The Role of Biomarkers in Adrenocortical Carcinoma: A Review of Current Evidence and Future Perspectives
Abstract
1. Introduction
2. Pathogenesis of Adrenocortical Cancer
3. Hormonal Work-Up
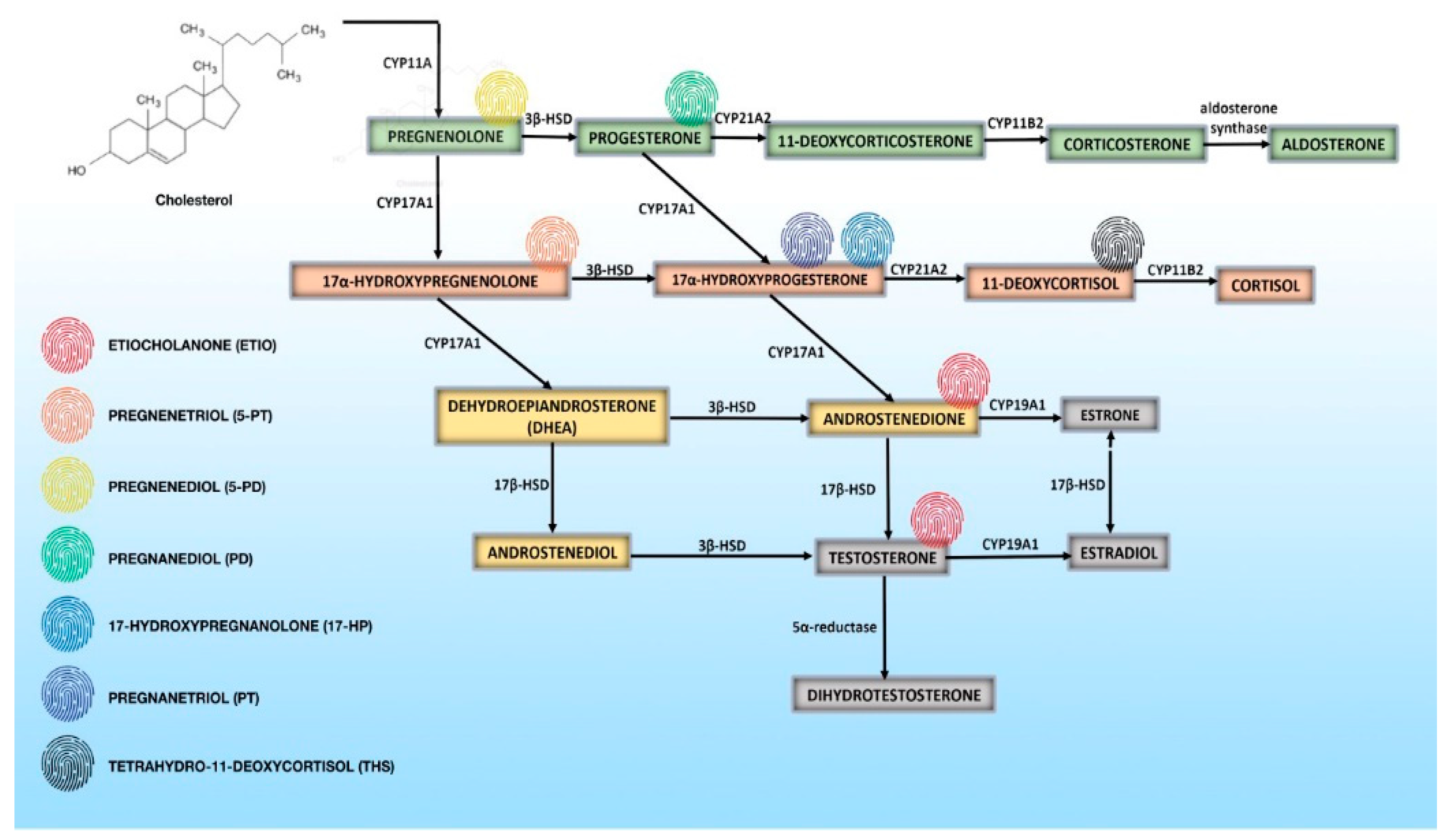
4. Steroid Metabolome Profiling
5. Pathological Approach to Adrenocortical Carcinoma
6. Circulating Tumor Biomarkers
7. Genetic Analysis
7.1. Genome Sequencing
7.2. MicroRNA
7.3. Novel Genomic Approaches
8. Treatment Options
Experimental Studies
9. Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Weiss Criteria ≥ 3 Criteria | Wieneke Criteria * ≥ 4 Criteria | Lin–Weiss–Bisceglia System **: |
|---|---|---|
| 1. High nuclear grade (III or IV) † 2. Mitotic rate greater than 5 per 50 high-power fields 3. Presence of atypical mitoses 4. Clear lipid-rich cells comprising less than 25% of the tumor 5. >33% diffuse architecture 6. necrosis 7. Invasion of venous structures 8. Invasion of sinusoidal structures 9. Invasion of the capsule | 1. Tumor weight >400 g 2. Tumor size >10.5 cm 3. Extension into periadrenal soft tissues and/or adjacent organs 4. Invasion into vena cava 5. Venous invasion 6. Capsular invasion 7. Presence of tumor necrosis 8. >15 mitoses per 20 HPF 9. Presence of atypical mitotic figures | Major criteria 1. Mitotic count >5 per 50 high- power fields 2.Atypical mitoses 3. Venous invasion Minor criteria 1. Size >10 cm and/or weight >200 g 2. Necrosis 3. Sinusoidal invasion 4. Capsular invasion |
References
- Else, T.; Kim, A.C.; Sabolch, A.; Raymond, V.M.; Kandathil, A.; Caoili, E.M.; Jolly, S.; Miller, B.S.; Giordano, T.J.; Hammer, G.D. Adrenocortical carcinoma. Endocr. Rev. 2014, 35, 282–326. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, T.; Goldberg, H.; Klaassen, Z.; Wallis, C.J.D.; Woon, D.T.S.; Herrera-Caceres, J.O.; Kulkarni, G.S.; Fleshner, N.E. The who, when, and why of primary adrenal malignancies: Insights into the epidemiology of a rare clinical entity. Cancer 2019, 125, 1050–1059. [Google Scholar] [CrossRef]
- Libé, R. Adrenocortical carcinoma (ACC): Diagnosis, prognosis, and treatment. Front. Cell Dev. Biol. 2015, 3, 45. [Google Scholar] [CrossRef]
- Xing, Z.; Luo, Z.; Yang, H.; Huang, Z.; Liang, X. Screening and identification of key biomarkers in adrenocortical carcinoma based on bioinformatics analysis. Oncol. Lett. 2019, 18, 4667–4676. [Google Scholar] [CrossRef]
- Vaidya, A.; Nehs, M.; Kilbridge, K. Treatment of Adrenocortical Carcinoma. Surg. Pathol. Clin. 2019, 12, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Cherradi, N. microRNAs as Potential Biomarkers in Adrenocortical Cancer: Progress and Challenges. Front. Endocrinol. 2016, 6, 195. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamazaki, Y.; Felizola, S.J.; Ise, K.; Morimoto, R.; Satoh, F.; Arai, Y.; Sasano, H. Adrenocortical carcinoma: Review of the pathologic features, production of adrenal steroids, and molecular pathogenesis. Endocrinol. Metab. Clin. N. Am. 2015, 44, 399–410. [Google Scholar] [CrossRef]
- Bourdeau, I.; MacKenzie-Feder, J.; Lacroix, A. Recent advances in adrenocortical carcinoma in adults. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 192–197. [Google Scholar] [CrossRef]
- Puglisi, S.; Perotti, P.; Pia, A.; Reimondo, G.; Terzolo, M. Adrenocortical Carcinoma with Hypercortisolism. Endocrinol. Metab. Clin. N. Am. 2018, 47, 395–407. [Google Scholar] [CrossRef]
- Komarowska, H.; Rucinski, M.; Tyczewska, M.; Sawicka-Gutaj, N.; Szyszka, M.; Hernik, A.; Klimont, A.; Milecka, P.; Migasiuk, L.; Biczysko, M.; et al. Ghrelin as a potential molecular marker of adrenal carcinogenesis: In vivo and in vitro evidence. Clin. Endocrinol. 2018, 89, 36–45. [Google Scholar] [CrossRef]
- Libè, R.; Fratticci, A.; Bertherat, J. Adrenocortical cancer: Pathophysiology and clinical management. Endocr. Relat. Cancer 2007, 14, 13–28. [Google Scholar] [CrossRef]
- Lodish, M. Genetics of Adrenocortical Development and Tumors. Endocrinol. Metab. Clin. N. Am. 2017, 46, 419–433. [Google Scholar] [CrossRef]
- Szyszka, P.; Grossman, A.B.; Diaz-Cano, S.; Sworczak, K.; Dworakowska, D. Molecular pathways of human adrenocortical carcinoma—Translating cell signalling knowledge into diagnostic and treatment options. Endokrynol. Pol. 2016, 67, 427–450. [Google Scholar]
- Creemers, S.G.; van Koetsveld, P.M.; van Kemenade, F.J.; Papathomas, T.G.; Franssen, G.J.H.; Dogan, F.; Eekhoff, E.M.; van der Valk, P.; de Herder, W.W.; Janssen, J.A.; et al. Methylation of IGF2 regulatory regions to diagnose adrenocortical carcinomas. Endocr. Relat. Cancer 2016, 23, 727–737. [Google Scholar] [CrossRef]
- Angelousi, A.; Kyriakopoulos, G.; Nasiri-Ansari, N.; Karageorgou, M.; Kassi, E. The role of epithelial growth factors and insulin growth factors in the adrenal neoplasms. Ann. Transl. Med. 2018, 6, 253. [Google Scholar] [CrossRef]
- Altieri, B.; Colao, A.; Faggiano, A. The role of insulin-like growth factor system in the adrenocortical tumors. Minerva Endocrinol. 2019, 44, 43–57. [Google Scholar] [CrossRef]
- Guillaud-Bataille, M.; Ragazzon, B.; de Reynies, A.; Chevalier, C.; Francillard, I.; Barreau, O.; Steunou, V.; Guillemot, J.; Tissier, F.; Rizk-Rabin, M.; et al. IGF2 promotes growth of adrenocortical carcinoma cells, but its overexpression does not modify phenotypic and molecular features of adrenocortical carcinoma. PLoS ONE 2014, 9, e103744. [Google Scholar] [CrossRef]
- Ilvesmaki, V.; Kahri, A.I.; Miettinen, P.J.; Voutilainen, R. Insulin-like growth factors (IGFs) and their receptors in adrenal tumors: High IGF-II expression in functional adrenocortical carcinomas. J. Clin. Endocrinol. Metab. 1993, 77, 852–858. [Google Scholar]
- Soon, P.S.; Gill, A.J.; Benn, D.E.; Clarkson, A.; Robinson, B.G.; McDonald, K.L.; Sidhuet, S.B. Microarray gene expression and immunohistochemistry analyses of adrenocortical tumors identify IGF2 and Ki-67 as useful in differentiating carcinomas from adenomas. Endocr. Relat. Cancer 2009, 16, 573–583. [Google Scholar] [CrossRef]
- Schmitt, A.; Saremaslani, P.; Schmid, S.; Rousson, V.; Montani, M.; Schmid, D.M.; Heitz, P.U.; Komminoth, P.; Perren, A. IGFII and MIB1 immunohistochemistry is helpful for the differentiation of benign from malignant adrenocortical tumours. Histopathology 2006, 49, 298–307. [Google Scholar] [CrossRef]
- Erickson, L.A.; Jin, L.; Sebo, T.J.; Lohse, C.; Pankratz, V.S.; Kendrick, M.L.; van Heerden, J.A.; Thompson, G.B.; Grant, C.S.; Lloyd, R.V. Pathologic features and expression of insulin-like growth factor-2 in adrenocortical neoplasms. Endocr. Pathol. 2001, 12, 429–435. [Google Scholar] [CrossRef]
- Pereira, S.S.; Monteiro, M.P.; Costa, M.M.; Moreira, Â.; Alves, M.G.; Oliveira, P.F.; Jarak, I.; Pignatelli, D. IGF2 role in adrenocortical carcinoma biology. Endocrine 2019, 66, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Gaujoux, S.; Launay, P.; Baudry, C.; Chokri, I.; Ragazzon, B.; Libé, R.; René-Corail, F.; Audebourg, A.; Vacher-Lavenuet, M.C.; et al. Wnt/β-Catenin Pathway Activation in Adrenocortical Adenomas Is Frequently due to Somatic CTNNB1-Activating Mutations, Which Are Associated with Larger and Nonsecreting Tumors: A Study in Cortisol-Secreting and -Nonsecreting Tumors. J. Clin. Endocrinol. Metab. 2011, 96, E419–E426. [Google Scholar] [CrossRef] [PubMed]
- Bonnet-Serrano, F.; Bertherat, J. Genetics of tumors of the adrenal cortex. Endocr. Relat. Cancer 2018, 25, R131–R152. [Google Scholar] [CrossRef]
- Rubin, B.; Regazzo, D.; Redaelli, M.; Mucignat, C.; Citton, M.; Iacobone, M.; Scaroni, C.; Betterle, C.; Mantero, F.; Fassina, A.; et al. Investigation of N-cadherin/β-catenin expression in adrenocortical tumors. Tumour Biol. 2016, 37, 13545–13555. [Google Scholar] [CrossRef]
- Nicolson, N.G.; Man, J.; Carling, T. Advances in understanding the molecular underpinnings of adrenocortical tumors. Curr. Opin. Oncol. 2018, 30, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.S.; Monteiro, M.P.; Bourdeau, I.; Lacroix, A.; Pignatelli, D. Mechanisms of endocrinology: Cell cycle regulation in adrenocortical carcinoma. Eur. J. Endocrinol. 2018, 179, R95–R110. [Google Scholar] [CrossRef]
- Wanis, K.N.; Kanthan, R. Diagnostic and prognostic features in adrenocortical carcinoma: A single institution case series and review of the literature. World J. Surg. Oncol. 2015, 13, 117. [Google Scholar] [CrossRef]
- Abiven, G.; Coste, J.; Groussin, L.; Anract, P.; Tissier, F.; Legmann, P.; Dousset, B.; Bertagna, X.; Bertherat, J. Clinical and biological features in the prognosis of adrenocortical cancer: Poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J. Clin. Endocrinol. Metab. 2006, 91, 2650–2655. [Google Scholar] [CrossRef]
- Fassnacht, M.; Allolio, B. Clinical management of adrenocortical carcinoma. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 273–289. [Google Scholar] [CrossRef]
- Puglisi, S.; Perotti, P.; Cosentini, D.; Roca, E.; Basile, V.; Berruti, A.; Terzolo, M. Decision-making for adrenocortical carcinoma: Surgical, systemic, and endocrine management options. Expert Rev. Anticancer Ther. 2018, 18, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Vanbrabant, T.; Fassnacht, M.; Assie, G.; Dekkers, O.M. Influence of hormonal functional status on survival in adrenocortical carcinoma: Systematic review and meta-analysis. Eur. J. Endocrinol. 2018, 179, 429–436. [Google Scholar] [CrossRef]
- Mantero, F.; Terzolo, M.; Arnaldi, G.; Osella, G.; Masini, A.M.; Ali, A.; Giovagnetti, M.; Opocher, G.; Angeli, A. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J. Clin. Endocrinol. Metab. 2000, 85, 637–644. [Google Scholar]
- Midorikawa, S.; Hashimoto, S.; Kuriki, M.; Katoh, K.; Watanabe, T.; Sasano, H.; Nishikawa, T. A patient with preclinical Cushing’s syndrome and excessive DHEA-S secretion having unilateral adrenal carcinoma and contralateral adenoma. Endocr. J. 1999, 46, 59–66. [Google Scholar] [CrossRef]
- Hofle, G.; Gasser, R.W.; Lhotta, K.; Janetschek, G.; Kreczy, A.; Finkenstedt, G. Adrenocortical carcinoma evolving after diagnosis of preclinical Cushing’s syndrome in an adrenal incidentaloma. A case report. Horm. Res. 1998, 50, 237–242. [Google Scholar]
- Fukai, N.; Hirono, Y.; Yoshimoto, T.; Doi, M.; Ohtsuka, Y.; Homma, K.; Shibata, H.; Sasano, H.; Hirata, Y. A case of estrogen-secreting adrenocortical carcinoma with subclinical Cushing’s syndrome. Endocr. J. 2006, 53, 237–245. [Google Scholar] [CrossRef][Green Version]
- Wong, L.M.; Li, C.H.; Chan, O.K.; Shek, C.C.; Kwong, N.S. A 12-year-old chinese girl with Cushing syndrome and virilization due to adrenocortical carcinoma. J. Pediatr. Endocrinol. Metab. 2011, 24, 193–196. [Google Scholar] [CrossRef]
- Ishikura, K.; Takamura, T.; Takeshita, Y.; Nakagawa, A.; Imaizumi, N.; Misu, H. Cushing’s syndrome and big IGF-II associated hypoglycaemia in a patient with adrenocortical carcinoma. BMJ Case Rep. 2010, 2010, bcr07.2009.2100. [Google Scholar] [CrossRef]
- Anderson, H. A tumor of the adrenal gland with fatal hypoglycemia. Am. J. Med. Sci. 1930, 180, 71–79. [Google Scholar] [CrossRef]
- Rustin, M.H.; Bowcock, S.J.; Coomes, E.N. Adrenocortical carcinoma with tumour-induced hypoglycaemia. J. R. Soc. Med. 1983, 76, 74–75. [Google Scholar] [CrossRef]
- Altieri, B.; Tirabassi, G.; Della Casa, S.; Ronchi, C.L.; Balercia, G.; Orio, F.; Pontecorvi, A.; Colao, A.; Muscogiuri, G. Adrenocortical tumors and insulin resistance: What is the first step? Int. J. Cancer 2016, 138, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Assie, G.; Baudin, E.; Eisenhofer, G.; de la Fouchardiere, C.; Haak, H.R.; de Krijger, R.; Porpiglia, F.; Terzolo, M.; Berruti, A. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1476–1490. [Google Scholar] [CrossRef]
- Song, G.; Joe, B.N.; Yeh, B.M.; Meng, M.V.; Westphalen, A.C.; Coakley, F.V. Risk of catecholamine crisis in patients undergoing resection of unsuspected pheochromocytoma. Int. Braz. J. Urol. 2011, 37, 35–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nieman, L.K.; Biller, B.M.; Findling, J.W.; Newell-Price, J.; Savage, M.O.; Stewart, P.M.; Montori, V.M. The diagnosis of Cushing’s syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2008, 93, 1526–1540. [Google Scholar] [CrossRef]
- Mazzaglia, P.J.; Habra, V.M.A. Evaluation and management of adrenal neoplasms: Endocrinologist and endocrine surgeon perspectives. Abdom. Radiol. 2020, 45, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, S.; Kunz, M.; Kurlbaum, M.; Vey, J.; Kendl, S.; Deutschbein, T.; Hahner, S.; Fassnacht, M.; Dandekar, T.; Kroiss, M. Plasma steroid metabolome profiling for the diagnosis of adrenocortical carcinoma. Eur. J. Endocrinol. 2019, 180, 117–125. [Google Scholar] [CrossRef]
- Arlt, W.; Biehl, M.; Taylor, A.E.; Hahner, S.; Libé, R.; Hughes, B.A.; Schneider, P.; Smith, D.J.; Stiekema, H.; Krone, N.; et al. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J. Clin. Endocrinol. Metab. 2011, 96, 3775–3784. [Google Scholar] [CrossRef]
- Chortis, V.; Bancos, I.; Nijman, T.; Gilligan, L.C.; Taylor, A.E.; Ronchi, C.L.; O’Reilly, M.W.; Schreiner, J.; Asia, M.; Riester, A.; et al. Urine Steroid Metabolomics as a Novel Tool for Detection of Recurrent Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2020, 105, e307–e318. [Google Scholar] [CrossRef]
- Pereira, S.S.; Costa, M.M.; Gomez-Sanchez, C.E.; Monteiro, M.P.; Pignatelli, D. Incomplete Pattern of Steroidogenic Protein Expression in Functioning Adrenocortical Carcinomas. Biomedicines 2020, 8, 256. [Google Scholar] [CrossRef]
- Bancos, I.; Arlt, W. Diagnosis of a malignant adrenal mass: The role of urinary steroid metabolite profiling. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 200–207. [Google Scholar] [CrossRef]
- Fanelli, F.; Di Dalmazi, G. Serum steroid profiling by mass spectrometry in adrenocortical tumors: Diagnostic implications. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 160–165. [Google Scholar] [CrossRef]
- McDonald, J.G.; Matthew, S.; Auchus, R.J. Steroid profiling by gas chromatography-mass spectrometry and high performance liquid chromatography-mass spectrometry for adrenal diseases. Horm. Cancer 2011, 2, 324–332. [Google Scholar] [CrossRef]
- Taylor, D.R.; Ghataore, L.; Couchman, L.; Vincent, R.P.; Whitelaw, B.; Lewis, D.; Diaz-Cano, S.; Galata, G.; Schulte, K.M.; Aylwin, S.; et al. A 13-Steroid Serum Panel Based on LC-MS/MS: Use in Detection of Adrenocortical Carcinoma. Clin. Chem. 2017, 63, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Cicalini, I.; Verrocchio, S.; Di Dalmazi, G.; Federici, L.; Bucci, I. The Potential of Steroid Profiling by Mass Spectrometry in the Management of Adrenocortical Carcinoma. Biomedicines 2020, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Rege, J.; Turcu, A.F.; Else, T.; Auchus, R.J.; Rainey, W.E. Steroid biomarkers in human adrenal disease. J. Steroid Biochem. Mol. Biol. 2019, 190, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, M.; Scarsbrook, A.; Abbas, A.; Fraser, S.; Limumpornpetch, P.; Dineen, R.; Stewart, P.M. Adrenal Incidentaloma. Endocr. Rev. 2020, 41, 6. [Google Scholar] [CrossRef] [PubMed]
- Lenders, N.F.; Greenfield, J.R. Urinary steroid profiling in diagnostic evaluation of an unusual adrenal mass. Endocrinol. Diabetes Metab. Case Rep. 2019, 2019, 19–0090. [Google Scholar] [CrossRef]
- Honour, J.W. Urinary Steroid Profiling in the Diagnosis of Adrenal Disorders. Clin. Chem. 2018, 64, 1257–1258. [Google Scholar] [CrossRef]
- Kerkhofs, T.M.; Kerstens, M.N.; Kema, I.P.; Willems, T.P.; Haak, H.R. Diagnostic Value of Urinary Steroid Profiling in the Evaluation of Adrenal Tumors. Horm. Cancer 2015, 6, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Velikanova, L.I.; Shafigullina, Z.R.; Lisitsin, A.A.; Vorokhobina, N.V.; Grigoryan, K.; Kukhianidze, E.A.; Strelnikova, E.G.; Krivokhizhina, N.S.; Krasnov, L.M.; Fedorov, E.A.; et al. Different Types of Urinary Steroid Profiling Obtained by High-Performance Liquid Chromatography and Gas Chromatography-Mass Spectrometry in Patients with Adrenocortical Carcinoma. Horm. Cancer 2016, 7, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Velikanova, L.I.; Shafigullina, Z.R.; Vorokhobina, N.V.; Malevanaya, E.V. Gas Chromatography-Mass Spectrometry Analysis of Urinary Steroid Metabolomics for Detection of Early Signs of Adrenal Neoplasm Malignancy in Patients with Cushing’s Syndrome. Bull. Exp. Biol. Med. 2019, 167, 676–680. [Google Scholar] [CrossRef]
- Calissendorff, J.; Calissendorff, F.; Falhammar, H. Adrenocortical cancer: Mortality, hormone secretion, proliferation and urine steroids—Experience from a single centre spanning three decades. BMC Endocr. Disord. 2016, 16, 15. [Google Scholar] [CrossRef]
- Hines, J.M.; Bancos, I.; Bancos, C.; Singh, R.D.; Avula, A.V.; Young, W.F.; Grebe, S.K.; Singh, R.J. High-Resolution, Accurate-Mass (HRAM) Mass Spectrometry Urine Steroid Profiling in the Diagnosis of Adrenal Disorders. Clin. Chem. 2017, 63, 1824–1835. [Google Scholar] [CrossRef]
- Suzuki, S.; Minamidate, T.; Shiga, A.; Ruike, Y.; Ishiwata, K.; Naito, K.; Ishida, A.; Deguchi, H.; Fujimoto, M.; Koide, H.; et al. Steroid metabolites for diagnosing and predicting clinicopathological features in cortisol-producing adrenocortical carcinoma. BMC Endocr. Disord. 2020, 20, 173. [Google Scholar] [CrossRef]
- Patel, D.; Thompson, M.D.; Manna, S.K.; Krausz, K.W.; Zhang, L.; Nilubol, N.; Gonzalez, F.J.; Kebebew, E. Unique and Novel Urinary Metabolomic Features in Malignant versus Benign Adrenal Neoplasms. Clin. Cancer Res. 2017, 23, 5302–5310. [Google Scholar] [CrossRef] [PubMed]
- Papathomas, T.G.; Sun, N.; Chortis, V.; Taylor, A.E.; Arlt, W.; Richter, S.; Eisenhofer, G.; Ruiz-Babot, G.; Guasti, L.; Walch, A.K. Novel methods in adrenal research: A metabolomics approach. Histochem. Cell Biol. 2019, 151, 201–216. [Google Scholar] [CrossRef]
- Tiu, S.C.; Chan, A.O.K.; Taylor, N.F.; Lee, C.Y.; Loung, P.Y.; Choi, C.H.; Shek, C.C. Use of urinary steroid profiling for diagnosing and monitoring adrenocortical tumours. Hong Kong Med. J. 2009, 15, 463–470. [Google Scholar]
- Bancos, I.; Taylor, A.E.; Chortis, V.; Sitch, A.J.; Jenkinson, C.; Davidge-Pitts, C.J.; Lang, K.; Tsagarakis, S.; Macech, M.; Riester, A.; et al. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: A prospective test validation study. Lancet Diabetes Endocrinol. 2020, 8, 773–781. [Google Scholar] [CrossRef]
- Biehl, M.; Schneider, P.; Smith, D.; Stiekema, H.; Taylor, A.; Hughes, B.; Shackleton, C.; Stewart, P.; Arlt, W. Matrix relevance LVQ in steroid metabolomics based classification of adrenal tumors. In Proceedings of the European Symposium on Artificial Neural Networks, Computational Intelligence and Machine Learning, Bruges, Belgium, 25–27 April 2012. [Google Scholar]
- Xu, S.; Hu, S.; Yu, X.; Zhang, M.; Yang, Y. 17α-hydroxylase/17,20-lyase deficiency in congenital adrenal hyperplasia: A case report. Mol. Med. Rep. 2017, 15, 339–344. [Google Scholar] [CrossRef]
- Sun, N.; Kunzke, T.; Sbiera, S.; Kircher, S.; Feuchtinger, A.; Aichler, M.; Herterich, S.; Ronchi, C.L.; Weigand, I.; Schlegel, N.; et al. Prognostic Relevance of Steroid Sulfation in Adrenocortical Carcinoma Revealed by Molecular Phenotyping Using High-Resolution Mass Spectrometry Imaging. Clin. Chem. 2019, 65, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, Y.; Wu, H.; Zhao, D.; Chen, J. Distinguishing adrenal cortical carcinomas and adenomas: A study of clinicopathological features and biomarkers. Histopathology 2014, 64, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, A.; Pakbaz, S.; Mete, O. A Diagnostic Approach to Adrenocortical Tumors. Surg. Pathol. Clin. 2019, 12, 967–995. [Google Scholar] [CrossRef]
- Aubert, S.; Wacrenier, A.; Leroy, X.; Devos, P.; Carnaille, B.; Proye, C.; Wemeau, J.L.; Lecomte-Houcke, M.; Leteurtre, E. Weiss system revisited: A clinicopathological and immunohistochemical study of 49 adrenocortical tumors. Am. J. Surg. Pathol. 2002, 26, 1612–1619. [Google Scholar] [CrossRef]
- Sasano, H.; Suzuki, T.; Moriya, T. Recent advances in surgical pathology of adrenal incidentaloma. Biomed. Pharmacother. 2000, 54, 169–174. [Google Scholar] [CrossRef]
- Almeida, M.Q.; Bezerra-Neto, J.E.; Mendonça, B.B.; Latronico, A.C.; Fragoso, M.C.B.V. Primary malignant tumors of the adrenal glands. Clinics 2018, 73 (Suppl. S1), e756s. [Google Scholar] [CrossRef]
- Kostiainen, I.; Hakaste, L.; Kejo, P.; Parviainen, H.; Laine, T.; Löyttyniemi, E.; Pennanen, M.; Arola, J.; Haglund, C.; Heiskanen, I.; et al. Adrenocortical carcinoma: Presentation and outcome of a contemporary patient series. Endocrine 2019, 65, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Duregon, E.; Cappellesso, R.; Maffeis, V.; Zaggia, B.; Ventura, L.; Berruti, A.; Terzolo, M.; Fassina, A.; Volante, M.; Papotti, M. Validation of the prognostic role of the “Helsinki Score” in 225 cases of adrenocortical carcinoma. Hum. Pathol. 2017, 62, 1–7. [Google Scholar] [CrossRef]
- Duregon, E.; Volante, M.; Giorcelli, J.; Terzolo, M.; Lalli, E.; Papotti, M. Diagnostic and prognostic role of steroidogenic factor 1 in adrenocortical carcinoma: A validation study focusing on clinical and pathologic correlates. Hum. Pathol. 2013, 44, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Anand Guntiboina, V.; Sengupta, M.; Islam, N.; Barman, S.; Krishna Biswas, S.; Chatterjee, U.; Kumar Mishra, P.; Roy, P.; Guha Mallick, M.; Datta, C. Diagnostic and prognostic utility of SF1, IGF2 and p57 immunoexpression in pediatric adrenal cortical tumors. J. Pediatr. Surg. 2019, 54, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, F.; Liu, Z.; Wu, K.; Zhu, Y.; Lu, Y. Prognostic Role of Ki-67 in Adrenocortical Carcinoma After Primary Resection: A Retrospective Mono-Institutional Study. Adv. Ther. 2019, 36, 2756–2768. [Google Scholar] [CrossRef]
- Papathomas, T.G.; Pucci, E.; Giordano, T.J.; Lu, H.; Duregon, E.; Volante, M.; Papotti, M.; Lloyd, R.V.; Tischler, A.S.; van Nederveen, F.H.; et al. An international Ki67 reproducibility study in adrenal cortical carcinoma. Am. J. Surg. Pathol. 2016, 40, 569–576. [Google Scholar] [CrossRef]
- Erickson, L.A. Challenges in surgical pathology of adrenocortical tumours. Histopathology 2018, 72, 82–96. [Google Scholar] [CrossRef]
- Mete, O.; Gucer, H.; Kefeli, M.; Asa, S.L. Diagnostic and Prognostic Biomarkers of Adrenal Cortical Carcinoma. Am. J. Surg. Pathol. 2018, 42, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Saiegh, L.; Sheikh-Ahmad, M.; Shechner, C.; Reut, M.; Darawsha, Y.; Zolotov, S.; Shefer, H.; Bejar, I.; Bejar, J. Metallothionein protein and minichromosome maintenance protein-2 expression in adrenocortical tumors. Ann. Endocrinol. 2019, 80, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Aporowicz, M.; Czopnik, P.; Kubicka, E.; Piotrowska, A.; Dziegiel, P.; Bolanowski, M.; Domoslawski, P. Minichromosome Maintenance Proteins MCM-3, MCM-5, MCM-7, and Ki-67 as Proliferative Markers in Adrenocortical Tumors. Anticancer Res. 2019, 39, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Habra, M.A.; Stephen, B.; Campbell, M.; Hess, K.; Tapia, C.; Xu, M.; Rodon Ahnert, J.; Jimenez, C.; Lee, J.E.; Perrier, N.D.; et al. Phase II clinical trial of pembrolizumab efficacy and safety in advanced adrenocortical carcinoma. J. Immunother. Cancer 2019, 7, 253. [Google Scholar] [CrossRef]
- Tierney, J.F.; Vogle, A.; Poirier, J.; Min, I.M.; Finnerty, B.; Zarnegar, R.; Pappas, S.G.; Scognamiglio, T.; Ghai, R.; Gattuso, P.; et al. Expression of programmed death ligand 1 and 2 in adrenocortical cancer tissues: An exploratory study. Surgery 2019, 165, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, A.M.F.; Soares, I.C.; Mariani, B.M.P.; Nishi, M.Y.; Bezerra-Neto, J.E.; Charchar, H.D.S.; Balderrama Brondani, V.; Tanno, F.; Srougi, V.; Chambo, J.L.; et al. Sterol O-Acyl Transferase 1 as a Prognostic Marker of Adrenocortical Carcinoma. Cancers 2020, 12, 247. [Google Scholar] [CrossRef]
- Weigand, I.; Altieri, B.; Lacombe, A.M.F.; Basile, V.; Kircher, S.; Landwehr, L.S.; Schreiner, J.; Zerbini, M.C.N.; Ronchi, C.L.; Megerle, F.; et al. Expression of SOAT1 in Adrenocortical Carcinoma and Response to Mitotane Monotherapy: An ENSAT Multicenter Study. J. Clin. Endocrinol. Metab. 2020, 105, dgaa293. [Google Scholar] [CrossRef] [PubMed]
- Grisanti, S.; Filice, A.; Basile, V.; Cosentini, D.; Rapa, I.; Albano, D.; Morandi, A.; Laganà, M.; Dalla Volta, A.; Bertagna, F.; et al. Treatment With 90Y/177Lu-DOTATOC in Patients With Metastatic Adrenocortical Carcinoma Expressing Somatostatin Receptors. J. Clin. Endocrinol. Metab. 2020, 105, dgz091. [Google Scholar] [CrossRef]
- Chifu, I.; Heinze, B.; Fuss, C.T.; Lang, K.; Kroiss, M.; Kircher, S.; Ronchi, C.L.; Altieri, B.; Schirbel, A.; Fassnacht, M.; et al. Impact of the Chemokine Receptors CXCR4 and CXCR7 on Clinical Outcome in Adrenocortical Carcinoma. Front. Endocrinol. 2020, 11, 597878. [Google Scholar] [CrossRef] [PubMed]
- Korol, P.; Jaranowska, M.; Pawlikowski, M. Immunohistochemical demonstration of LH/CG receptors in non-neoplastic human adrenal cortex and adrenocortical tumors. Folia Histochem. Cytobiol. 2019, 57, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Ruggiero, C.; Cantini, G.; Canu, L.; Baroni, G.; Armignacco, R.; Jouinot, A.; Santi, R.; Ercolino, T.; Ragazzon, B.; et al. Fascin-1 Is a Novel Prognostic Biomarker Associated With Tumor Invasiveness in Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2019, 104, 1712–1724. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liu, Z.; Wei, X.; Zhou, L.; Tang, Y.; Zhou, C.; Wu, K.; Zhang, F.; Zhang, F.; Lu, Y.; et al. Expression of FSCN1 and FOXM1 are associated with poor prognosis of adrenocortical carcinoma patients. BMC Cancer 2019, 19, 1165. [Google Scholar] [CrossRef]
- Roca, E.; Berruti, A.; Sbiera, S.; Rapa, I.; Oneda, E.; Sperone, P.; Ronchi, C.L.; Ferrari, L.; Grisanti, S.; Germano, A.; et al. Topoisomerase 2α and thymidylate synthase expression in adrenocortical cancer. Endocr. Relat. Cancer 2017, 24, 319–327. [Google Scholar] [CrossRef]
- De Martino, M.C.; van Koetsveld, P.M.; Feelders, R.A.; de Herder, W.W.; Dogan, F.; Janssen, J.A.M.J.L.; Op Bruinink, D.H.; Pivonello, C.; Waaijers, A.M.; Colao, A.; et al. IGF and mTOR pathway expression and in vitro effects of linsitinib and mTOR inhibitors in adrenocortical cancer. Endocrine 2019, 64, 673–684. [Google Scholar] [CrossRef]
- Brown, T.C.; Nicolson, N.G.; Stenman, A.; Juhlin, C.C.; Gibson, C.E.; Callender, G.G.; Korah, R.; Carling, T. Insulin-Like Growth Factor and SLC12A7 Dysregulation: A Novel Signaling Hallmark of Non-Functional Adrenocortical Carcinoma. J. Am. Coll. Surg. 2019, 229, 305–315. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Z.; Pei, T.; Xiao, Y.; Zhou, L.; Tang, Y.; Zhou, C.; Wu, K.; Zhang, F.; Zhang, F.; et al. Clinicopathological and Prognostic Characteristics of CD276 (B7-H3) Expression in Adrenocortical Carcinoma. Dis. Mark. 2020, 2020, 5354825. [Google Scholar] [CrossRef]
- Pennanen, M.; Hagström, J.; Heiskanen, I.; Sane, T.; Mustonen, H.; Arola, J.; Haglund, C. C-myc expression in adrenocortical tumours. J. Clin. Pathol. 2018, 71, 129–134. [Google Scholar] [CrossRef]
- Germano, A.; Rapa, I.; Duregon, E.; Votta, A.; Giorcelli, J.; Buttigliero, C.; Scagliotti, G.V.; Volante, M.; Terzolo, M.; Papotti, M. Tissue Expression and Pharmacological In Vitro Analyses of mTOR and SSTR Pathways in Adrenocortical Carcinoma. Endocr. Pathol. 2017, 28, 95–102. [Google Scholar] [CrossRef]
- Romero Arenas, M.A.; Whitsett, T.G.; Aronova, A.; Henderson, S.A.; LoBello, J.; Habra, M.A.; Grubbs, E.G.; Lee, J.E.; Sircar, K.; Zarnegar, R.; et al. Protein Expression of PTTG1 as a Diagnostic Biomarker in Adrenocortical Carcinoma. Ann. Surg. Oncol. 2018, 25, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Lionti, S.; Ieni, A.; Cannavò, S.; Barresi, V. Immunohistochemical expression of glypican-3 in adrenocortical carcinoma: A potential cause of diagnostic pitfalls. Ann. Diagn. Pathol. 2018, 35, 92–93. [Google Scholar] [CrossRef]
- Park, W.Y.; Seo, H.I.; Choi, K.U.; Kim, A.; Kim, Y.K.; Lee, S.J.; Hun Lee, C.; Yeong Huh, G.; Youn Park, D. Three cases of adrenocortical tumors mistaken for hepatocellular carcinomas/diagnostic pitfalls and differential diagnosis. Ann. Diagn. Pathol. 2017, 31, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Papathomas, T.G.; Duregon, E.; Korpershoek, E.; Restuccia, D.F.; van Marion, R.; Cappellesso, R.; Sturm, N.; Rossi, G.; Coli, A.; Zucchini, N.; et al. Sarcomatoid adrenocortical carcinoma: A comprehensive pathological, immunohistochemical, and targeted next-generation sequencing analysis. Hum. Pathol. 2016, 58, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Sbiera, S.; Della Casa, S.; Weigand, I.; Wild, V.; Steinhauer, S.; Fadda, G.; Kocot, A.; Bekteshi, M.; Mambretti, E.M.; et al. Livin/BIRC7 expression as malignancy marker in adrenocortical tumors. Oncotarget 2017, 8, 9323–9338. [Google Scholar] [CrossRef] [PubMed]
- Liu-Chittenden, Y.; Jain, M.; Gaskins, K.; Wang, S.; Merino, M.J.; Kotian, S.; Kumar Gara, S.; Davis, S.; Zhang, L.; Kebebew, E. RARRES2 functions as a tumor suppressor by promoting β-catenin phosphorylation/degradation and inhibiting p38 phosphorylation in adrenocortical carcinoma. Oncogene 2017, 36, 3541–3552. [Google Scholar] [CrossRef] [PubMed]
- Babińska, A.; Pęksa, R.; Wiśniewski, P.; Świątkowska-Stodulska, R.; Sworczak, K. Diagnostic and prognostic role of SF1, IGF2, Ki67, p53, adiponectin, and leptin receptors in human adrenal cortical tumors. J. Surg. Oncol. 2017, 116, 427–433. [Google Scholar] [CrossRef]
- Aronova, A.; Min, I.M.; Crowley, M.J.P.; Panjwani, S.J.; Finnerty, B.M.; Scognamiglio, T.; Liu, Y.F.; Whitsett, T.G.; Garg, S.; Demeure, M.J.; et al. STMN1 is Overexpressed in Adrenocortical Carcinoma and Promotes a More Aggressive Phenotype In Vitro. Ann. Surg. Oncol. 2018, 25, 792–800. [Google Scholar] [CrossRef]
- Pinheiro, C.; Granja, S.; Longatto-Filho, A.; Faria, A.M.; Fragoso, M.C.B.V.; Lovisolo, S.M.; Lerário, A.M.; Almeida, M.Q.; Baltazar, F.; Zerbini, M.C.N. Metabolic reprogramming: A new relevant pathway in adult adrenocortical tumors. Oncotarget 2015, 6, 44403–44421. [Google Scholar] [CrossRef] [PubMed]
- Sbiera, S.; Sbiera, I.; Ruggiero, C.; Doghman-Bouguerra, M.; Korpershoek, E.; de Krijger, R.R.; Ettaieb, H.; Haak, H.; Volante, M.; Papotti, M.; et al. Assessment of VAV2 Expression Refines Prognostic Prediction in Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2017, 102, 3491–3498. [Google Scholar] [CrossRef] [PubMed]
- Murtha, T.D.; Brown, T.C.; Rubinstein, J.C.; Haglund, F.; Juhlin, C.C.; Larsson, C.; Korah, R.; Carling, T. Overexpression of cytochrome P450 2A6 in adrenocortical carcinoma. Surgery 2017, 161, 1667–1674. [Google Scholar] [CrossRef]
- Kieler, M.; Müllauer, L.; Koperek, O.; Bianconi, D.; Unseld, M.; Raderer, M.; Prager, G.W. Analysis of 10 Adrenocortical Carcinoma Patients in the Cohort of the Precision Medicine Platform MONDTI. Oncology 2018, 94, 306–310. [Google Scholar] [CrossRef]
- Pereira, S.S.; Costa, M.M.; Guerreiro, S.G.; Monteiro, M.P.; Pignatelli, D. Angiogenesis and Lymphangiogenesis in the Adrenocortical. Tumors Pathol. Oncol. Res. 2018, 24, 689–693. [Google Scholar] [CrossRef]
- Zlatibor, L.; Paunovic, I.; Zivaljevic, V.; Dundjerovic, D.; Tatic, S.; Djukic, V. Prognostic significance of immunohistochemical markers in adrenocortical carcinoma. Acta Chir. Belg. 2020, 120, 23–29. [Google Scholar] [CrossRef]
- Laufs, V.; Altieri, B.; Sbiera, S.; Kircher, S.; Steinhauer, S.; Beuschlein, F.; Quinkler, M.; Willenberg, H.S.; Rosenwald, A.; Fassnacht, M.; et al. ERCC1 as predictive biomarker to platinum-based chemotherapy in adrenocortical carcinomas. Eur. J. Endocrinol. 2018, 178, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Dong, B.; Huang, J.; Kong, W.; Xue, W.; Zhu, Y.; Zhang, J.; Huang, Y. Sphingosine kinase 1 is overexpressed and promotes adrenocortical carcinoma progression. Oncotarget 2016, 7, 3233–3244. [Google Scholar] [CrossRef]
- Pereira, S.S.; Máximo, V.; Coelho, R.; Batista, R.; Soares, P.; Guerreiro, S.G.; Sobrinho-Simões, M.; Monteiro, M.P.; Pignatelli, D. Telomerase and N-Cadherin Differential Importance in Adrenocortical Cancers and Adenomas. J. Cell Biochem. 2017, 118, 2064–2071. [Google Scholar] [CrossRef] [PubMed]
- Pennanen, M.; Tynninen, O.; Kytölä, S.; Ellonen, P.; Mustonen, H.; Heiskanen, I.; Haglund, C.; Arola, J. IDH1 Expression via the R132H Mutation-Specific Antibody in Adrenocortical Neoplasias-Prognostic Impact in Carcinomas. J. Endocr. Soc. 2020, 4, bvaa018. [Google Scholar] [CrossRef] [PubMed]
- Tierney, J.F.; Vogle, A.; Finnerty, B.; Zarnegar, R.; Ghai, R.; Gattuso, P.; Fahey, T.J., 3rd; Keutgen, X.M. Indoleamine 2,3-Dioxygenase-1 Expression in Adrenocortical Carcinoma. J. Surg. Res. 2020, 256, 90–95. [Google Scholar] [CrossRef]
- Bisceglia, M.; Ludovico, O.; Di Mattia, A.; Ben-Dor, D.; Sandbank, J.; Pasquinelli, G.; Lau, S.K.; Weiss, L.M. Adrenocortical oncocytic tumors: Report of 10 cases and review of the literature. Int. J. Surg. Pathol. 2004, 12, 231–243. [Google Scholar] [CrossRef]
- Das, S.; Sengupta, M.; Islam, N.; Roy, P.; Datta, C.; Kumar Mishra, P.; Banerjee, S.; Chaudhuri, M.K.; Chatterjee, U. Weineke criteria, Ki-67 index and p53 status to study pediatric adrenocortical tumors: Is there a correlation? J. Pediatr. Surg. 2016, 51, 1795–1800. [Google Scholar] [CrossRef]
- Chatterjee, G.; DasGupta, S.; Mukherjee, G.; Sengupta, M.; Roy, P.; Arun, I. Usefulness of Wieneke criteria in assessing morphologic characteristics of adrenocortical tumors in children. Pediatr. Surg. Int. 2015, 31, 563–571. [Google Scholar] [CrossRef]
- Pennanen, M.; Heiskanen, I.; Sane, T.; Remes, S.; Mustonen, H.; Haglund, C.; Arola, J. Helsinki score-a novel model for prediction of metastases in adrenocortical carcinomas. Hum. Pathol. 2015, 46, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Kawahara, T.; Takamoto, D.; Makiyama, K.; Hattori, Y.; Teranishi, J.I.; Miyoshi, Y.; Yumura, Y.; Yao, M.; Uemura, H. The neutrophil-to-lymphocyte ratio (NLR) predicts adrenocortical carcinoma and is correlated with the prognosis. BMC Urol. 2017, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Gaitanidis, A.; Wiseman, D.; El Lakis, M.; Nilubol, N.; Kebebew, E.; Patel, D. Preoperative systemic inflammatory markers are prognostic indicators in recurrent adrenocortical carcinoma. J. Surg. Oncol. 2019, 120, 1450–1455. [Google Scholar] [CrossRef]
- Sisman, P.; Bicer, B.; Oz Gul, O.; Cander, S.; Ersoy, C.; Saraydaroglu, O.; Erturk, E. May hemocytometer parameters be a biomarker in distinguishing between adrenal adenomas and carcinomas and in prognosis of adrenocortical carcinomas? Acta Clin. Croat. 2020, 59, 439–444. [Google Scholar]
- Babińska, A.; Kaszubowski, M.; Kmieć, P.; Sworczak, K. Adipokine and cytokine levels in patients with adrenocortical cancer, subclinical Cushing’s syndrome and healthy controls. Steroids 2018, 140, 39–44. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, S.; Bi, J.; Kong, C. Construction of a risk signature for adrenocortical carcinoma using immune-related genes. Transl. Androl. Urol. 2020, 9, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Kołomecki, K.; Stepień, H.; Bartos, M.; Narebski, J. Evaluation of MMP-1, MMP-8, MMP-9 serum levels in patients with adrenal tumors prior to and after surgery. Neoplasma 2001, 48, 116–121. [Google Scholar]
- Liu-Chittenden, Y.; Patel, D.; Gaskins, K.; Giordano, T.J.; Assie, G.; Bertherat, J.; Kebebew, E. Serum RARRES2 Is a Prognostic Marker in Patients With Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2016, 101, 3345–3352. [Google Scholar] [CrossRef]
- Hofland, J.; Feelders, R.A.; van der Wal, R.; Kerstens, M.N.; Haak, H.R.; de Herder, W.W.; de Jong, F.H. Serum inhibin pro-αC is a tumor marker for adrenocortical carcinomas. Eur. J. Endocrinol. 2012, 166, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, C.L.; Sbiera, S.; Leich, E.; Tissier, F.; Steinhauer, S.; Deutschbein, T. Low SGK1 expression in human adrenocortical tumors is associated with ACTH-independent glucocorticoid secretion and poor prognosis. J. Clin. Endocrinol. Metab. 2012, 97, E2251–E2260. [Google Scholar] [CrossRef] [PubMed]
- Ide, H.; Terado, Y.; Tokiwa, S.; Nishio, K.; Saito, K.; Isotani, S.; Kamiyama, Y.; Muto, S.; Imamura, T.; Horie, S. Novel germ line mutation p53-P177R in adult adrenocortical carcinoma producing neuron-specific enolase as a possible marker. Jpn. J. Clin. Oncol. 2010, 40, 815–818. [Google Scholar] [CrossRef]
- Jouinot, A.; Lippert, J.; Fassnacht, M.; de La Villeon, B.; Septier, A.; Neou, M.; Perlemoine, K.; Appenzeller, S.; Sibony, M.; Gaujoux, S.; et al. Intratumor heterogeneity of prognostic DNA-based molecular markers in adrenocortical carcinoma. Endocr. Connect. 2020, 9, 705–714. [Google Scholar]
- Gicquel, C.; Leblond-Francillard, M.; Bertagna, X.; Louvel, A.; Chapuis, Y.; Luton, J.P.; Girard, F.; Le Bouc, Y. Clonal analysis of human adrenocortical carcinomas and secreting adenomas. Clin. Endocrinol. 1994, 40, 465–477. [Google Scholar] [CrossRef]
- Beuschlein, F.; Reincke, M.; Karl, M.; Travis, W.D.; Jaursch-Hancke, C.; Abdelhamid, S.; Chrousos, G.P.; Allolio, B. Clonal composition of human adrenocortical neoplasms. Cancer Res. 1994, 54, 4927–4932. [Google Scholar]
- Jouinot, A.; Assie, G.; Libe, R.; Fassnacht, M.; Papathomas, T.; Barreau, O.; de la Villeon, B.; Faillot, S.; Hamzaoui, N.; Neou, M.; et al. DNA Methylation Is an Independent Prognostic Marker of Survival in Adrenocortical Cancer. J. Clin. Endocrinol. Metab. 2017, 102, 923–932. [Google Scholar] [CrossRef]
- Barreau, O.; Assié, G.; Wilmot-Roussel, H.; Ragazzon, B.; Baudry, C.; Perlemoine, K.; René-Corail, F.; Bertagna, X.; Dousset, B.; Hamzaoui, N.; et al. Identification of a CpG Island Methylator Phenotype in Adrenocortical Carcinomas. J. Clin. Endocrinol. Metab. 2013, 98, E174–E184. [Google Scholar] [CrossRef] [PubMed]
- Ettaieb, M.; Kerkhofs, T.; van Engeland, M.; Haak, H. Past, Present and Future of Epigenetics in Adrenocortical Carcinoma. Cancers 2020, 12, 1218. [Google Scholar] [CrossRef]
- Ikeya, A.; Nakashima, M.; Yamashita, M.; Kakizawa, K.; Okawa, Y.; Saitsu, H.; Sasaki, S.; Sasano, H.; Suda, T.; Oki, Y. CCNB2 and AURKA overexpression may cause atypical mitosis in Japanese cortisol-producing adrenocortical carcinoma with TP53 somatic variant. PLoS ONE 2020, 15, e0231665. [Google Scholar] [CrossRef]
- Xiao, H.; Xu, D.; Chen, P.; Zeng, G.; Wang, X.; Zhang, X. Identification of Five Genes as a Potential Biomarker for Predicting Progress and Prognosis in Adrenocortical Carcinoma. Cancer 2018, 9, 4484–4495. [Google Scholar] [CrossRef]
- Else, T.; Marcondes Lerario, A.; Everett, J.; Haymon, L.; Wham, D.; Mullane, M.; LeShan Wilson, T.; Rainville, I.; Rana, H.; Worth, A.J.; et al. Adrenocortical carcinoma and succinate dehydrogenase gene mutations: An observational case series. Eur. J. Endocrinol. 2017, 177, 439–444. [Google Scholar] [CrossRef]
- Bie, J.; Liu, K.; Song, G.; Hu, X.; Xiong, R.; Zhang, X.; Shi, X.; Wang, Z. ENST00000489707.5 Is a Preferred Alternative Splicing Variant of PTK7 in Adrenocortical Cancer and Shows Potential Prognostic Value. Med. Sci. Monit. 2019, 25, 8326–8334. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.H.; Qu, Y.Y.; Zhang, H.L.; Ye, D.W. Screening and Identification of Potential Prognostic Biomarkers in Adrenocortical Carcinoma. Front. Genet. 2019, 10, 821. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, C.; Cohen, M.S. Over expression of DNA damage and cell cycle dependent proteins are associated with poor survival in patients with adrenocortical carcinoma. Surgery 2019, 165, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Gara, S.K.; Tyagi, M.V.; Patel, D.T.; Gaskins, K.; Lack, J.; Liu, Y.; Kebebew, E. GATA3 and APOBEC3B are prognostic markers in adrenocortical carcinoma and APOBEC3B is directly transcriptionally regulated by GATA3. Oncotarget 2020, 11, 3354–3370. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Sbiera, S.; Herterich, S.; De Francia, S.; Della Casa, S.; Calabrese, A.; Pontecorvi, A.; Quinkler, M.; Kienitz, T.; Mannelli, M.; et al. Effects of Germline CYP2W1*6 and CYP2B6*6 Single Nucleotide Polymorphisms on Mitotane Treatment in Adrenocortical Carcinoma: A Multicenter ENSAT Study. Cancers 2020, 12, 359. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.C.; Murtha, T.D.; Rubinstein, J.; Korah, R.; Carling, T. SLC12A7 alters adrenocortical carcinoma cell adhesion properties to promote an aggressive invasive behavior. Cell Commun. Signal. 2018, 16, 27. [Google Scholar] [CrossRef]
- Brown, T.C.; Nicolson, N.G.; Korah, R.; Carling, T. BCL9 Upregulation in Adrenocortical Carcinoma: A Novel Wnt/β-Catenin Activating Event Driving Adrenocortical Malignancy. J. Am. Coll. Surg. 2018, 226, 988–995. [Google Scholar] [CrossRef]
- Pittaway, J.F.H.; Guasti, L. Pathobiology and genetics of adrenocortical carcinoma. J. Mol. Endocrinol. 2019, 62, R105–R119. [Google Scholar] [CrossRef]
- Lippert, J.; Appenzeller, S.; Liang, R.; Sbiera, S.; Kircher, S.; Altieri, B.; Nanda, I.; Weigand, I.; Gehrig, A.; Steinhauer, S.; et al. Targeted Molecular Analysis in Adrenocortical Carcinomas: A Strategy Toward Improved Personalized Prognostication. J. Clin. Endocrinol. Metab. 2018, 103, 4511–4523. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.C.; Gardiner, J.R.; Renaud, Y.; Chauhan, R.; Weinstein, Y.; Gomez-Sanchez, C.; Lefrançois-Martinez, A.M.; Bertherat, J.; Val, P.; Swain, A. HOX genes promote cell proliferation and are potential therapeutic targets in adrenocortical tumours. Br. J. Cancer 2020. [Google Scholar] [CrossRef]
- Bedrose, S.; Daher, M.; Altameemi, L.; Habra, M.A. Adjuvant Therapy in Adrenocortical Carcinoma: Reflections and Future Directions. Cancers 2020, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Catalano, R.; Giardino, E.; Treppiedi, D.; Mangili, F.; Morelli, V.; Elli, F.M.; Serban, A.L.; Luconi, M.; Mannelli, M.; Spada, A.; et al. The cytoskeleton actin binding protein filamin A impairs both IGF2 mitogenic effects and the efficacy of IGF1R inhibitors in adrenocortical cancer cells. Cancer Lett. 2021, 497, 77–88. [Google Scholar] [CrossRef]
- Bulzico, D.; Barreto Pires, B.R.; Silvestre, D.E.; Faria, P.A.; Vieira Neto, L.; Abdelhay, E. Twist1 Correlates With Epithelial-Mesenchymal Transition Markers Fibronectin and Vimentin in Adrenocortical Tumors. Anticancer Res. 2019, 39, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Murtha, T.D.; Korah, R.; Carling, T. Suppression of cytochrome P450 4B1: An early event in adrenocortical tumorigenesis. Surgery 2017, 161, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Haase, M.; Thiel, A.; Scholl, U.I.; Ashmawy, H.; Schott, M.; Ehlers, M. Subcellular localization of fibroblast growth factor receptor type 2 and correlation with CTNNB1 genotype in adrenocortical carcinoma. BMC Res. Notes 2020, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, S.; Qian, B.; Wang, Y.; Duan, R.; Jiang, W.; Kang, Y.; Dou, Y.; Yang, G.; Shene, L.; et al. Integrative omics analysis reveals relationships of genes with synthetic lethal interactions through a pan-cancer analysis. Comput. Struct. Biotechnol. J. 2020, 18, 3243–3254. [Google Scholar] [CrossRef]
- Hu, C.; Fang, D.; Xu, H.; Wang, Q.; Xia, H. The androgen receptor expression and association with patient’s survival in different cancers. Genomics 2020, 112, 1926–1940. [Google Scholar] [CrossRef]
- Zheng, S.; Cherniack, A.D.; Dewal, N.; Moffitt, R.A.; Danilova, L.; Murray, B.A.; Lerario, A.M.; Else, T.; Knijnenburg, T.A.; Ciriello, G.; et al. Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma. Cancer Cell 2016, 30, 363. [Google Scholar] [CrossRef]
- Fernandez-Ranvier, G.G.; Weng, J.; Yeh, R.F.; Khanafshar, E.; Suh, I.; Barker, C.; Yang Duh, Q.; Clark, O.H.; Kebebew, E. Identification of biomarkers of adrenocortical carcinoma using genomewide gene expression profiling. Arch. Surg. 2008, 143, 841–846. [Google Scholar] [CrossRef]
- Ross, J.S.; Wang, K.; Rand, J.V.; Gay, L.; Presta, M.J.; Sheehan, C.E.; Ali, S.M.; Elvin, J.A.; Labrecque, E.; Hiemstra, C.; et al. Next-generation sequencing of adrenocortical carcinoma reveals new routes to targeted therapies. J. Clin. Pathol. 2014, 67, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Alshabi, A.M.; Vastrad, B.; Shaikh, I.A.; Vastrad, C. Identification of important invasion and proliferation related genes in adrenocortical carcinoma. Med. Oncol. 2019, 36, 73. [Google Scholar] [CrossRef] [PubMed]
- Fojo, T.; Huff, L.; Litman, T.; Im, K.; Edgerly, M.; Del Rivero, J.; Pittaluga, S.; Merino, M.; Bates, S.E.; Dean, M. Metastatic and recurrent adrenocortical cancer is not defined by its genomic landscape. BMC Med. Genom. 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhi, Q.; Xie, Q.; Wu, X.; Gao, Y.; Chen, X.; Shi, L. Reduced expression of ferroportin1 and ceruloplasmin predicts poor prognosis in adrenocortical carcinoma. J. Trace Elem. Med. Biol. 2019, 56, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Assié, G.; Jouinot, A.; Fassnacht, M.; Libé, R.; Garinet, S.; Jacob, L.; Hamzaoui, N.; Neou, M.; Sakat, J.; De La Villéon, B.; et al. Value of Molecular Classification for Prognostic Assessment of Adrenocortical Carcinoma. JAMA Oncol. 2019, 5. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Xu, Z.; Zhang, Z.; Zheng, Y.; Qi, F. Identification of prognostic genes in adrenocortical carcinoma microenvironment based on bioinformatic methods. Cancer Med. 2020, 9, 1161–1172. [Google Scholar] [CrossRef]
- Yuan, L.; Qian, G.; Chen, L.; Wu, C.L.; Dan, H.C.; Xiao, Y. Co-expression Network Analysis of Biomarkers for Adrenocortical Carcinoma. Front. Genet. 2018, 9, 328. [Google Scholar] [CrossRef]
- Gao, Z.; Man, X.; Li, Z.; Bi, J.; Liu, X.; Li, Z.; Zhu, Y.; Zhang, Z.; Kong, C. Expression profiles analysis identifies the values of carcinogenesis and the prognostic prediction of three genes in adrenocortical carcinoma. Oncol. Rep. 2019, 41, 2440–2452. [Google Scholar] [CrossRef]
- Darabi, S.; Braxton, D.R.; Eisenberg, B.L.; Demeure, M.J. Molecular genomic profiling of adrenocortical cancers in clinical practice. Surgery 2021, 169, 138–144. [Google Scholar] [CrossRef]
- Rahane, C.S.; Kutzner, A.; Heese, K. Establishing a human adrenocortical carcinoma (ACC)-specific gene mutation signature. Cancer Genet. 2019, 230, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lalli, E.; Sasano, H. 5th International ACC Symposium: An Outlook to Current and Future Research on the Biology of Adrenocortical Carcinoma: Diagnostic and Therapeutic Applications. Horm. Cancer 2016, 7, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Decmann, Á.; Perge, P.; Nagy, Z.; Butz, H.; Patócs, A.; Igaz, P. Circulating microRNAs in the diagnostics of endocrine neoplasms. Orv. Hetil. 2017, 158, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Igaz, P. Circulating microRNAs in adrenal tumors. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Chehade, M.; Bullock, M.; Glover, A.; Hutvagner, G.; Sidhu, S. Key MicroRNA’s and Their Targetome in Adrenocortical Cancer. Cancers 2020, 12, 2198. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Soon, P.S.H.; Feige, J.J.; Lalli, E.; Sidhuet, S.B. Dysregulation of microRNAs in adrenocortical tumors. Mol. Cell. Endocrinol. 2012, 351, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Salvianti, F.; Canu, L.; Poli, G.; Armignacco, R.; Scatena, C.; Cantini, G.; Di Franco, A.; Gelmini, S.; Ercolino, T.; Pazzagli, M.; et al. New insights in the clinical and translational relevance of miR483–5p in adrenocortical cancer. Oncotarget 2017, 8, 65525–65533. [Google Scholar] [CrossRef]
- Perge, P.; Butz, H.; Pezzani, R.; Bancos, I.; Nagy, Z.; Pálóczi, K.; Nyírő, G.; Decmann, A.; Pap, E.; Luconi, M.; et al. Evaluation and diagnostic potential of circulating extracellular vesicle-associated microRNAs in adrenocortical tumors. Sci. Rep. 2017, 7, 5474. [Google Scholar] [CrossRef] [PubMed]
- Agosta, C.; Laugier, J.; Guyon, L.; Denis, J.; Bertherat, J.; Libé, R.; Boisson, B.; Sturm, N.; Feige, J.-J.; Chabreet, O.; et al. MiR-483–5p and miR-139–5p promote aggressiveness by targeting N-myc downstream-regulated gene family members in adrenocortical cancer. Int. J. Cancer 2018, 143, 944–957. [Google Scholar] [CrossRef]
- Tömböl, Z.; Szabo, P.M.; Molnar, V.; Wiener, Z.; Tolgyesi, G.; Horanyi, J.; Riesz, P.; Reismann, P.; Patócs, A.; Likó, I.; et al. Integrative molecular bioinformatics study of human adrenocortical tumors:microRNA, tissue-specific target prediction, and pathway analysis. Endocr. Relat. Cancer 2009, 16, 895–906. [Google Scholar] [CrossRef]
- Koperski, Ł.; Kotlarek, M.; Świerniak, M.; Kolanowska, M.; Kubiak, A.; Górnicka, B.; Jażdżewski, K.; Wójcicka, A. Next-generation sequencing reveals microRNA markers of adrenocortical tumors malignancy. Oncotarget 2017, 8, 49191–49200. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.E.; Holloway, A.K.; Weng, J.; Fojo, T.; Kebebew, E. MicroRNAprofiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer 2011, 117, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Chabre, O.; Libé, R.; Assie, G.; Barreau, O.; Bertherat, J.; Bertagna, X.; Feige, J.J.; Cherradi, N. Serum miR-483–5p and miR-195 are predictive of recurrence risk in adrenocortical cancer patients. Endocr. Relat. Cancer 2013, 20, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Oreglia, M.; Sbiera, S.; Fassnacht, M.; Guyon, L.; Denis, J.; Cristante, J.; Chabre, O.; Cherradi, N. Early Postoperative Circulating miR-483–5p is a Prognosis Marker for Adrenocortical Cancer. Cancers 2020, 12, 724. [Google Scholar] [CrossRef]
- Decmann, A.; Bancos, I.; Khanna, A.; Thomas, M.A.; Turai, P.; Perge, P.; Zsolt Pintér, J.; Tóth, M.; Patócs, A.; Igaz, P. Comparison of plasma and urinary microRNA-483–5p for the diagnosis of adrenocortical malignancy. J. Biotechnol. 2019, 297, 49–53. [Google Scholar] [CrossRef]
- Pinzani, P.; Scatena, C.; Salvianti, F.; Corsini, E.; Canu, L.; Poli, G.; Paglierani, M.; Piccini, V.; Pazzagli, M.; Nesi, G.; et al. Detection of circulat-ing tumor cells in patients withadrenocortical carcinoma: Amonocentric preliminary study. J. Clin. Endocrinol. Metab. 2013, 98, 3731–3738. [Google Scholar] [CrossRef]
- Cantini, G.; Canu, L.; Armignacco, R.; Salvianti, F.; De Filpo, G.; Ercolino, T. Prognostic and Monitoring Value of Circulating Tumor Cells in Adrenocortical Carcinoma: A Preliminary Monocentric Study. Cancers 2020, 12, 3176. [Google Scholar] [CrossRef]
- Creemers, S.G.; Korpershoek, E.; Atmodimedjo, P.N.; Dinjens, W.N.M.; van Koetsveld, P.M.; Feelders, R.A.; Hofland, L.J. Identification of Mutations in Cell-Free Circulating Tumor DNA in Adrenocortical Carcinoma: A Case Series. J. Clin. Endocrinol. Metab. 2017, 102, 3611–3615. [Google Scholar] [CrossRef]
- Garinet, S.; Nectoux, J.; Neou, M.; Pasmant, E.; Jouinot, A.; Sibony, M.; Orhant, L.; Pipoli da Fonseca, J.; Perlemoine, K.; Bricaire, L.; et al. Detection and monitoring of circulating tumor DNA in adrenocortical carcinoma. Endocr. Relat. Cancer 2018, 25, L13–L17. [Google Scholar] [CrossRef]
- Schmitt, A.M.; Chang, H.Y. Long Noncoding RNAs in Cancer Pathways Cancer Cell. Cancer Cell 2016, 29, 452–463. [Google Scholar] [CrossRef]
- Yan, J.; Song, J.; Qiao, M.; Zhao, X.; Li, R.; Jiao, J.; Sun, Q. Long noncoding RNA expression profile and functional analysis in psoriasis. Mol. Med. Rep. 2019, 19, 3421–3430. [Google Scholar] [CrossRef]
- Buishand, F.O.; Liu-Chittenden, Y.; Fan, Y.; Tirosh, A.; Gara, S.K.; Patel, D.; Meerzaman, D.; Kebebew, E. Adrenocortical tumors have a distinct, long, non-coding RNA expression profile and LINC00271 is downregulated in malignancy. Surgery 2020, 167, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, A.; Chander, P.; Howe, P.H. Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes: Focus on hnRNP E1′s multifunctional regulatory roles. RNA 2010, 16, 1449–1462. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Shen, S.; Dai, D.; Cheng, S.; Dong, X.; Sun, L.; Guo, X. Pan-cancer analysis of alternative splicing regulator heterogeneous nuclear ribonucleoproteins (hnRNPs) family and their prognostic potential. J. Cell. Mol. Med. 2020, 24, 11111–11119. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Wang, Q.; Nan, F.; Ge, L.; Dang, Y.; Sun, X.; Li, N.; Dong, H.; Han, H.; Zhang, G.; et al. OSacc: Gene Expression-Based Survival Analysis Web Tool For Adrenocortical Carcinoma. Cancer Manag. Res. 2019, 11, 9145–9152. [Google Scholar] [CrossRef]
- Ye, B.; Shi, J.; Kang, H.; Oyebamiji, O.; Hill, D.; Yu, H.; Ness, S.; Ye, F.; Ping, J.; He, J.; et al. Advancing Pan-cancer Gene Expression Survial Analysis by Inclusion of Non-coding RNA. RNA Biol. 2020, 17, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Polistina, F.A.; Farruggio, A.; Gasparin, P.; Pasquale, S.; Frego, M. Spontaneously metachronous ruptures of adrenocortical carcinoma and its contralateral adrenal metastasis. Int. Cancer Conf. J. 2015, 5, 90–97. [Google Scholar] [CrossRef]
- Bronswijk, M.J.H.; Laenen, A.; Bechter, O.E. Clinical presentation, treatment modalities and outcome in patients with adrenocortical carcinoma: A single center experience. Neoplasma 2020, 67, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Megerle, F.; Kroiss, M.; Hahner, S.; Fassnacht, M. Advanced Adrenocortical Carcinoma—What to do when First-Line Therapy Fails? Exp. Clin. Endocrinol. Diabetes 2019, 127, 109–116. [Google Scholar] [CrossRef]
- Sbiera, S.; Leich, E.; Liebisch, G.; Sbiera, I.; Schirbel, A.; Wiemer, L.; Matysik, S.; Eckhardt, C.; Gardill, F.; Gehl, A.; et al. Mitotane inhibits sterol-o-Acyl transferase 1 triggering lipid-mediated endoplasmic reticulum stress and apoptosis in adrenocortical carcinoma cells. Endocrinology 2015, 156, 3895–3908. [Google Scholar] [CrossRef]
- Puglisi, S.; Calabrese, A.; Basile, V.; Ceccato, F.; Scaroni, C.; Altieri, B.; Della Casa, S.; Loli, P.; Pivonello, R.; De Martino, M.C.; et al. Mitotane Concentrations Influence Outcome in Patients with Advanced Adrenocortical Carcinoma. Cancers 2020, 12, 740. [Google Scholar] [CrossRef]
- Puglisi, S.; Calabrese, A.; Basile, V.; Pia, A.; Reimondo, G.; Perotti, P.; Terzolo, M. New perspectives for mitotane treatment of adrenocortical carcinoma. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101415. [Google Scholar] [CrossRef]
- Jung, S.; Nagy, Z.; Fassnacht, M.; Zambetti, G.; Weiss, M.; Reincke, M.; Igaz, P.; Beuschlein, F.; Hantel, C. Preclinical progress and first translational steps for a liposomal chemotherapy protocol against adrenocortical carcinoma. Endocr. Relat. Cancer 2016, 23, 825–837. [Google Scholar] [CrossRef][Green Version]
- Altieri, B.; Ronchi, C.L.; Kroiss, M.; Fassnacht, M. Next-generation therapies for adrenocortical carcinoma. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101434. [Google Scholar] [CrossRef] [PubMed]
- Ardolino, L.; Hansen, A.; Ackland, S.; Joshua, A. Advanced Adrenocortical Carcinoma (ACC): A Review with Focus on Second-Line Therapies. Horm. Cancer 2020, 11, 155–169. [Google Scholar] [CrossRef] [PubMed]
- De Filpo, G.; Mannelli, M.; Canu, L. Adrenocortical carcinoma: Current treatment options. Curr. Opin. Oncol. 2021, 33, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.C.; Chintakuntlawar, A.V.; Hilger, C.; Bancos, I.; Morris, J.C.; Ryder, M.; Smith, C.Y.; Jenkins, S.M.; Bible, K.C. Salvage Therapy With Multikinase Inhibitors and Immunotherapy in Advanced Adrenal Cortical Carcinoma. J. Endocr. Soc. 2020, 4, bvaa069. [Google Scholar] [CrossRef]
- Crona, J.; Baudin, E.; Terzolo, M.; Chrisoulidou, A.; Angelousi, A.G.; Ronchi, C.L.; Oliveira, C.L.; Nieveen van Dijkum, E.J.M.; Ceccato, F.; Borson-Chazot, F.; et al. ENSAT Registry-Based Randomized Clinical Trials for Adrenocortical Carcinoma. Eur. J. Endocrinol. 2021, 184, R51–R59. [Google Scholar] [CrossRef]
- Jasim, S.; Habra, M.A. Management of Adrenocortical Carcinoma. Curr. Oncol. Rep. 2019, 21, 20. [Google Scholar] [CrossRef]
- Elfiky, A.A.; Krishnan Nair, H.K. Assessment and management of advanced adrenocortical carcinoma using a precision oncology care model. Discov. Med. 2016, 21, 49–56. [Google Scholar] [PubMed]
- Sasano, H.; Satoh, F.; Nakamura, Y. Roles of the pathologist in evaluating surrogate markers for medical therapy in adrenocortical carcinoma. Endocr. Pathol. 2014, 25, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Kroiss, M.; Megerle, F.; Kurlbaum, M.; Zimmermann, S.; Wendler, J.; Jimenez, C.; Lapa, C.; Quinkler, M.; Scherf-Clavel, M.; Habra, M.A.; et al. Objective Response and Prolonged Disease Control of Advanced Adrenocortical Carcinoma with Cabozantinib. J. Clin. Endocrinol. Metab. 2020, 105, dgz318. [Google Scholar] [CrossRef] [PubMed]
- Brabo, E.P.; Moraes, A.B.; Neto, L.V. The role of immune checkpoint inhibitor therapy in advanced adrenocortical carcinoma revisited: Review of literature. J. Endocrinol. Investig. 2020, 43, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; Meric-Bernstam, F.; Stephen, B.; Karp, D.D.; Hajjar, J.; Rodon Ahnert, J.; Piha-Paul, A.S.; Colen, R.R.; Jimenez, C.; Raghav, K.P.; et al. Phase 2 study of pembrolizumab in patients with advanced rare cancers. J. Immunother. Cancer 2020, 8, e000347. [Google Scholar] [CrossRef] [PubMed]
- Raj, N.; Zheng, Y.; Kelly, V.; Katz, S.S.; Chou, J.; Do, R.K.G.; Capanu, M.; Zamarin, D.; Saltz, L.B.; Ariyan, C.E.; et al. PD-1 Blockade in Advanced Adrenocortical Carcinoma. J. Clin. Oncol. 2020, 38, 71–80. [Google Scholar] [CrossRef]
- Carneiro, B.A.; Konda, B.; Costa, R.B.; Costa, R.L.B.; Sagar, V.; Gursel, D.B.; Kirschner, L.S.; Chae, Y.K.; Abdulkadir, S.A.; Rademaker, A.; et al. Nivolumab in Metastatic Adrenocortical Carcinoma: Results of a Phase 2 Trial. J. Clin. Endocrinol. Metab. 2019, 104, 6193–6200. [Google Scholar] [CrossRef]
- Poli, G.; Cantini, G.; Armignacco, R.; Fucci, R.; Santi, R.; Canu, L.; Nesi, G.; Mannelli, M.; Luconi, M. Metformin as a new anti-cancer drug in adrenocortical carcinoma. Oncotarget 2016, 7, 49636–49648. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E.; Buryanek, J.; McGuire, M.F. Metformin and Melatonin in Adrenocortical Carcinoma: Morphoproteomics and Biomedical Analytics Provide Proof of Concept in a Case Study. Ann. Clin. Lab. Sci. 2017, 47, 457–465. [Google Scholar]
- Trotta, F.; Avena, P.; Chimento, A.; Rago, V.; De Luca, A.; Sculco, S.; Nocito, M.C.; Malivindi, R.; Fallo, F.; Pezzani, R.; et al. Statins Reduce Intratumor Cholesterol Affecting Adrenocortical Cancer Growth. Mol. Cancer Ther. 2020, 19, 1909–1921. [Google Scholar] [CrossRef]
- Wang, T.; Subramanian, C.; Blagg, B.S.J.; Cohen, M.S. A novel heat shock protein 90 inhibitor potently targets adrenocortical carcinoma tumor suppression. Surgery 2020, 167, 233–240. [Google Scholar] [CrossRef]
- Chortis, V.; Taylor, A.E.; Doig, C.L.; Walsh, M.D.; Meimaridou, E.; Jenkinson, C.; Rodriguez-Blanco, G.; Ronchi, C.L.; Jafri, A.; Metherell, L.A.; et al. Nicotinamide Nucleotide Transhydrogenase as a Novel Treatment Target in Adrenocortical Carcinoma. Endocrinology 2018, 159, 2836–2849. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, M.; Zhao, X.; Zhang, L.; Wu, Y.; Wang, B.; Hu, W. Rottlerin as a novel chemotherapy agent for adrenocortical carcinoma. Oncotarget 2017, 8, 22825–22834. [Google Scholar] [CrossRef]
- Silveira, E.; Cavalcante, I.P.; Kremer, J.L.; de Mendonça, P.O.R.; Pacicco Lotfi, C.F. The tyrosine kinase inhibitor nilotinib is more efficient than mitotane in decreasing cell viability in spheroids prepared from adrenocortical carcinoma cells. Cancer Cell Int. 2018, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Weigand, I.; Lippert, J.; Kircher, S.; Altieri, B.; Steinhauer, S.; Hantel, C.; Rost, S.; Rosenwald, A.; Kroiss, M.; et al. Targeted Gene Expression Profile Reveals CDK4 as Therapeutic Target for Selected Patients With Adrenocortical Carcinoma. Front. Endocrinol. 2020, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Hasanovic, A.; Simsir, M.; Choveau, F.S.; Lalli, E.; Mus-Veteau, I. Astemizole Sensitizes Adrenocortical Carcinoma Cells to Doxorubicin by Inhibiting Patched Drug Efflux Activity. Biomedicines 2020, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, C.; Zhang, H.; Gallagher, R.; Hammer, G.; Timmermann, B.; Cohen, M. Withanolides are potent novel targeted therapeutic agents against adrenocortical carcinomas. World J. Surg. 2014, 38, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- White, P.T.; Subramanian, C.; Motiwala, H.F.; Cohen, M.S. Natural Withanolides in the Treatment of Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 329–373. [Google Scholar] [PubMed]
- Rubin, B.; Pilon, C.; Pezzani, R.; Rebellato, A.; Fallo, F. The effects of mitotane and 1alpha,25-dihydroxyvitamin D (3) on Wnt/beta-catenin signaling in human adrenocortical carcinoma cells. J. Endocrinol. Investig. 2020, 43, 357–367. [Google Scholar] [CrossRef]
- Smith, D.C.; Kroiss, M.; Kebebew, E.; Habra, M.A.; Chugh, R.; Schneider, B.J.; Fassnacht, M.; Jafarinasabian, P.; Ijzerman, M.M.; Lin, V.H.; et al. A phase 1 study of nevanimibe HCl, a novel adrenal-specific sterol O-acyltransferase 1 (SOAT1) inhibitor, in adrenocortical carcinoma. Investig. New Drugs 2020, 38, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Reimondo, G.; Muller, A.; Ingargiola, E.; Puglisi, S.; Terzolo, M. Is Follow-up of Adrenal Incidentalomas Always Mandatory? Endocrinol. Metab. 2020, 35, 26–35. [Google Scholar] [CrossRef]
- Belmihoub, I.; Silvera, S.; Sibony, M.; Dousset, B.; Legmann, P.; Bertagna, X.; Tabarin, A.; Terzolo, M.; Tsagarakis, S.; Dekkers, O.M. From benign adrenal incidentaloma to adrenocortical carcinoma: An exceptional random event. Eur. J. Endocrinol. 2017, 176, K15–K19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Permana, H.; Darmawan, G.; Ritonga, E.; Kusumawati, M.; Miftahurachman, M.; Soetedjo, N.N. An Interesting Case of Hepatic Adrenocortical Carcinoma. Acta Med. Indones 2018, 50, 257–259. [Google Scholar] [PubMed]
- Wieneke, J.A.; Thompson, L.D.R.; Heffess, C.S. Adrenal cortical neoplasms in the pediatric population: A clinicopathologic and immunophenotypic analysis of 83 patients. Am. J. Surg. Pathol. 2003, 27, 867–881. [Google Scholar] [CrossRef] [PubMed]
| Indication | Assays | Specific Question |
|---|---|---|
| All adrenal masses with no overt Cushing (clinically) | 1 mg dexamethasone suppression test | Exclusion of glucocorticoid excess |
| Adrenal masses with clinical signs of Cushing or pathological 1 mg dexamethasone test | 1 mg dexamethasone suppression test | Characterization of glucocorticoid excess |
| Free cortisol in 24-h urine | ||
| Basal ACTH (plasma) | ||
| Any adrenal mass suspected to be an ACC | DHEA-S | Sex steroids precursors excess |
| 17-OH progesterone | ||
| Androstenedione | ||
| Testosterone (only in women) | ||
| 17-beta-oestradiol (only in men and postmenopausal women) | ||
| 11-deoxycortisol (if available) | ||
| Any adrenal masses with hypertension and/or hypokalemia | Potassium | Mineralocorticoid excess |
| Aldosterone/renin ratio |
| Marker | Definition/Role | Clinical Significance/Result | Number of Patients with Adrenocortical Carcinoma | Ref. |
|---|---|---|---|---|
| Metallothionein protein (MT) Minichromosome maintenance protein-2 (MCM2) | MT: scavengers of intracellular reactive oxygen species; overexpressed in various human tumors; MCM2: involved in the initiation of eukaryotic genome replication | -MT: no correlation with stage IV carcinoma -MCM2: positive correlation with Weiss revisited score, mitotic rate on histology, stage IV carcinoma | 14 | [85] |
| Minichromosome maintenance protein complex MCM-3, 5, 7 | Replication-licensing proteins; increased levels of MCM are observed in dysplastic and neoplastic cells | -higher levels in ACC of MCM-3, MCM-7, but not MCM-5; -proliferative and diagnostic markers in discerning benign and malignant adrenocortical tumors. | 3 | [86] |
| Programmed death ligand (PD-L1 and 2) | Regulation of immune response; highly expressed in several cancers | -all tumor specimens were negative for PD-L1 expression; -PD-L2 is expressed commonly in adrenocortical adenomas samples | 14; 34 | [87,88] |
| Sterol-O-acyl transferase 1 (SOAT1) | Involved in cholesterol esterification and lipid droplet formation; SOAT1 inhibition leads to impaired steroidogenesis and cell viability in ACC | -37.5% of the ACCs demonstrated a strong SOAT1 protein expression (score > 2) -Strong SOAT1 protein expression correlated with features of high aggressiveness in ACC -SOAT1 expression was not correlated with recurrence-free survival, progression-free survival and disease-specific survival in ACC patients with mitotane monotherapy | 112; 231 | [89,90] |
| Somatostatin receptors (SSTRs) | Expressed in both normal tissues and solid tumors; part of distinct signaling cascades | -ACC can express SSTRs; SSTRs-based peptide receptor radionuclide therapy may represent a potential treatment opportunity for a minority of patients with advanced ACC | 19 | [91] |
| Chemokine receptor (CXCR 4 and 7) | Chemokine receptors have a negative impact on tumor progression in several human cancers | -High expression of CXCR4 and CXCR7 in both healthy and malignant adrenal tissue; strong membrane expression of CXCR4 and CXCR7 in 50% of ACC; -strong cytoplasmic CXCR4 staining was more frequent in metastases compared to primaries and local recurrences; -CXCR4 staining positively and CXCR7 negatively correlated with Ki67. | 187 | [92] |
| LH/CGR | Luteinizing hormone and/or chorionic gonadotropin (LH/CG) exert direct actions on the adrenal cortex and are involved in the adrenal pathology | -positive in the whole cytoplasm, but weak or absent in cell membranes; the loss of membrane localization of LH/CGR in adrenocortical cancer suggests the alteration of receptors’ function. | 5 | [93] |
| Fascin-1 (FSCN1) and FOXM1 | Epithelial–mesenchymal transition (EMT) related genes | -FSCN1 and FOXM1 over-expression in ACC; -novel independent prognostic markers in ACC; -potential therapeutic target to block tumor spread | 37; 51 | [94,95] |
| Topoisomerase II alpha (TOP2A); thymidylate synthase (TS) | Prognostic parameters in several tumors and also predictors of efficacy of anthracyclines, topoisomerase inhibitors and fluoropirimidines | -TOP2A expression was associated with better after EDP-M (etoposide, doxorubicin and cisplatin plus mitotane) -TOP2A and TS were neither prognostic nor predictive of mitotane efficacy in ACC patients | 39 | [96] |
| Insulin like growth factor 2 (IGF2) IGF1 receptor (IGF1R) | Main pathway in ACC tumorigenesis | -in addition to IGF2 and IGF1R, ACC express IGF2R, IRA and several IGFBPs, suggesting that the interplay between the different components of the IGF pathway in ACC could be more complex than previously considered -IGF1 overexpression was associated with SLC12A7 overexpression and non-functional, early-stage and larger tumors | 17; 33 | [97,98] |
| CD276-(B7-H3) | Inhibitory role in adaptive immunity; in malignant tissues, B7-H3 is an immune checkpoint molecule | -positive expression on the cell membrane and in the cytoplasm of cancer cells or tumor-associated vascular cells -vascular expression of CD276 associated with local aggression | 48 | [99] |
| c-myc | Proto-oncogene | -strong cytoplasmic c-myc expression and weak nuclear expression in ACC associated with malignancy and shorter survival | 31 | [100] |
| Phosphorylated mTOR | Part of signaling pathway | -p-mTOR expression in 32% cases, with a moderate or strong cytoplasmic reactivity -p-mTOR was also negative in tumors with high Weiss Score, | 58 | [101] |
| Pituitary-tumor transforming gene (PTTG1) | Modulate cancer invasiveness and response to therapy | -increased nuclear protein expression of PTTG1 in ACC -PTTG1 correlated with Ki-67 | 20; 14 | [102] |
| Glypicin-3 (GPC-3) | Role in the control of cell division and growth regulation; role in distinguishing hepatic lesions | -GPC-3 positivity rare in ACC, but possible, especially of extra-adrenal ACC | 1; 2 | [103,104] |
| E-/P-/N-cadherins, MMP-2/-9 and caveolin-1 ZEB-1/-2, Slug Oct3/4, LIN28, SOX2, SO17, NANOG, CD133, nestin | Epithelial–mesenchymal transition (EMT)-associated markers (E-/P-/N-cadherins, MMP-2/-9 and caveolin-1) Downstream transcriptional regulators of EMT-related signaling pathways (ZEB-1/-2, Slug) Stem cell factors (Oct3/4, LIN28, SOX2, SO17, NANOG, CD133, nestin) Markers of adrenocortical origin/tumorigenesis (SF-1, β-catenin, p53) | ACC with sarcomatous areas: -SF-1 and E-/P-/N-cadherins positive only in the epithelial component of all cases, whereas the nonepithelial components were mainly enriched for nestin, ZEB-1, and MMP-2/-9 -β-Catenin demonstrated an aberrant nuclear localization in the sarcomatoid component whereas p53 was strongly positive in the nonepithelial constituent | 6 | [105] |
| Livin/BIRC7 | Member of the inhibitors of apoptosis proteins family, which are involved in tumor development through the inhibition of caspases | -over-expressed in ACC, localized in both cytoplasm and nuclei.-the ratio between cytoplasmic and nuclear staining was significantly higher in ACC than in ACA | 192 | [106] |
| Retinoic acid receptor responder 2 (RARRES2) | An immune-dependent tumor suppressor | -compared to normal adrenocortical tissues, expression was significantly lower in benign tumors, and even lower in ACC samples. | 19 | [107] |
| Adiponectin receptors | Adiponectin: involved in regulating glucose levels as well as fatty acid breakdown | -the expression of Adipo R1 and R2 receptors was associated with ACC diagnosis | 20 | [108] |
| Stathmin1 (STMN1) | Cytosolic protein involved in microtubule dynamics; implicated in carcinogenesis and aggressive behavior in multiple malignancies | -significantly higher expression of STMN1 protein in ACC compared with normal and benign tissues | 13 | [109] |
| MCT1, MCT2, MCT4, CD147, CD44, GLUT1 CAIX | MCT1 and MCT4 mediate monocarboxylate efflux from cells, while MCT2 is involved in monocarboxylate uptake; these transporters require co-expression with chaperones for proper plasma membrane localization and activity; the main chaperone is CD147; CD44 has also been recently described as a MCT chaperone | -increased membranous expression of MCT4, GLUT1 and CAIX in ACC -MCT1, GLUT1 and CAIX expressions associated with poor prognostic variables -MCT2 membranous expression was associated with favorable prognostic parameters. -cytoplasmic expression of CD147 was identified as an independent predictor of longer overall survival -cytoplasmic expression of CAIX as an independent predictor of longer disease-free survival. | 78 | [110] |
| VAV2 | VAV2 -guanine nucleotide exchange factor; oncogene | -VAV2 expression correlated with Ki-67 index and progression free survival and overall survival | 171 | [111] |
| Cytochrome P450 genes | P450 overexpression potentiates adrenocortical carcinoma chemoresistance. | -analysis confirmed protein overexpression | 29 | [112] |
| Steroidogenic acute regulatory protein (StAR), CYP11B1, CYP11B2, YP17A1 | Key proteins involved in the steroidogenesis cascade | -CYP11B1, StAR and CYP17A1 expression was lower in ACC; ACC presented co-staining cells for CYP11B1 and CYP11B2; CYP11B1 had the most discriminative power to distinguish ACC from ACA with a sensitivity of 100%, specificity of 92% | 14 | [49] |
| Progesterone receptor | Protein activated by the steroid hormone progesterone | -in 50% samples, IHC (immunohistochemistry) revealed a weak expression of progesterone receptor | 8 | [113] |
| D2-40; CD-31 | D2-40 antibody for lymph vessels CD-31 antibody for blood vessels | -D2-40 expression lower in ACC and correlated positively with the expression of StAR; -CD31 expression higher in ACC | 15 | [114] |
| Inhibin, D2-40, synaptophysin | Inhibin downregulates FSH (follicle-stimulating hormone) synthesis and secretion; D2-40 may be a useful marker for distinguishing primary adrenal cortical neoplasms from both metastatic renal cell carcinoma and pheochromocytoma; Synaptophysin: major synaptic vesicle protein | -patients with any negative staining had shorter cancer-specific survival than ones with positive staining -negative staining for inhibin, D2-40 and synaptophysin and Ki-67 expression ≥7% were associated with poorer prognosis | 30 | [115] |
| Excision repair cross complementing group 1 (ERCC1) | Role in the repair of platinum-induced DNA damage | -high ERCC1 expression was observed in 66% of ACC samples | 146 | [116] |
| Sphingosine kinase 1 (SphK1) | Oncogene | -over-expression of SphK1 protein in the carcinomas compared with adenomas | 24 | [117] |
| N-cadherin | Aberrant expression of N-cadherin plays important role in ACC tumorigenesis | -N-cadherin downregulation was observed in 100 % of ACC | 24; 15 | [25,118] |
| Telomerase reverse transcriptase (TERT) | Catalytic subunit of the telomerase complex | -telomerase nuclear expression was present in 26.6% of ACC and in 45.5% of non-functioning adenomas | 15 | [118] |
| Isocitrate dehydrogenase (IDH) R132H mutation | Metabolic enzyme, ubiquitous in all cells; mutations of IDH play a prognostic or predictive role in several neoplasms | -positive IDH1 R132H staining correlated with a better prognosis among patients with ACC; it did not distinguish between local and metastasized tumors. | 33 | [119] |
| Indoleamine 2,3-dioxygenase 1 (IDO-1) | An immune checkpoint molecule | -IDO-1 is expressed in a majority of ACC samples; its expression in tumor tissue is associated with PD-L2 expression, and expression in stroma is associated with CD8+ cell infiltration. | 32 | [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizdrak, M.; Tičinović Kurir, T.; Božić, J. The Role of Biomarkers in Adrenocortical Carcinoma: A Review of Current Evidence and Future Perspectives. Biomedicines 2021, 9, 174. https://doi.org/10.3390/biomedicines9020174
Mizdrak M, Tičinović Kurir T, Božić J. The Role of Biomarkers in Adrenocortical Carcinoma: A Review of Current Evidence and Future Perspectives. Biomedicines. 2021; 9(2):174. https://doi.org/10.3390/biomedicines9020174
Chicago/Turabian StyleMizdrak, Maja, Tina Tičinović Kurir, and Joško Božić. 2021. "The Role of Biomarkers in Adrenocortical Carcinoma: A Review of Current Evidence and Future Perspectives" Biomedicines 9, no. 2: 174. https://doi.org/10.3390/biomedicines9020174
APA StyleMizdrak, M., Tičinović Kurir, T., & Božić, J. (2021). The Role of Biomarkers in Adrenocortical Carcinoma: A Review of Current Evidence and Future Perspectives. Biomedicines, 9(2), 174. https://doi.org/10.3390/biomedicines9020174







