Bisphosphonates for the Treatment of Calcinosis Cutis—A Retrospective Single-Center Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patient’s Characteristics
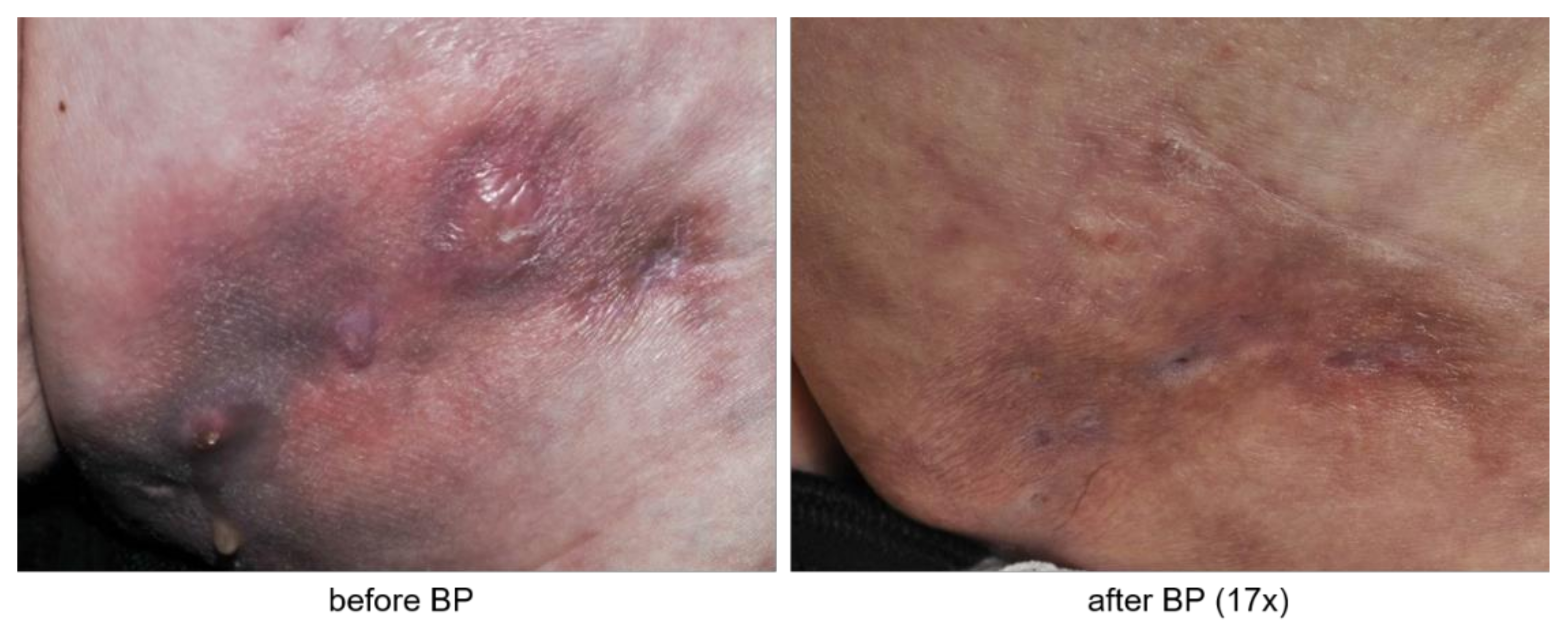
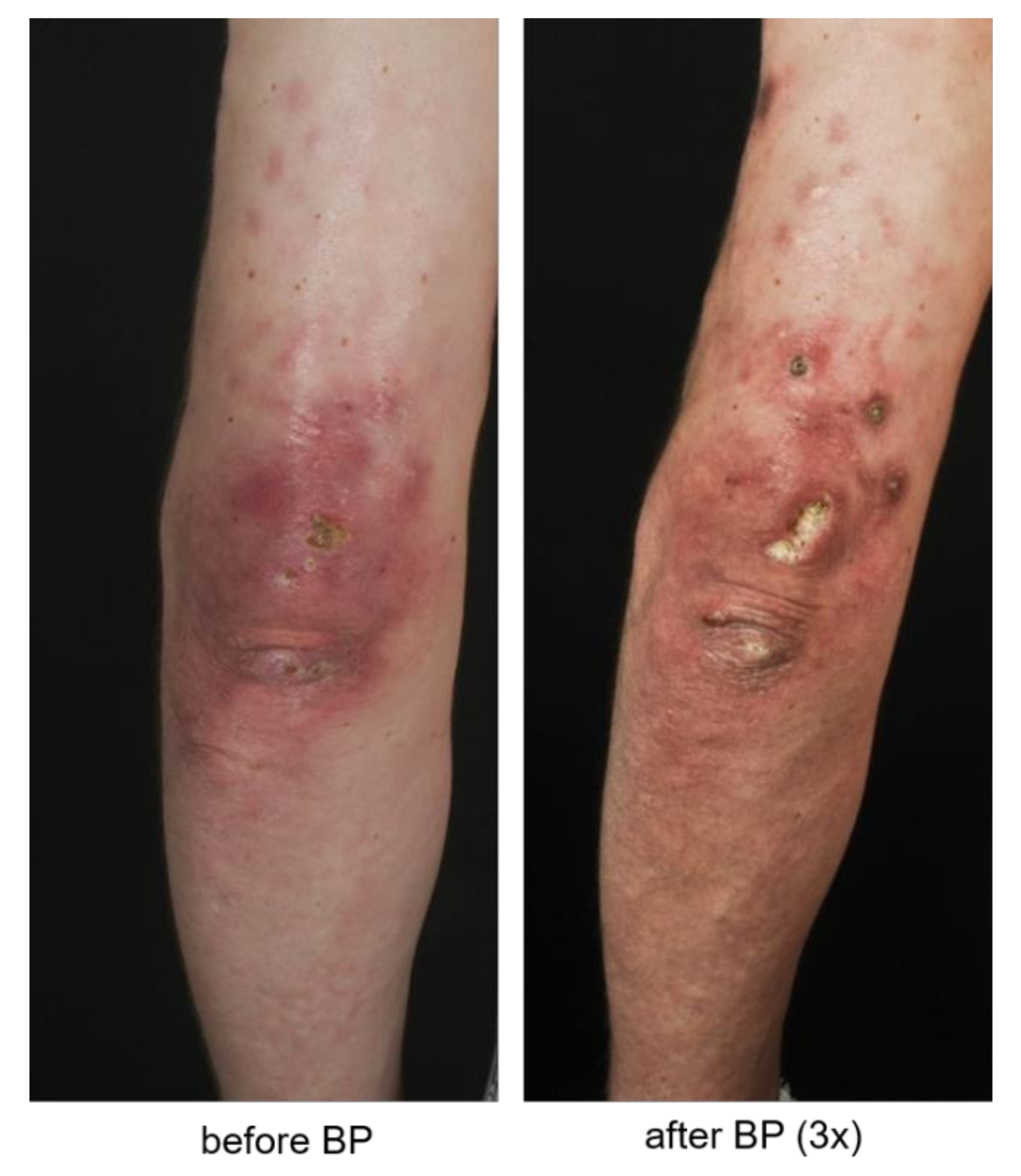
3.2. Patients Evaluate Bisphosphonate Treatment Positively
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reiter, N.; El-Shabrawi, L.; Leinweber, B.; Berghold, A.; Aberer, E. Calcinosis cutis: Part I. Diagnostic pathway. J. Am. Acad. Dermatol. 2011, 65, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A., Jr.; Wetter, D.A. Calcinosis cutis in autoimmune connective tissue diseases. Dermatol. Ther. 2012, 25, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, A.; Baron, M.; Canadian Scleroderma Research Group; Herrick, A.L.; Proudman, S.; Stevens, W.; Australian Scleroderma Interest Group; Rodriguez-Reyna, T.S.; Vacca, A.; Medsger, T.A., Jr.; et al. Calcinosis is associated with digital ulcers and osteoporosis in patients with systemic sclerosis: A Scleroderma Clinical Trials Consortium study. Semin. Arthritis Rheum. 2016, 46, 344–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenzuela, A.; Song, P.; Chung, L. Calcinosis in scleroderma. Curr. Opin. Rheumatol. 2018, 30, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, N.; Hamano, T.; Isaka, Y.; Ito, T.; Imai, E. Risedronate: A possible treatment for extraosseous calcification. Clin. Calcium 2005, 15 (Suppl. 1), 75–78. [Google Scholar] [PubMed]
- Rabens, S.F.; Bethune, J.E. Disodium etidronate therapy for dystrophic cutaneous calcification. Arch. Dermatol. 1975, 111, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Metzger, A.L.; Singer, F.R.; Bluestone, R.; Pearson, C.M. Failure of disodium etidronate in calcinosis due to dermatomyositis and scleroderma. N. Engl. J. Med. 1974, 291, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Reiter, N.; El-Shabrawi, L.; Leinweber, B.; Berghold, A.; Aberer, E. Calcinosis cutis: Part II. Treatment options. J. Am. Acad. Dermatol. 2011, 65, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Mlinaric, A.; Horvat, M.; Supak Smolcic, V. Dealing with the positive publication bias: Why you should really publish your negative results. Biochem. Med. 2017, 27, 030201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Gallo, D.; Ossorio-Garcia, L.; Linares-Barrios, M. Calcinosis Cutis and Calciphylaxis. Actas Dermosifiliogr. 2015, 106, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.L.; Holen, I. Tumour macrophages as potential targets of bisphosphonates. J. Transl. Med. 2011, 9, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roelofs, A.J.; Thompson, K.; Ebetino, F.H.; Rogers, M.J.; Coxon, F.P. Bisphosphonates: Molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr. Pharm. Des. 2010, 16, 2950–2960. [Google Scholar] [CrossRef] [PubMed]


| Participant ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| sex | male | female | female | female | female | female | female |
| diagnosis | dermato-myositis | dermato-myositis | mixed connective tissue disease | mixed connective tissue disease | systemic sclerosis | mixed connective tissue disease | systemic sclerosis |
| age at disease onset (years) | 26 | 46 | 48 | 10 | 58 | 47 | 38 |
| age at onset of calcinosis cutis (years) | 26 | unknown | 50 | 14 | 59 | 49 | unknown |
| time between beginning of calcinosis cutis and initiation of bisphospho-nate treatment (years) | 8 | unknown | 8 | 13 | 4 | 2 | unknown |
| number of bisphospho-nate cycles | 7 | 12 | 3 | 6 | 12 | 18 | 2 |
| dosage (mg) | 70 | 75 | 75 | 75 | 75 | 75 | 75 |
| previous treatments | intravenous immuno-globulins cyclophosphamid methyl-prednisolone azathioprine | methotrexate prednisolone azathioprine | intravenous immuno-globuline methotrexate prednisolone azathioprine chloroquine PUVA, iloprost | methotrexate prednisolone cyclosporine chloroquine | bosentan pentoxi-fylline | hydroxy-chloroquine azathioprin mycophenolate mofetil intravenous immuno-globulins prednisolone iloprost | prednisolone |
| treatment during bisphospho-nate therapy | methyl-prednisolone | methotrexate prednisolone | intravenous immuno-globulines methotrexate prednisolone | methotrexate prednisolone | pentoxi-fylline | - | prednisolone |
| adverse events | - | fever low blood pressure | - | fever shivering limb pain | fever osteonecrosis of the jaw | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauch, L.; Hein, R.; Biedermann, T.; Eyerich, K.; Lauffer, F. Bisphosphonates for the Treatment of Calcinosis Cutis—A Retrospective Single-Center Study. Biomedicines 2021, 9, 1698. https://doi.org/10.3390/biomedicines9111698
Rauch L, Hein R, Biedermann T, Eyerich K, Lauffer F. Bisphosphonates for the Treatment of Calcinosis Cutis—A Retrospective Single-Center Study. Biomedicines. 2021; 9(11):1698. https://doi.org/10.3390/biomedicines9111698
Chicago/Turabian StyleRauch, Lilian, Rüdiger Hein, Tilo Biedermann, Kilian Eyerich, and Felix Lauffer. 2021. "Bisphosphonates for the Treatment of Calcinosis Cutis—A Retrospective Single-Center Study" Biomedicines 9, no. 11: 1698. https://doi.org/10.3390/biomedicines9111698
APA StyleRauch, L., Hein, R., Biedermann, T., Eyerich, K., & Lauffer, F. (2021). Bisphosphonates for the Treatment of Calcinosis Cutis—A Retrospective Single-Center Study. Biomedicines, 9(11), 1698. https://doi.org/10.3390/biomedicines9111698






