The Role of Neuropeptide Y in Adipocyte-Macrophage Crosstalk during High Fat Diet-Induced Adipose Inflammation and Liver Steatosis
Abstract
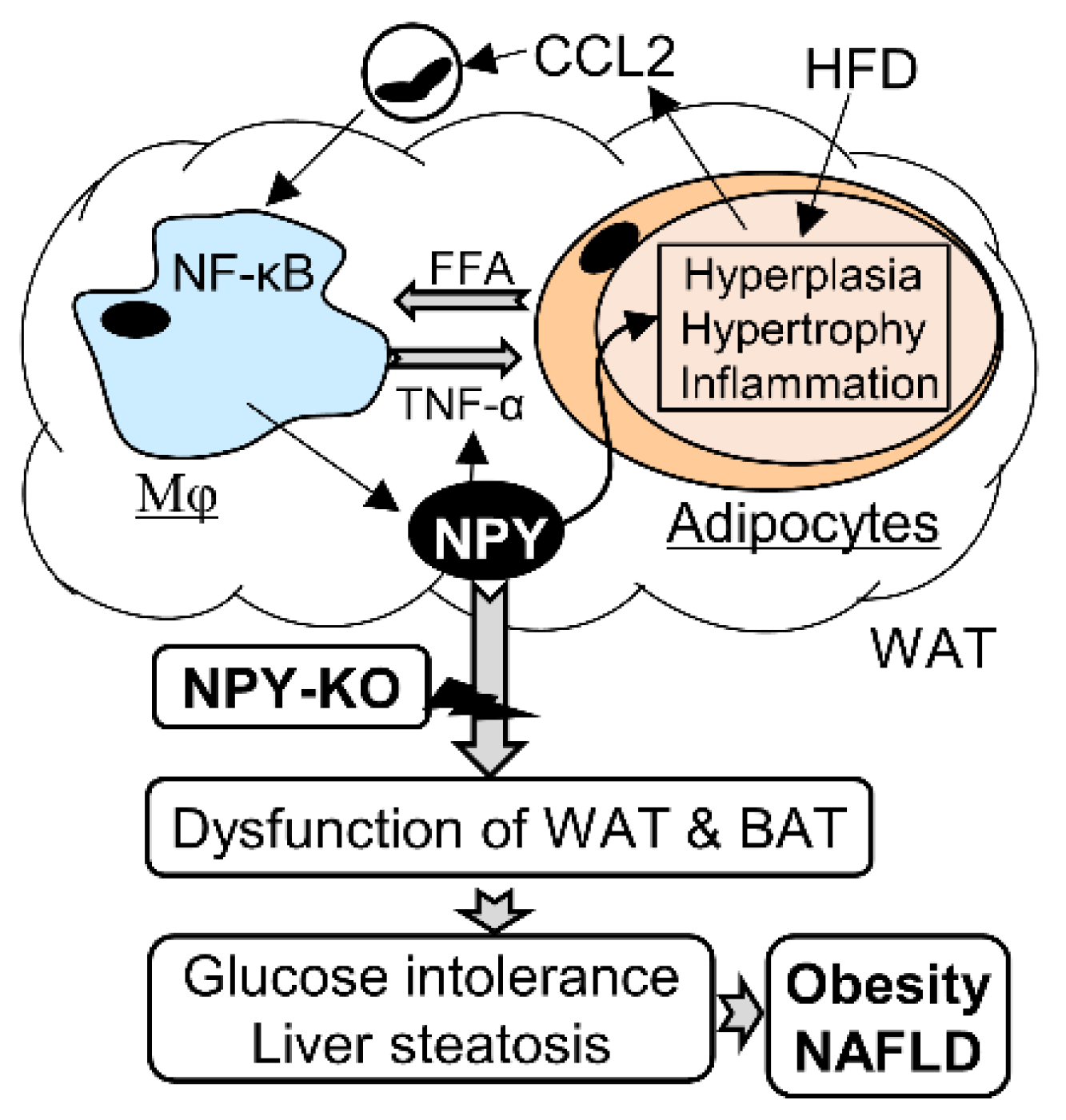
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Metabolic Studies
2.3. Insulin and FFA Quantification
2.4. Liver Triglyceride (TG) Content
2.5. Quantitative Real-Time PCR
2.6. Semi-Qunatitative RT-PCR
2.7. Histological Analyzes
2.8. Cell Culture
2.9. Primary Peritoneal Macrophage Preparation
2.10. Co-Culture of 3T3-L1 Adipocytes and Macrophages
2.11. Statistical Analysis
3. Results
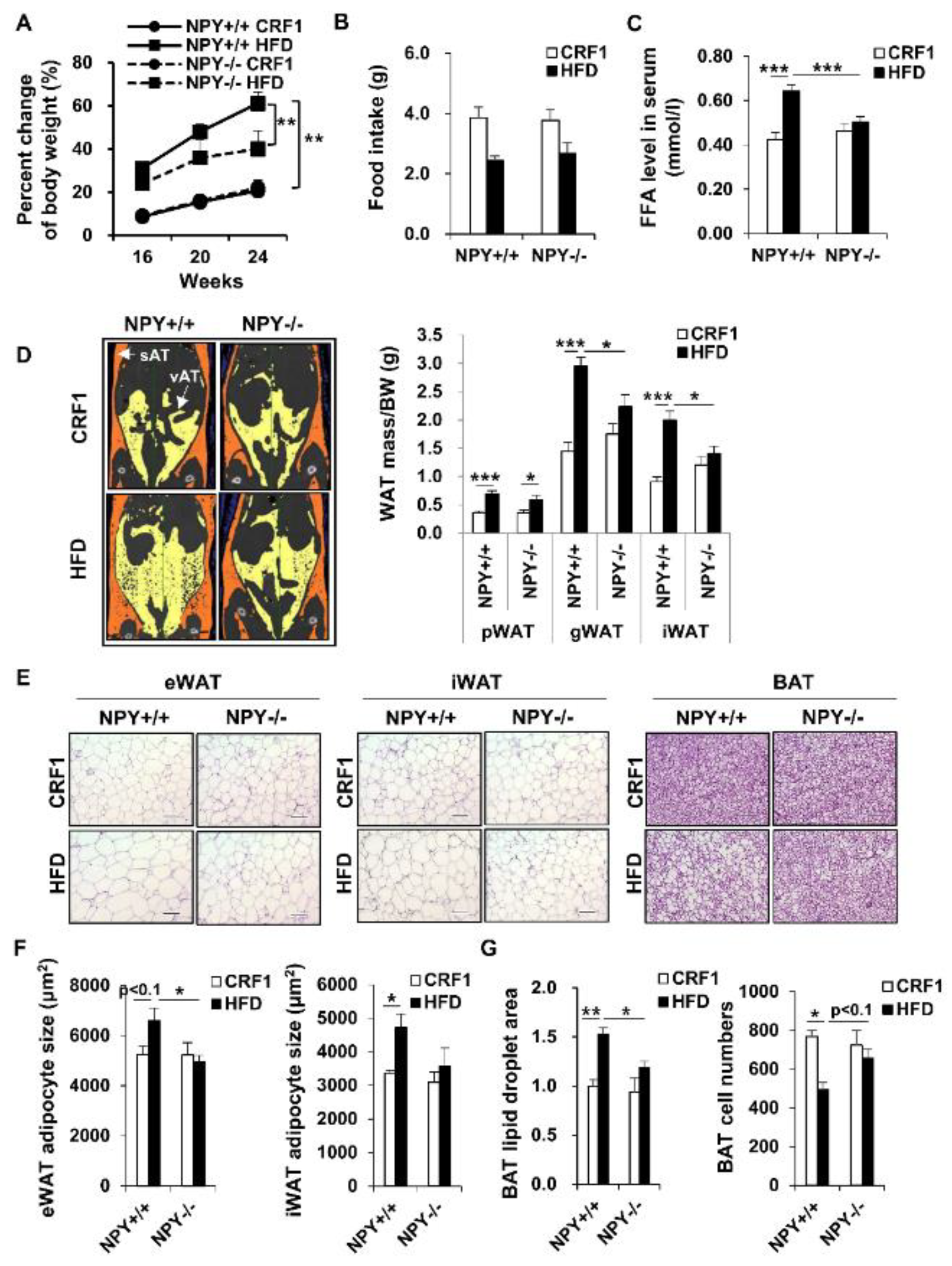
3.1. Deletion of NPY in Mouse Protects against High-Fat Diet (HFD)-Induced Obesity
3.2. Loss of NPY Results in an Alteration of the Energy Source
3.3. Deletion of NPY Partly Activates Thermogenesis in BAT
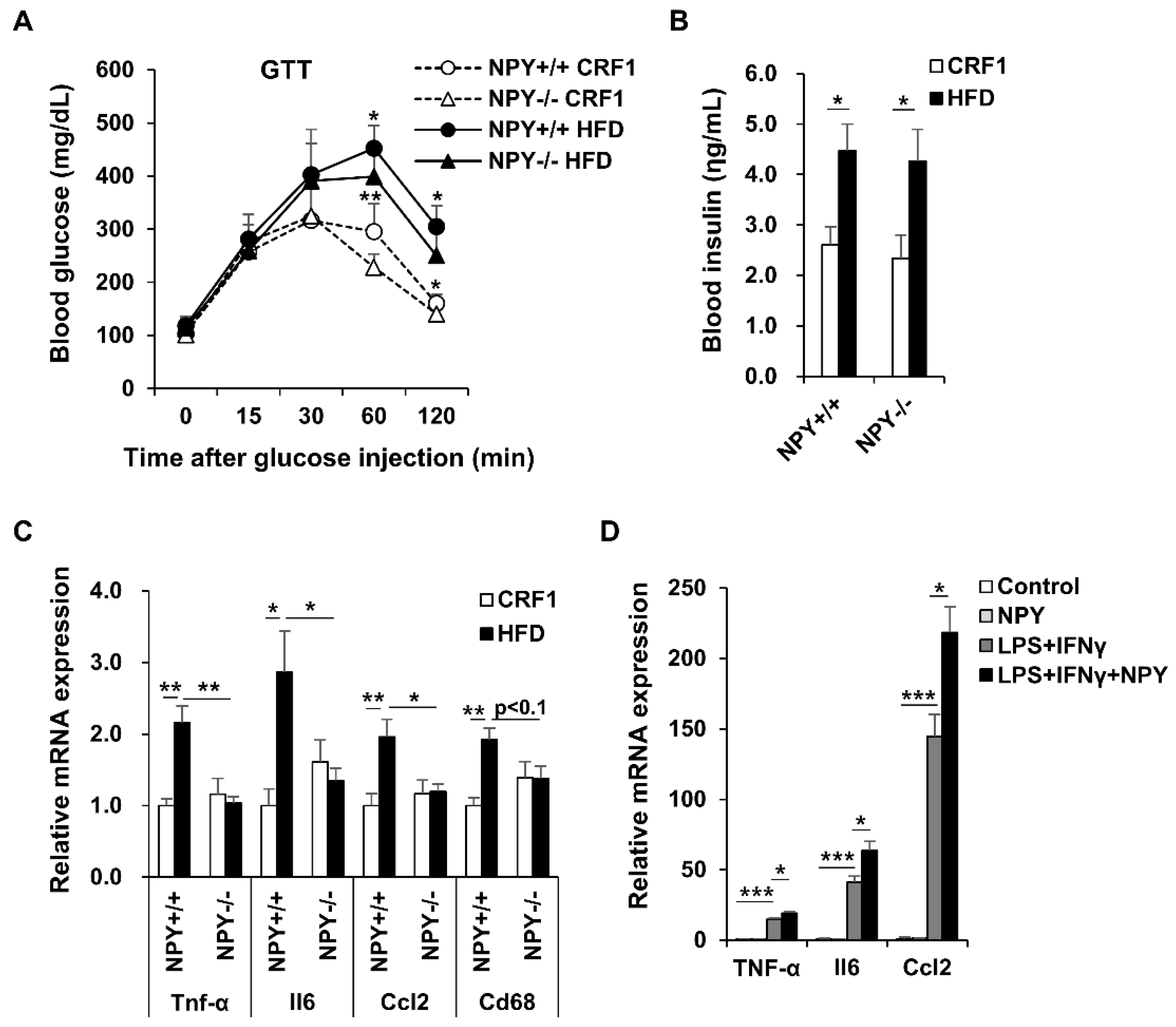
3.4. NPY Ablation Improves Glucose Tolerance and Attenuates Adipose Tissue Inflammation
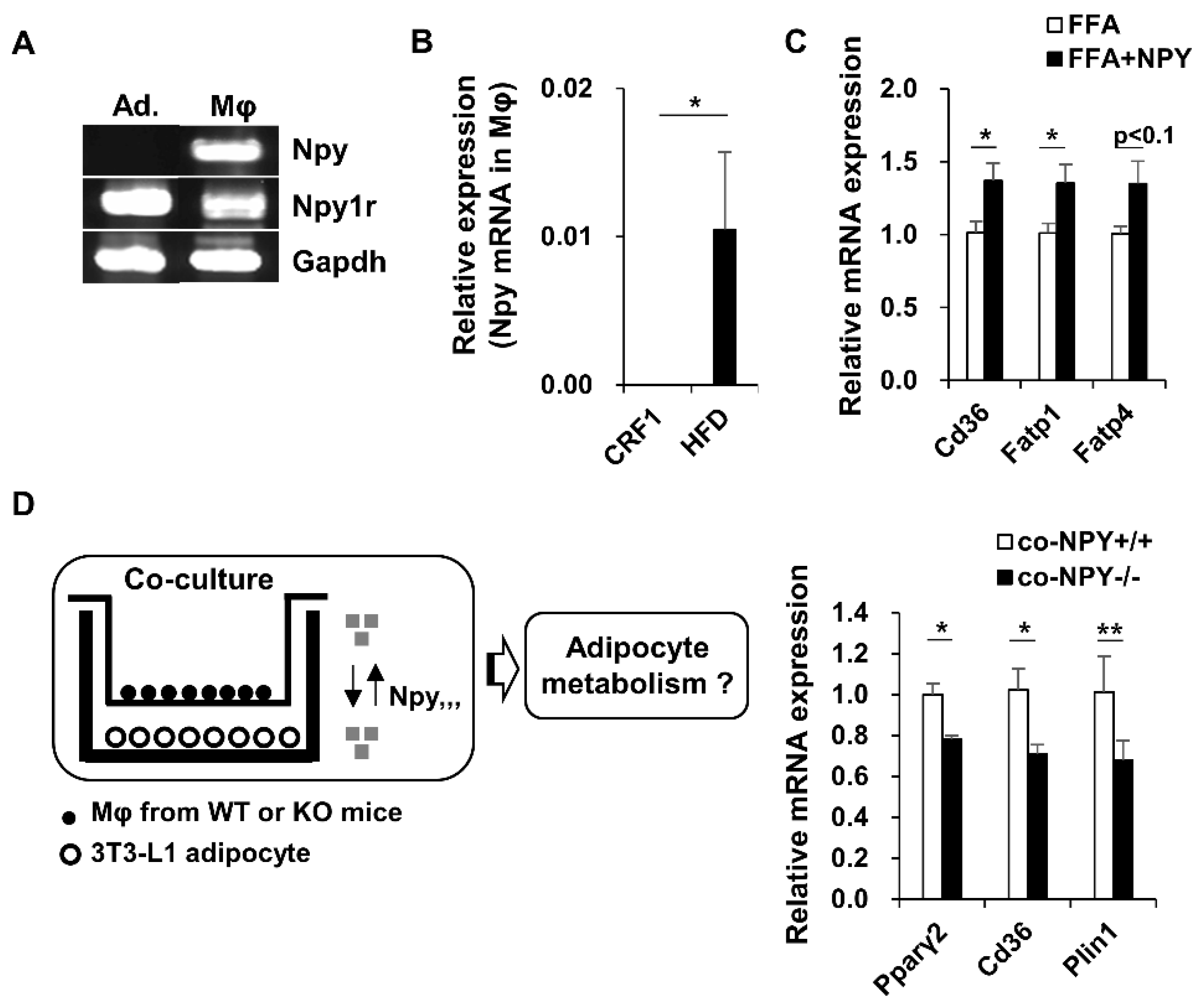
3.5. NPY Regulates Adipocyte-Macrophage Crosstalk
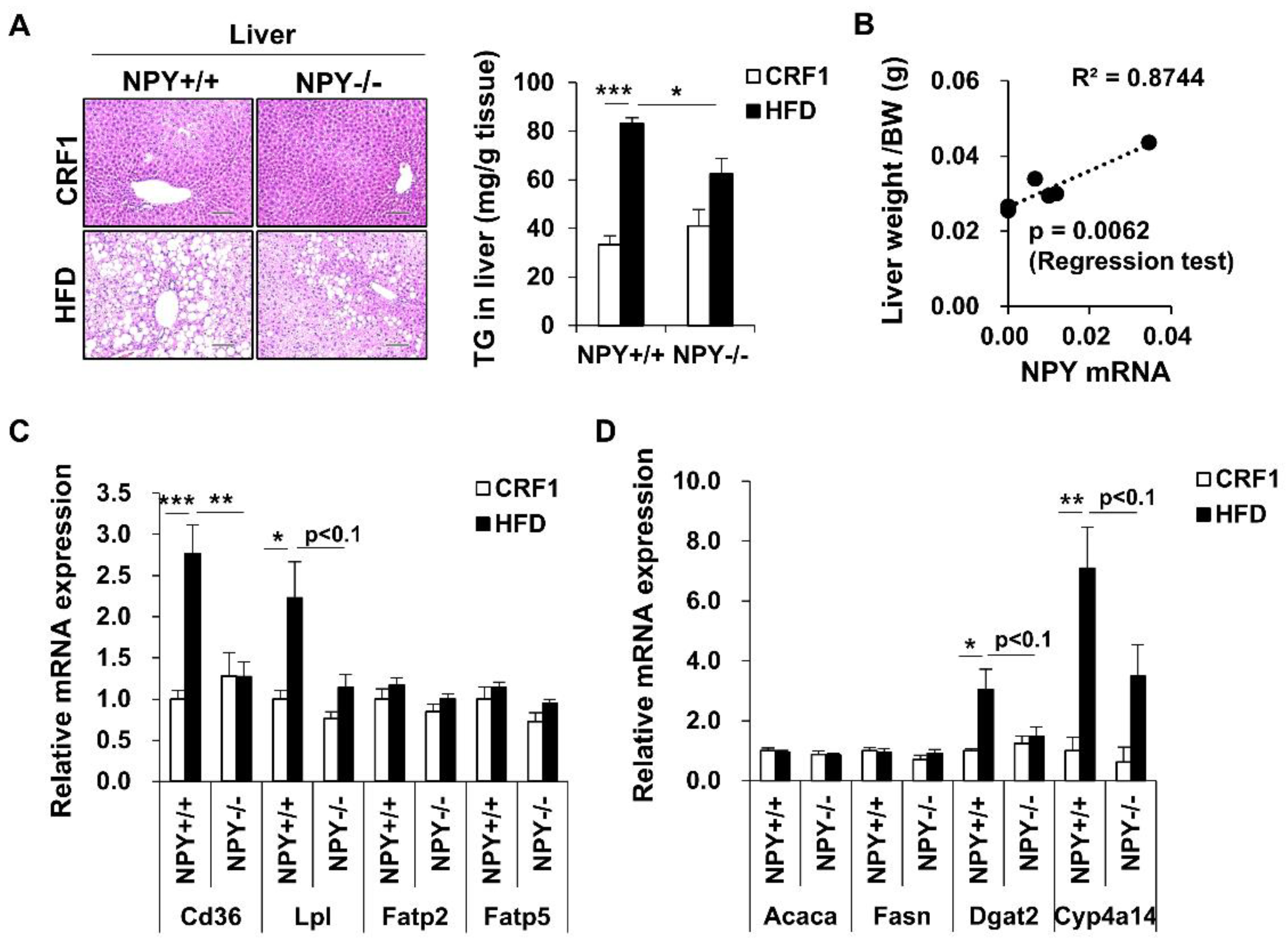
3.6. Inhibition of NPY Ameliorates High Fat Diet-Induced Steatosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lafontan, M.; Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid Res. 2009, 48, 275–297. [Google Scholar] [CrossRef]
- Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900,000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef] [Green Version]
- Global BMI Mortality Collaboration; Di Angelantonio, E.; Bhupathiraju, S.; Wormser, D.; Gao, P.; Kaptoge, S.; Berrington de Gonzalez, A.; Cairns, B.; Huxley, R.; Jackson, C.; et al. Body-mass index and all-causemortality: Individual-participant-datametaanalysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef] [Green Version]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Reilly, J.J.; El-Hamdouchi, A.; Diouf, A.; Monyeki, A.; Somda, S.A. Determining the worldwide prevalence of obesity. Lancet 2018, 391, 1773–1774. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- Musso, G.; Gambino, R.; Cassader, M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog. Lipid Res. 2009, 48, 1–26. [Google Scholar] [CrossRef]
- Castoldi, A.; Naffah de Souza, C.; Olsen Saraiva Câmara, N.; Moraes-Vieira, P.M. The Macrophage Switch in Obesity Development. Front. Immunol. 2016, 6, 637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercer, R.E.; Chee, M.J.; Colmers, W.F. The role of NPY in hypothalamic mediated food intake. Front. Neuroendocrinol. 2011, 32, 398–415. [Google Scholar] [CrossRef]
- Ruohonen, S.T.; Vähätalo, L.H.; Savontaus, E. Diet-induced obesity in mice overexpressing neuropeptide y in noradrenergic neurons. Int. J. Pept. 2012, 2012, 452524. [Google Scholar] [CrossRef]
- Zarjevski, N.; Cusin, I.; Vettor, R.; Rohner-Jeanrenaud, F.; Jeanrenaud, B. Chronic intracerebroventricular neuropeptide-Y administration to normal rats mimics hormonal and metabolic changes of obesity. Endocrinology 1993, 133, 1753–1758. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.T.; Yang, L.; Aja, S.; Moran, T.H.; Bi, S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab. 2011, 13, 573–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishihara, A.; Kanatani, A.; Mashiko, S.; Tanaka, T.; Hidaka, M.; Gomori, A.; Iwaasa, H.; Murai, N.; Egashira, S.; Murai, T.; et al. A neuropeptide Y Y5 antagonist selectively ameliorates body weight gain and associated parameters in diet-induced obese mice. Proc. Natl. Acad. Sci. USA 2006, 103, 7154–7158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Fujishita, C.; Komatsu, T.; Kim, S.E.; Chiba, T.; Mori, R.; Shimokawa, I. NPY antagonism reduces adiposity and attenuates age-related imbalance of adipose tissue metabolism. FASEB J. 2014, 28, 5337–5348. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Komatsu, T.; Kim, S.E.; Tanaka, K.; Hayashi, H.; Mori, R.; Shimokawa, I. Neuropeptide Y resists excess loss of fat by lipolysis in calorie-restricted mice: A trait potential for the life-extending effect of calorie restriction. Aging Cell 2017, 16, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked IR. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Chandrasekharan, B.; Jeppsson, S.; Pienkowski, S.; Belsham, D.D.; Sitaraman, S.V.; Merlin, D.; Kokkotou, E.; Nusrat, A.; Tansey, M.G.; Srinivasan, S. Tumor necrosis factor-neuropeptide Y cross talk regulates inflammation, epithelial barrier functions, and colonic motility. Inflamm. Bowel Dis. 2013, 19, 2535–2546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheway, J.; Mackay, C.R.; Newton, R.A.; Sainsbury, A.; Boey, D.; Herzog, H.; Mackay, F. A fundamental bimodal role for neuropeptide Y1 receptor in the immune system. J. Exp. Med. 2005, 202, 1527–1538. [Google Scholar] [CrossRef]
- He, J.; Lee, J.H.; Febbraio, M.; Xie, W. The emerging roles of fatty acid translocase/CD36 and the aryl hydrocarbon receptor in fatty liver disease. Exp. Biol. Med. 2011, 236, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.J.; Kuchibhotla, S.; Westfall, K.M.; Silverstein, R.L.; Morton, R.E.; Febbraio, M. A CD36-dependent pathway enhances macrophage and adipose tissue inflammation and impairs insulin signalling. Cardiovasc. Res. 2011, 89, 604–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rada, P.; González-Rodríguez, Á.; García-Monzón, C.; Valverde, Á.M. Understanding lipotoxicity in NAFLD pathogenesis: Is CD36 a key driver? Cell Death Dis. 2020, 11, 802. [Google Scholar] [CrossRef]
- Herman, M.A.; Peroni, O.D.; Villoria, J.; Schön, M.R.; Abumrad, N.A.; Blüher, M.; Klein, S.; Kahn, B.B. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 2012, 484, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Guerra, C.; Koza, R.A.; Walsh, K.; Kurtz, F.M.; Wood, P.A.; Kozak, L.P. Abnormal nonshivering thermogenesis in mice with inherited defects of fatty acid oxidation. J. Clin. Investig. 1998, 102, 1724–1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.H.; Lundh, M.; Fu, A.; Kriszt, R.; Huang, T.L.; Lynes, M.D.; Leiria, L.O.; Shamsi, F.; Darcy, J.; Greenwood, B.P. CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Sci. Transl. Med. 2020, 12, eaaz8664. [Google Scholar] [CrossRef]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E444–E452. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Nayantai, E.; Komatsu, T.; Hayashi, H.; Mori, R.; Shimokawa, I. NPY Deficiency Prevents Postmenopausal Adiposity by Augmenting Estradiol-Mediated Browning. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1042–1049. [Google Scholar] [CrossRef]
- Ikeda, K.; Yamada, T. UCP1 Dependent and Independent Thermogenesis in Brown and Beige Adipocytes. Front. Endocrinol. 2020, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Bertholet, A.M.; Kazak, L.; Chouchani, E.T.; Bogaczyńska, M.G.; Paranjpe, I.; Wainwright, G.L.; Bétourné, A.; Kajimura, S.; Spiegelman, B.M.; Kirichok, Y. Mitochondrial Patch Clamp of Beige Adipocytes Reveals UCP1-Positive and UCP1-Negative Cells Both Exhibiting Futile Creatine Cycling. Cell Metab. 2017, 25, 811–822. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Cline, M.A.; Gilbert, E.R. Hypothalamus-adipose tissue crosstalk: Neuropeptide Y and the regulation of energy metabolism. Nutr. Metab. 2014, 11, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Z.; Madden, C.J.; Brooks, V.L. Arcuate neuropeptide Y inhibits sympathetic nerve activity via multiple neuropathways. J. Clin. Investig. 2017, 127, 2868–2880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, K.; Morris, D.L.; Oatmen, K.E.; Wang, T.; DelProposto, J.; Mergian, T.; Cho, K.W.; Lumeng, C.N. Neuropeptide Y is produced by adipose tissue macrophages and regulates obesity-induced inflammation. PLoS ONE 2013, 8, e57929. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Komatsu, T.; Hayashi, H.; Mori, R.; Shimokawa, I. The Role of Neuropeptide Y in Adipocyte-Macrophage Crosstalk during High Fat Diet-Induced Adipose Inflammation and Liver Steatosis. Biomedicines 2021, 9, 1739. https://doi.org/10.3390/biomedicines9111739
Park S, Komatsu T, Hayashi H, Mori R, Shimokawa I. The Role of Neuropeptide Y in Adipocyte-Macrophage Crosstalk during High Fat Diet-Induced Adipose Inflammation and Liver Steatosis. Biomedicines. 2021; 9(11):1739. https://doi.org/10.3390/biomedicines9111739
Chicago/Turabian StylePark, Seongjoon, Toshimitsu Komatsu, Hiroko Hayashi, Ryoichi Mori, and Isao Shimokawa. 2021. "The Role of Neuropeptide Y in Adipocyte-Macrophage Crosstalk during High Fat Diet-Induced Adipose Inflammation and Liver Steatosis" Biomedicines 9, no. 11: 1739. https://doi.org/10.3390/biomedicines9111739
APA StylePark, S., Komatsu, T., Hayashi, H., Mori, R., & Shimokawa, I. (2021). The Role of Neuropeptide Y in Adipocyte-Macrophage Crosstalk during High Fat Diet-Induced Adipose Inflammation and Liver Steatosis. Biomedicines, 9(11), 1739. https://doi.org/10.3390/biomedicines9111739




