacoustic imaging of skin and burn. IEEE Trans. Med. Imaging 2014, 33, 535–545. [Google Scholar] [CrossRef]
- Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L.V. Imaging acute thermal burns by photoacoustic microscopy. J. Biomed. Opt. 2006, 11, 054033. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Z.; Deng, Y.; Chen, S.L. Photoacoustic microscopy for evaluating a lipopolysaccharide-induced inflammation model in mice. J. Biophotonics 2019, 12, e201800251. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Lee, S.; Wang, Z.; Kim, D.; Stubblefield, B.; Gilbert, E.; Murthy, N. Maltodextrin-based imaging probes detect bacteria in vivo with high sensitivity and specificity. Nat. Mater. 2011, 10, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Zlitni, A.; Gowrishankar, G.; Steinberg, I.; Haywood, T.; Sam Gambhir, S. Maltotriose-based probes for fluorescence and photoacoustic imaging of bacterial infections. Nat. Commun. 2020, 11, 1250. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.; Hindelang, B.; Berezhnoi, A.; Darsow, U.; Lauffer, F.; Eyerich, K.; Biedermann, T.; Ntziachristos, V. Assessing nailfold microvascular structure with ultra-wideband raster-scan optoacoustic mesoscopy. Photoacoustics 2018, 10, 31–37. [Google Scholar] [CrossRef]
- Aguirre, J.; Schwarz, M.; Soliman, D.; Buehler, A.; Omar, M.; Ntziachristos, V. Broadband mesoscopic optoacoustic tomography reveals skin layers. Opt. Lett. 2014, 39, 6297–6300. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.H.; Schon, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Aguirre, J.; Schwarz, M.; Garzorz, N.; Omar, M.; Buehler, A.; Eyerich, K.; Ntziachristos, V. Precision assessment of label-free psoriasis biomarkers with ultra-broadband optoacoustic mesoscopy. Nat. Biomed. Eng. 2017, 1, 0068. [Google Scholar] [CrossRef]
- Yew, Y.W.; Dinish, U.S.; Yu Kuan, A.H.; Li, X.; Dev, K.; Ebrahim Attia, A.B.; Bi, R.; Moothanchery, M.; Balasundaram, G.; Aguirre, J.; et al. Raster-scanning optoacoustic mesoscopy (RSOM) imaging as an objective disease severity tool in atopic dermatitis patients. J. Am. Acad. Dermatol. 2020, 84, 1121–1123. [Google Scholar] [CrossRef]
- Yew, Y.W.; Dinish, U.S.; Choi, E.C.E.; Bi, R.; Ho, C.J.H.; Dev, K.; Li, X.; Attia, A.B.E.; Wong, M.K.W.; Balasundaram, G.; et al. Investigation of morphological, vascular and biochemical changes in the skin of an atopic dermatitis (AD) patient in response to dupilumab using raster scanning optoacoustic mesoscopy (RSOM) and handheld confocal Raman spectroscopy (CRS). J. Dermatol. Sci. 2019, 95, 123–125. [Google Scholar] [CrossRef]
- Brillant, N.; Elmasry, M.; Burton, N.C.; Rodriguez, J.M.; Sharkey, J.W.; Fenwick, S.; Poptani, H.; Kitteringham, N.R.; Goldring, C.E.; Kipar, A.; et al. Dynamic and accurate assessment of acetaminophen-induced hepatotoxicity by integrated photoacoustic imaging and mechanistic biomarkers in vivo. Toxicol. Appl. Pharm. 2017, 332, 64–74. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, L.; Zeng, F.; Wu, S. A conjugated-polymer-based ratiometric nanoprobe for evaluating in-vivo hepatotoxicity induced by herbal medicine via MSOT imaging. Photoacoustics 2019, 13, 6–17. [Google Scholar] [CrossRef]
- Sun, L.; Wu, Y.; Chen, J.; Zhong, J.; Zeng, F.; Wu, S. A Turn-On Optoacoustic Probe for Imaging Metformin-Induced Upregulation of Hepatic Hydrogen Sulfide and Subsequent Liver Injury. Theranostics 2019, 9, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qi, Y.; Zhan, C.; Zeng, F.; Wu, S. Diagnosing Drug-Induced Liver Injury by Multispectral Optoacoustic Tomography and Fluorescence Imaging Using a Leucine-Aminopeptidase-Activated Probe. Anal. Chem. 2019, 91, 8085–8092. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, P.J.; Bansal, R.; Daoudi, K.; Steenbergen, W.; Prakash, J. Preclinical detection of liver fibrosis using dual-modality photoacoustic/ultrasound system. Biomed. Opt. Express 2016, 7, 5081–5091. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Johnson, L.A.; Huang, Z.; Rubin, J.M.; Yuan, J.; Lei, H.; Ni, J.; Wang, X.; Higgins, P.D.R.; Xu, G. Identifying intestinal fibrosis and inflammation by spectroscopic photoacoustic imaging: An animal study. Biomed. Opt. Express 2018, 9, 1590–1600. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Johnson, L.A.; Liu, S.; Moons, D.S.; Ma, T.; Zhou, Q.; Rice, M.D.; Ni, J.; Wang, X.; Higgins, P.D.; et al. Characterizing intestinal inflammation and fibrosis in Crohn’s disease by photoacoustic imaging: Feasibility study. Biomed. Opt. Express 2016, 7, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Kempski, K.M.; Wiacek, A.; Graham, M.; Gonzalez, E.; Goodson, B.; Allman, D.; Palmer, J.; Hou, H.; Beck, S.; He, J.; et al. In vivo photoacoustic imaging of major blood vessels in the pancreas and liver during surgery. J. Biomed. Opt. 2019, 24, 1–12. [Google Scholar] [CrossRef]
- Yang, J.M.; Favazza, C.; Chen, R.; Yao, J.; Cai, X.; Maslov, K.; Zhou, Q.; Shung, K.K.; Wang, L.V. Simultaneous functional photoacoustic and ultrasonic endoscopy of internal organs in vivo. Nat. Med. 2012, 18, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Knieling, F.; Neufert, C.; Hartmann, A.; Claussen, J.; Urich, A.; Egger, C.; Vetter, M.; Fischer, S.; Pfeifer, L.; Hagel, A.; et al. Multispectral Optoacoustic Tomography for Assessment of Crohn’s Disease Activity. N. Engl. J. Med. 2017, 376, 1292–1294. [Google Scholar] [CrossRef] [PubMed]
- Waldner, M.J.; Knieling, F.; Egger, C.; Morscher, S.; Claussen, J.; Vetter, M.; Kielisch, C.; Fischer, S.; Pfeifer, L.; Hagel, A.; et al. Multispectral Optoacoustic Tomography in Crohn’s Disease: Noninvasive Imaging of Disease Activity. Gastroenterology 2016, 151, 238–240. [Google Scholar] [CrossRef]
- Beziere, N.; von Schacky, C.; Kosanke, Y.; Kimm, M.; Nunes, A.; Licha, K.; Aichler, M.; Walch, A.; Rummeny, E.J.; Ntziachristos, V.; et al. Optoacoustic imaging and staging of inflammation in a murine model of arthritis. Arthritis Rheumatol. 2014, 66, 2071–2078. [Google Scholar] [CrossRef]
- Fournelle, M.; Bost, W.; Tarner, I.H.; Lehmberg, T.; Weiß, E.; Lemor, R.; Dinser, R. Antitumor necrosis factor-α antibody-coupled gold nanorods as nanoprobes for molecular optoacoustic imaging in arthritis. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, R.; Luo, Y.; Liu, S.; Tang, T.; Yang, F.; Zhu, L.; He, X.; Yang, M.; Jiang, Y. Multimodal VEGF-Targeted Contrast-Enhanced Ultrasound and Photoacoustic Imaging of Rats with Inflammatory Arthritis: Using Dye-VEGF-Antibody-Loaded Microbubbles. Ultrasound Med. Biol. 2020, 46, 2400–2411. [Google Scholar] [CrossRef] [PubMed]
- Hallasch, S.; Giese, N.; Stoffels, I.; Klode, J.; Sondermann, W. Multispectral optoacoustic tomography might be a helpful tool for noninvasive early diagnosis of psoriatic arthritis. Photoacoustics 2021, 21, 100225. [Google Scholar] [CrossRef]
- Jo, J.; Xu, G.; Schiopu, E.; Chamberland, D.; Gandikota, G.; Wang, X. Imaging of enthesitis by an LED-based photoacoustic system. J. Biomed. Opt. 2020, 25. [Google Scholar] [CrossRef]
- Daoudi, K.; Kersten, B.E.; van den Ende, C.H.M.; van den Hoogen, F.H.J.; Vonk, M.C.; de Korte, C.L. Photoacoustic and high-frequency ultrasound imaging of systemic sclerosis patients. Arthritis Res. 2021, 23, 22. [Google Scholar] [CrossRef]
- Park, S.J.; Ho, C.J.H.; Arai, S.; Samanta, A.; Olivo, M.; Chang, Y.T. Visualizing Alzheimer’s Disease Mouse Brain with Multispectral Optoacoustic Tomography using a Fluorescent probe, CDnir7. Sci. Rep. 2019, 9, 12052. [Google Scholar] [CrossRef]
- Ichkova, A.; Rodriguez-Grande, B.; Zub, E.; Saudi, A.; Fournier, M.L.; Aussudre, J.; Sicard, P.; Obenaus, A.; Marchi, N.; Badaut, J. Early cerebrovascular and long-term neurological modifications ensue following juvenile mild traumatic brain injury in male mice. Neurobiol. Dis. 2020, 141, 104952. [Google Scholar] [CrossRef]
- Regensburger, A.P.; Fonteyne, L.M.; Jungert, J.; Wagner, A.L.; Gerhalter, T.; Nagel, A.M.; Heiss, R.; Flenkenthaler, F.; Qurashi, M.; Neurath, M.F.; et al. Detection of collagens by multispectral optoacoustic tomography as an imaging biomarker for Duchenne muscular dystrophy. Nat. Med. 2019, 25, 1905–1915. [Google Scholar] [CrossRef]
- Buehler, A.; Herzog, E.; Razansky, D.; Ntziachristos, V. Video rate optoacoustic tomography of mouse kidney perfusion. Opt. Lett. 2010, 35, 2475–2477. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Peng, W.; Ning, F.; Zhang, Y.; Wang, Y.; Xie, W.; Zhang, J.; Xin, H.; Li, C.; Zhang, X. Non-invasive detection of the early phase of kidney injury by photoacoustic/computed tomography imaging. Nanotechnology 2018, 29, 265101. [Google Scholar] [CrossRef] [PubMed]
- Hysi, E.; He, X.; Fadhel, M.N.; Zhang, T.; Krizova, A.; Ordon, M.; Farcas, M.; Pace, K.T.; Mintsopoulos, V.; Lee, W.L.; et al. Photoacoustic imaging of kidney fibrosis for assessing pretransplant organ quality. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Lawrence, D.J.; Escott, M.E.; Myers, L.; Intapad, S.; Lindsey, S.H.; Bayer, C.L. Spectral photoacoustic imaging to estimate in vivo placental oxygenation during preeclampsia. Sci. Rep. 2019, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Yamaleyeva, L.M.; Brosnihan, K.B.; Smith, L.M.; Sun, Y. Preclinical Ultrasound-Guided Photoacoustic Imaging of the Placenta in Normal and Pathologic Pregnancy. Mol. Imaging 2018, 17, 1536012118802721. [Google Scholar] [CrossRef]
- Huda, K.; Wu, C.; Sider, J.G.; Bayer, C.L. Spherical-view photoacoustic tomography for monitoring in vivo placental function. Photoacoustics 2020, 20, 100209. [Google Scholar] [CrossRef]
- Hacker, L.; Brunker, J.; Smith, E.S.J.; Quiros-Gonzalez, I.; Bohndiek, S.E. Photoacoustics resolves species-specific differences in hemoglobin concentration and oxygenation. J. Biomed. Opt. 2020, 25. [Google Scholar] [CrossRef]
- Laufer, J.; Elwell, C.; Delpy, D.; Beard, P. In vitro measurements of absolute blood oxygen saturation using pulsed near-infrared photoacoustic spectroscopy: Accuracy and resolution. Phys. Med. Biol. 2005, 50, 4409–4428. [Google Scholar] [CrossRef]
- Laufer, J.; Delpy, D.; Elwell, C.; Beard, P. Quantitative spatially resolved measurement of tissue chromophore concentrations using photoacoustic spectroscopy: Application to the measurement of blood oxygenation and haemoglobin concentration. Phys. Med. Biol. 2007, 52, 141–168. [Google Scholar] [CrossRef] [PubMed]
- David, H.; Ughetto, A.; Gaudard, P.; Plawecki, M.; Paiyabhroma, N.; Zub, E.; Colson, P.; Richard, S.; Marchi, N.; Sicard, P. Experimental Myocardial Infarction Elicits Time-Dependent Patterns of Vascular Hypoxia in Peripheral Organs and in the Brain. Front. Cardiovasc. Med. 2020, 7, 615507. [Google Scholar] [CrossRef]
- Brown, E.; Brunker, J.; Bohndiek, S.E. Photoacoustic imaging as a tool to probe the tumour microenvironment. Dis. Models Mech. 2019, 12. [Google Scholar] [CrossRef]
- Galanzha, E.I.; Zharov, V.P. In vivo photoacoustic and photothermal cytometry for monitoring multiple blood rheology parameters. Cytom. A 2011, 79, 746–757. [Google Scholar] [CrossRef]
- Van den Berg, P.J.; Daoudi, K.; Steenbergen, W. Review of photoacoustic flow imaging: Its current state and its promises. Photoacoustics 2015, 3, 89–99. [Google Scholar] [CrossRef]
- Brunker, J.; Beard, P. Velocity measurements in whole blood using acoustic resolution photoacoustic Doppler. Biomed. Opt. Express 2016, 7, 2789–2806. [Google Scholar] [CrossRef]
- Zharov, V.P.; Galanzha, E.I.; Shashkov, E.V.; Kim, J.W.; Khlebtsov, N.G.; Tuchin, V.V. Photoacoustic flow cytometry: Principle and application for real-time detection of circulating single nanoparticles, pathogens, and contrast dyes in vivo. J. Biomed. Opt. 2007, 12, 051503. [Google Scholar] [CrossRef]
- Cai, C.; Carey, K.A.; Nedosekin, D.A.; Menyaev, Y.A.; Sarimollaoglu, M.; Galanzha, E.I.; Stumhofer, J.S.; Zharov, V.P. In vivo photoacoustic flow cytometry for early malaria diagnosis. Cytom. A 2016, 89, 531–542. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Stary, H.C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W., Jr.; Rosenfeld, M.E.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arter. Thromb. Vasc. Biol. 1995, 15, 1512–1531. [Google Scholar] [CrossRef]
- Park, Y.M. CD36, a scavenger receptor implicated in atherosclerosis. Exp. Mol. Med. 2014, 46, e99. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef]
- Bi, R.; Dinish, U.S.; Goh, C.C.; Imai, T.; Moothanchery, M.; Li, X.; Kim, J.Y.; Jeon, S.; Pu, Y.; Kim, C.; et al. In vivo label-free functional photoacoustic monitoring of ischemic reperfusion. J. Biophotonics 2019, 12, e201800454. [Google Scholar] [CrossRef]
- Moustakidis, S.; Omar, M.; Aguirre, J.; Mohajerani, P.; Ntziachristos, V. Fully automated identification of skin morphology in raster-scan optoacoustic mesoscopy using artificial intelligence. Med. Phys. 2019, 46, 4046–4056. [Google Scholar] [CrossRef]
- Schwarz, M.; Buehler, A.; Aguirre, J.; Ntziachristos, V. Three-dimensional multispectral optoacoustic mesoscopy reveals melanin and blood oxygenation in human skin in vivo. J. Biophotonics 2016, 9, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhan, Y.; Harris, L.M.; Khan, S.; Xia, J. A portable three-dimensional photoacoustic tomography system for imaging of chronic foot ulcers. Quant. Imaging Med. Surg. 2019, 9, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.; Chen, F.; Moore, C.; Jokerst, J.V. Noninvasive staging of pressure ulcers using photoacoustic imaging. Wound Repair Regen. 2019, 27, 488–496. [Google Scholar] [CrossRef]
- Wu, Z.; Duan, F.; Zhang, J.; Li, S.; Ma, H.; Nie, L. In vivo dual-scale photoacoustic surveillance and assessment of burn healing. Biomed. Opt. Express 2019, 10, 3425–3433. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, L.; Xu, M.; Zheng, J.; Yuan, Z. Dual-Modal In Vivo Fluorescence/Photoacoustic Microscopy Imaging of Inflammation Induced by GFP-Expressing Bacteria. Sensors 2019, 19, 238. [Google Scholar] [CrossRef]
- Bhutiani, N.; Grizzle, W.E.; Galandiuk, S.; Otali, D.; Dryden, G.W.; Egilmez, N.K.; McNally, L.R. Noninvasive Imaging of Colitis Using Multispectral Optoacoustic Tomography. J. Nucl. Med. 2017, 58, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Atreya, R.; Neumann, H.; Neufert, C.; Waldner, M.J.; Billmeier, U.; Zopf, Y.; Willma, M.; App, C.; Munster, T.; Kessler, H.; et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat. Med. 2014, 20, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Rath, T.; Bojarski, C.; Neurath, M.F.; Atreya, R. Molecular imaging of mucosal alpha4beta7 integrin expression with the fluorescent anti-adhesion antibody vedolizumab in Crohn’s disease. Gastrointest. Endosc. 2017, 86, 406–408. [Google Scholar] [CrossRef]
- Shen, B.; Kochhar, G.; Navaneethan, U.; Farraye, F.A.; Schwartz, D.A.; Iacucci, M.; Bernstein, C.N.; Dryden, G.; Cross, R.; Bruining, D.H.; et al. Practical guidelines on endoscopic treatment for Crohn’s disease strictures: A consensus statement from the Global Interventional Inflammatory Bowel Disease Group. Lancet Gastroenterol. Hepatol. 2020, 5, 393–405. [Google Scholar] [CrossRef]
- Crespi, M.; Dulbecco, P.; De Ceglie, A.; Conio, M. Strictures in Crohn’s Disease: From Pathophysiology to Treatment. Dig. Dis. Sci. 2020, 65, 1904–1916. [Google Scholar] [CrossRef]
- Lei, H.; Johnson, L.A.; Eaton, K.A.; Liu, S.; Ni, J.; Wang, X.; Higgins, P.D.R.; Xu, G. Characterizing intestinal strictures of Crohn’s disease. Biomed. Opt. Express 2019, 10, 2542–2555. [Google Scholar] [CrossRef] [PubMed]
- Bettenworth, D.; Bokemeyer, A.; Baker, M.; Mao, R.; Parker, C.E.; Nguyen, T.; Ma, C.; Panes, J.; Rimola, J.; Fletcher, J.G.; et al. Assessment of Crohn’s disease-associated small bowel strictures and fibrosis on cross-sectional imaging: A systematic review. Gut 2019, 68, 1115–1126. [Google Scholar] [CrossRef]
- Knieling, F.; Gonzales Menezes, J.; Claussen, J.; Schwarz, M.; Neufert, C.; Fahlbusch, F.B.; Rath, T.; Thoma, O.M.; Kramer, V.; Menchicchi, B.; et al. Raster-Scanning Optoacoustic Mesoscopy for Gastrointestinal Imaging at High Resolution. Gastroenterology 2018, 154, 807–809.e803. [Google Scholar] [CrossRef]
- Heichler, C.; Scheibe, K.; Schmied, A.; Geppert, C.I.; Schmid, B.; Wirtz, S.; Thoma, O.M.; Kramer, V.; Waldner, M.J.; Büttner, C.; et al. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut 2020, 69, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Regensburger, A.P.; Wagner, A.L.; Claussen, J.; Waldner, M.J.; Knieling, F. Shedding light on pediatric diseases: Multispectral optoacoustic tomography at the doorway to clinical applications. Mol. Cell. Pediatr. 2020, 7, 3. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017, 389, 2328–2337. [Google Scholar] [CrossRef]
- Razansky, D.; Klohs, J.; Ni, R. Multi-scale optoacoustic molecular imaging of brain diseases. Eur. J. Nucl. Med. Mol. Imaging 2021. [Google Scholar] [CrossRef]
- Kang, N.Y.; Park, S.J.; Ang, X.W.; Samanta, A.; Driessen, W.H.; Ntziachristos, V.; Vasquez, K.O.; Peterson, J.D.; Yun, S.W.; Chang, Y.T. A macrophage uptaking near-infrared chemical probe CDnir7 for in vivo imaging of inflammation. Chem. Commun. 2014, 50, 6589–6591. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.L.; Danko, V.; Federle, A.; Klett, D.; Simon, D.; Heiss, R.; Jungert, J.; Uder, M.; Schett, G.; Neurath, M.F.; et al. Precision of handheld multispectral optoacoustic tomography for muscle imaging. Photoacoustics 2021, 21, 100220. [Google Scholar] [CrossRef]
- Noris, M.; Perico, N.; Remuzzi, G. Mechanisms of disease: Pre-eclampsia. Nat. Clin. Pract. Nephrol. 2005, 1, 98–114, quiz 120. [Google Scholar] [CrossRef] [PubMed]
- Brochu, F.M.; Brunker, J.; Joseph, J.; Tomaszewski, M.R.; Morscher, S.; Bohndiek, S.E. Towards Quantitative Evaluation of Tissue Absorption Coefficients Using Light Fluence Correction in Optoacoustic Tomography. IEEE Trans. Med. Imaging 2017, 36, 322–331. [Google Scholar] [CrossRef]
- Joseph, J.; Tomaszewski, M.R.; Quiros-Gonzalez, I.; Weber, J.; Brunker, J.; Bohndiek, S.E. Evaluation of Precision in Optoacoustic Tomography for Preclinical Imaging in Living Subjects. J. Nucl. Med. 2017, 58, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Bohndiek, S. Addressing photoacoustics standards. Nat. Photonics 2019, 13, 298. [Google Scholar] [CrossRef]
- Mercep, E.; Jeng, G.; Morscher, S.; Li, P.C.; Razansky, D. Hybrid optoacoustic tomography and pulse-echo ultrasonography using concave arrays. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 1651–1661. [Google Scholar] [CrossRef]
- Mercep, E.; Dean-Ben, X.L.; Razansky, D. Combined Pulse-Echo Ultrasound and Multispectral Optoacoustic Tomography With a Multi-Segment Detector Array. IEEE Trans. Med. Imaging 2017, 36, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals 2016, 29, 365–376. [Google Scholar] [CrossRef]
- Conjusteau, A.; Liopo, A.; Tsyboulski, D.; Ermilov, S.; Elliott, W.; Barsalou, N.; Maswadi, S.; Glickman, R.; Oraevsky, A. Optoacoustic Sensor for Nanoparticle Linked Immunosorbent Assay (NanoLISA); SPIE: Bellingham, WA, USA, 2011; Volume 7899. [Google Scholar]
- Longo, D.L.; Stefania, R.; Aime, S.; Oraevsky, A. Melanin-Based Contrast Agents for Biomedical Optoacoustic Imaging and Theranostic Applications. Int. J. Mol. Sci. 2017, 18, 1719. [Google Scholar] [CrossRef]
- Liopo, A.; Oraevsky, A. Nanoparticles as Contrast Agents for Optoacoustic Imaging. In Nanotechnology for Biomedical Imaging and Diagnostics: From Nanoparticle Design to Clinical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 111–149. [Google Scholar] [CrossRef]
- Hannah, A.; Luke, G.; Wilson, K.; Homan, K.; Emelianov, S. Indocyanine green-loaded photoacoustic nanodroplets: Dual contrast nanoconstructs for enhanced photoacoustic and ultrasound imaging. ACS Nano 2014, 8, 250–259. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Van de Sompel, D.; Bohndiek, S.E.; Gambhir, S.S. Cellulose Nanoparticles are a Biodegradable Photoacoustic Contrast Agent for Use in Living Mice. Photoacoustics 2014, 2, 119–127. [Google Scholar] [CrossRef] [PubMed]
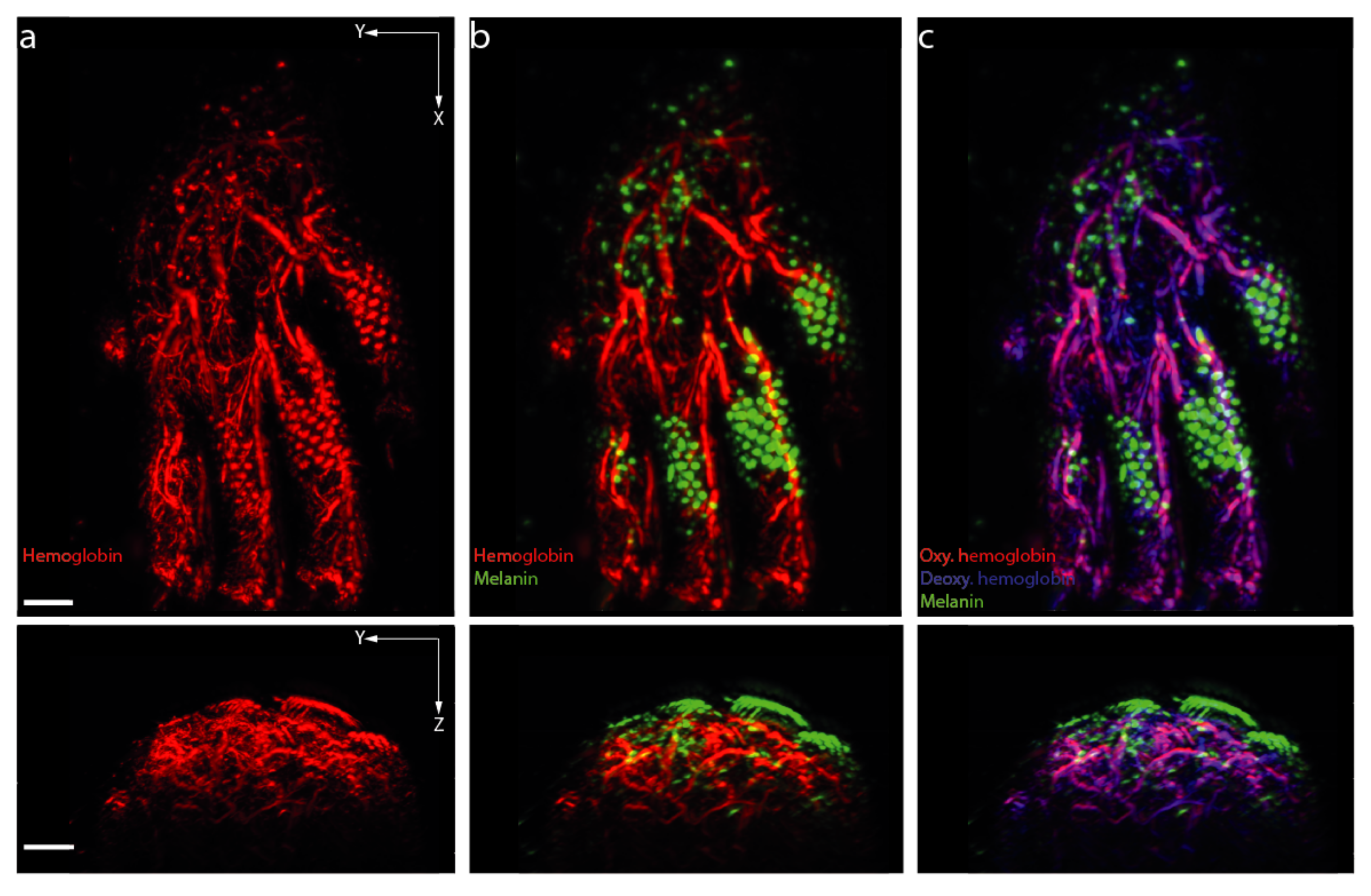
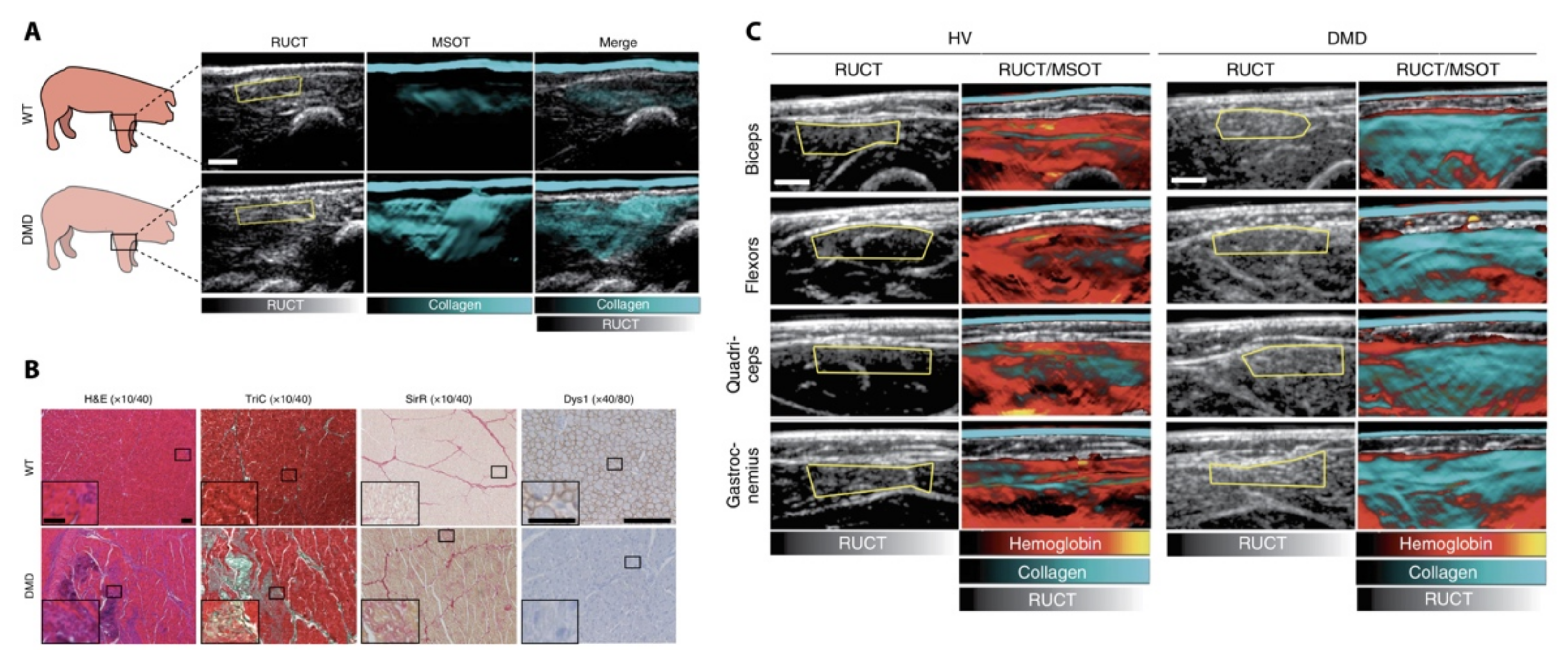
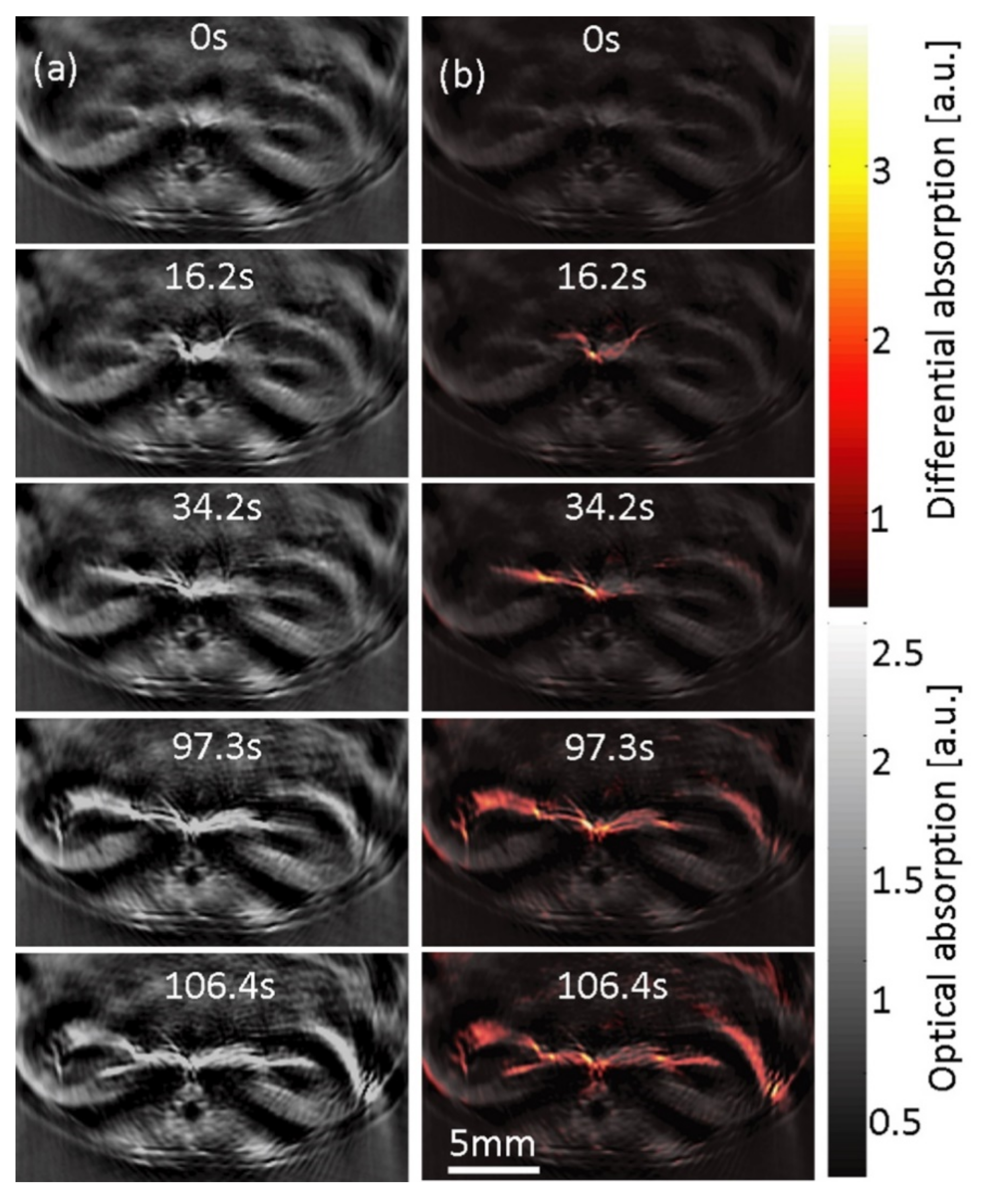
| Technique | Resolution | Penetration Depth | Laser Source | Focus | References | |
|---|---|---|---|---|---|---|
| Microscopy | Optical resolution | ~1 µm ~85 µm | >1 mm | Visible/NIR | US < Optical | [30] |
| Acoustic resolution | ~3 mm | Visible/NIR | US > Optical | |||
| Mesoscopy | ~10 µm | 1–4 mm | Visible | US > Optical | [33] | |
| Macroscopy/ Tomography | ~250 µm | 2–5 cm | NIR/exNIR | None | [36] |
| Diseases | Condition | Stage | Target/Contrast | Reference |
|---|---|---|---|---|
| Cardiovascular | Atherosclerosis | Preclinical | Lipid | [47,48,49,50,51,52,53,54,55] |
| Atherosclerosis | Preclinical | CD36 targeted NP | [56] | |
| Atherosclerosis | Preclinical | ICG loaded NETs | [57] | |
| Atherosclerosis | Preclinical | Gold nanoparticles | [58] | |
| Foot vasculature | Clinical | Hemoglobin | [59] | |
| Vascular malformations | Clinical | Hemoglobin | [60] | |
| Carotid arteries | Clinical | Hemoglobin | [61] | |
| Peripheral artery disease | Clinical | Hemoglobin | [62] | |
| Dermatologic | Thermal injuries | Preclinical | Hemoglobin | [63,64,65] |
| LPS-induced wound inflammation | Preclinical | Hemoglobin | [66] | |
| Bacterial wound infection | Preclinical | Targeted sugars | [67,68] | |
| Skin microvasculature, layers | Clinical | Hemoglobin | [69,70] | |
| Psoriasis | Clinical | Hemoglobin | [71,72] | |
| Atopic dermatitis | Clinical | Hemoglobin | [73,74] | |
| Gastrointestinal | Acute liver damage | Preclinical | ICG Perfusion | [75] |
| Acute liver damage | Preclinical | Probes, NO, H2S and leucine aminopeptidase | [76,77,78] | |
| Liver fibrosis | Preclinical | Collagen | [79] | |
| Intestinal strictures | Preclinical | Collagen | [80,81] | |
| Intestinal inflammation | Preclinical | Hemoglobin | [82,83] | |
| Image-guided surgery | Preclinical | Hemoglobin | [82] | |
| Intestinal vasculature and lymphatic vessels | Preclinical | Hemoglobin, Evans blue dye | [83] | |
| Intestinal inflammation | Clinical | Hemoglobin | [84,85] | |
| Musculoskeletal | Arthritis | Preclinical | NP: targeting L-selectin/P-selectin, TNFα, VEGF | [86,87,88] |
| Rheumatoid arthritis | Clinical | Hemoglobin | [89] | |
| Enthesitis | Clinical | Hemoglobin | [90] | |
| Systemic sclerosis | Clinical | Hemoglobin | [60,91] | |
| Neurodegenerative | Alzheimer’s diseases | Preclinical | CDnir7 | [92] |
| Cerebrovascular damage | Preclinical | Hemoglobin | [93] | |
| Muscular dystrophy | Clinical | Collagen | [94] | |
| Kidney | Organ perfusion | Preclinical | ICG Perfusion | [95] |
| Acute injury | Preclinical | NP | [96] | |
| Organ transplant | Clinical | Collagen | [97] | |
| Gynecologic | Preeclampsia | Preclinical | Hemoglobin | [98,99] |
| Preeclampsia | Preclinical | ICG targeting FRα | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regensburger, A.P.; Brown, E.; Krönke, G.; Waldner, M.J.; Knieling, F. Optoacoustic Imaging in Inflammation. Biomedicines 2021, 9, 483. https://doi.org/10.3390/biomedicines9050483
Regensburger AP, Brown E, Krönke G, Waldner MJ, Knieling F. Optoacoustic Imaging in Inflammation. Biomedicines. 2021; 9(5):483. https://doi.org/10.3390/biomedicines9050483
Chicago/Turabian StyleRegensburger, Adrian P., Emma Brown, Gerhard Krönke, Maximilian J. Waldner, and Ferdinand Knieling. 2021. "Optoacoustic Imaging in Inflammation" Biomedicines 9, no. 5: 483. https://doi.org/10.3390/biomedicines9050483
APA StyleRegensburger, A. P., Brown, E., Krönke, G., Waldner, M. J., & Knieling, F. (2021). Optoacoustic Imaging in Inflammation. Biomedicines, 9(5), 483. https://doi.org/10.3390/biomedicines9050483






