Versatile Role of Prokineticins and Prokineticin Receptors in Neuroinflammation
Abstract
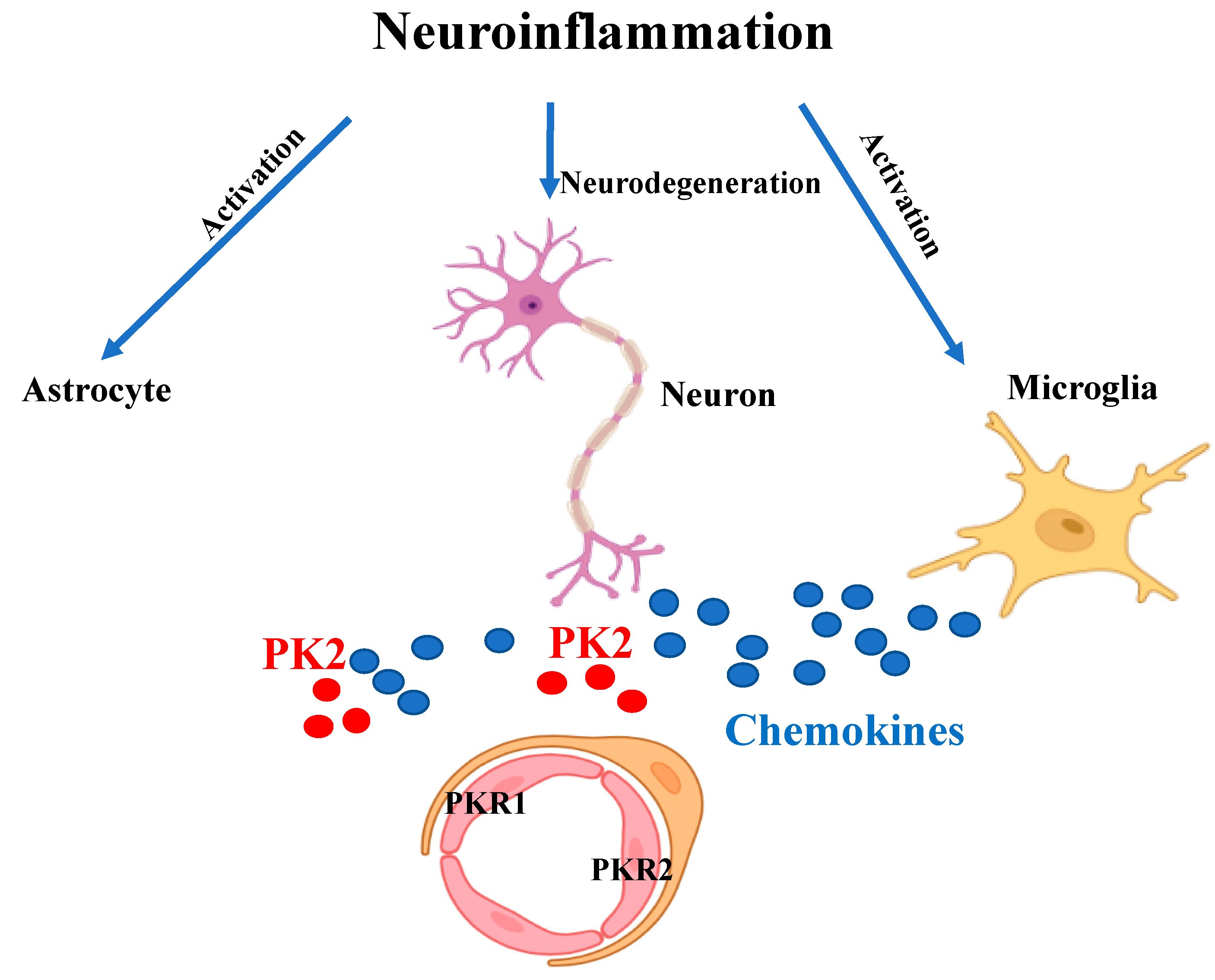
1. Introduction
2. Inflammatory and Neuropathic Pain
3. Neurological Diseases
3.1. Alzheimer’s Disease
3.2. Parkinson’s Disease
3.3. Multiple Sclerosis
3.4. Stroke
4. Control of Energy Metabolism
4.1. Obesity
4.2. Diabetes
5. Gastro-Intestinal Inflammation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gozales, H.; Pacheco, R. T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. J. Neuroinfl. 2014, 11, 201. [Google Scholar]
- Bagyinszkya, E.; Van Giaua, V.; Shima, K.; Sukb, K.; Ana, S.S.A.; Kim, S.Y. Role of inflammatory molecules in the Alzheimer’s disease progression and diagnosis. J. Neurol. Sci. 2017, 376, 242–254. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015, 16, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive astrocytes: Production, function, and therapeutic potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Kaser, A.; Winklmayr, M.; Lepperdinger, G.; Kreil, G. The AVIT protein family. Secreted cysteine-rich vertebrate proteins with diverse functions. EMBO Rep. 2003, 4, 469–473. [Google Scholar] [CrossRef]
- Miele, R.; Lattanzi, R.; Bonaccorsi di Patti, M.C.; Paiardini, A.; Negri, L.; Barra, D. Expression of Bv8 in Pichia pastoris to identify structural features for receptor binding. Protein Expr. Purif. 2010, 73, 10–14. [Google Scholar] [CrossRef]
- Lattanzi, R.; Sacerdote, P.; Franchi, S.; Canestrelli, M.; Miele, R.; Barra, D.; Visentin, S.; DeNuccio, C.; Porreca, F.; De Felice, M.; et al. Pharmacological activity of a Bv8 analogue modified in position 24. Br. J. Pharmacol. 2012, 166, 950–963. [Google Scholar] [CrossRef]
- Marsango, S.; Bonaccorsi di Patti, M.C.; Barra, D.; Miele, R. Evidence that prokineticin receptor 2 exists as a dimer in vivo. Cell. Mol. Life Sci. 2011, 68, 2919–2929. [Google Scholar] [CrossRef] [PubMed]
- Sposini, S.; Caltabiano, G.; Hanyaloglu, A.C.; Miele, R. Identification of transmembrane domains that regulate spatial arrangements and activity of prokineticin receptor 2 dimers. Mol. Cell. Endocrinol. 2015, 399, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Désaubry, L.; Kanthasamy, A.G.; Nebigil, C.G. Prokineticin signaling in heart-brain developmental axis: Therapeutic options for heart and brain injuries. Pharm. Res. 2020, 160, 105190. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kuei, C.; Sutton, S.; Wilson, S.; Yu, J.; Kamme, F.; Mazur, C.; Lovenberg, T.; Liu, C. Identification and pharmacological characterization of prokineticin 2 beta as a selective ligand for prokineticin receptor 1. Mol. Pharmacol. 2005, 67, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, R.; Maftei, D.; Negri, L.; Fusco, I.; Miele, R. PK2β ligand, a splice variant of prokineticin 2, is able to modulate and drive signaling through PKR1 receptor. Neuropeptides 2018, 71, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Negri, L.; Ferrara, N. The prokineticins: Neuromodulators and mediators of inflammation and myeloid cell-dependent angiogenesis. Physiol. Rev. 2018, 98, 1055–1082. [Google Scholar] [CrossRef]
- Cheng, M.Y.; Leslie, F.M.; Zhou, Q.Y. Expression of prokineticins and their receptors in the adult mouse brain. J. Comp. Neurol. 2006, 498, 796–809. [Google Scholar] [CrossRef]
- Nebigil, C.G. Prokineticin receptors in cardiovascular function: Foe or friend. Trends Cardiovasc. Med. 2009, 19, 55–60. [Google Scholar] [CrossRef]
- Cheng, M.Y.; Bullock, C.M.; Li, C.; Lee, A.G.; Bermak, J.C.; Belluzzi, J.; Weaver, D.R.; Leslie, F.M.; Zhou, Q.Y. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachi- asmatic nucleus. Nature 2006, 417, 405–410. [Google Scholar] [CrossRef]
- LeCouter, J.; Zlot, C.; Tejada, M.; Peale, F.; Ferrara, N. Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization. Proc. Natl. Acad. Sci. USA 2004, 101, 16813–16818. [Google Scholar] [CrossRef]
- Monnier, J.; Samson, M. Prokineticins in angiogenesis and cancer. Cancer Lett. 2010, 296, 144–149, Epub 14 July 2010. [Google Scholar] [CrossRef]
- Meng, L.; Yang, H.; Jin, C.; Quan, S. miR-28–5p suppresses cell proliferation and weakens the progression of polycystic ovary syndrome by targeting prokineticin-1. Mol. Med. Rep. 2019, 20, 2468–2475, Epub 1 July 2019. [Google Scholar] [CrossRef]
- Alfaidy, N.; Brouillet, S.; Rajaraman, G.; Kalionis, B.; Hoffmann, P.; Barjat, Y.; Benharouga, M.; Murthi, P. The Emerging Role of the Prokineticins and Homeobox Genes in the Vascularization of the Placenta: Physiological and Pathological Aspects. Front. Physiol. 2020, 12, 591850. [Google Scholar] [CrossRef] [PubMed]
- Dodé, C.; Rondard, P. PROK2/PROKR2 Signaling and Kallmann Syndrome. Front. Endocrinol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ferrer, M.; Torroglosa, A.; Núñez-Torres, R.; De Agustín, J.C.; Antiñolo, G.; Borrego, S. Expression of PROKR1 and PROKR2 in Human Enteric Neural Precursor Cells and Identification of Sequence Variants Suggest a Role in HSCR. PLoS ONE 2011, 6, e23475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martucci, C.; Franchi, S.; Giannini, E.; Tian, H.; Melchiorri, P.; Negri, L.; Sacerdote, P. Bv8, the amphibian homologue of the mammalian prokineticins, induces a proinflammatory phenotype of mouse macrophages. Br. J. Pharmacol. 2006, 147, 225–234. [Google Scholar] [CrossRef]
- Neal, M.; Luo, D.; Harischandra, D.S.; Gordon, R.; Sarkar, S.; Jin, H.; Anantharam, V.; Désaubry, L.; Kanthasamy, A.; Kanthasamy, A. Prokineticin-2 promotes chemotaxis and alternative A2 reactivity of astrocytes. Glia 2018, 66, 2137–2157. [Google Scholar] [CrossRef]
- Ma, M.; Li, H.; Wu, J.; Zhang, Y.; Shen, H.; Li, X.; Wang, Z.; Chen, G. Roles of Prokineticin 2 in Subarachnoid Hemorrhage-Induced Early Brain Injury via Regulation of Phenotype Polarization in Astrocytes. Mol. Neurobiol. 2020, 57, 3744–3758. [Google Scholar] [CrossRef]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef]
- Franchi, S.; Sacerdote, P.; Panerai, P. The prokineticin system: An interface between neural inflammation and pain. Neurol. Sci. 2017, 38, S27–S30. [Google Scholar] [CrossRef]
- Shojaei, F.; Ferrara, N. Role of the microenvironment in tumor growth and in refrac- toriness/resistance to anti-angiogenic therapies. Drug Resist. Updat. 2008, 11, 219–230. [Google Scholar] [CrossRef]
- Maftei, D.; Vellani, V.; Artico, M.; Giacomoni, C.; Severini, C.; Lattanzi, R. Abnormal Pain Sensation in Mice Lacking the Prokineticin Receptor PKR2: Interaction of PKR2 with Transient Receptor Potential TRPV1 and TRPA1. J. Neurosci. 2020, 427, 16–28. [Google Scholar] [CrossRef]
- Ren, C.; Qiu, C.Y.; Gan, X.; Liu, T.T.; Qu, Z.W.; Rao, Z.; Hu, W.P. Prokineticin 2 facilitates mechanical allodynia induced by α,β-methylene ATP in rats. Eur. J. Pharmacol. 2015, 767, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.C.; Li, X.M.; Wang, X.J.; Liu, Y.Q.; Qiu, F.; Wu, D.; Gan, Y.B.; Wang, B.H.; Hu, W.P. Prokineticin 2 suppresses GABA-activated current in rat primary sensory neurons. Neuropharmacology 2010, 59, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Ingves, M.V.; Ferguson, A.V. Prokineticin 2 modulates the excitability of area postrema neurons in vitro in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R617–R626. [Google Scholar] [CrossRef] [PubMed]
- Montague-Cardoso, K.; Malcangio, M. Changes in blood–spinal cord barrier permeability and neuroimmune interactions in the underlying mechanisms of chronic pain. Pain Rep. 2021, 6, e879, PMCID:PMC8108584. [Google Scholar] [CrossRef] [PubMed]
- Congiu, C.; Onnis, V.; Deplano, A.; Salvadori, S.; Marconi, V.; Maftei, D.; Negri, L.; Lattanzi, R.; Balboni, G. A new convenient synthetic method and preliminary pharmacological characterization of triazinediones as prokineticin receptor antagonists. Eur. J. Med. Chem. 2014, 81, 334–340. [Google Scholar] [CrossRef]
- Moschetti, G.; Amodeo, G.; Maftei, D.; Lattanzi, R.; Procacci, P.; Sartori, P.; Balboni, G.; Onnis, V.; Conte, V.; Panerai, A.; et al. Targeting prokineticin system counteracts hypersensitivity, neuroinflammation, and tissue damage in a mouse model of bortezomib-induced peripheral neuropathy. Neuroinflammation 2019, 16, 89. [Google Scholar] [CrossRef]
- Moschetti, G.; Amodeo, G.; Paladini, M.S.; Molteni, R.; Balboni, G.; Panerai, A.; Sacerdote, P.; Franchi, S. Prokineticin 2 promotes and sustains neuroinflammation in vincristine treated mice: Focus on pain and emotional like behavior. Brain Behaviur Immun. 2019, 82, 422–431. [Google Scholar] [CrossRef]
- Moschetti, G.; Kalpachidou, T.; Amodeo, G.; Lattanzi, R.; Sacerdote, P.; Kress, M.; Franchi, S. Prokineticin Receptor Inhibition with PC1 protects Mouse Primary Sensory Neurons FromNeurotoxic Effects of Chemotherapeutic Drugs in vitro. Front. Immunol. 2020, 11, 2119. [Google Scholar] [CrossRef]
- DáMesquita, S.; Ferreira, A.C.; Sousa, J.C.; Correia-Neves, M.; Sousa, N.; Marques, F. Insights on the pathophysiology of Alzheimer’s disease: The crosstalk between amyloid pathology, neuroinflammation and the peripheral immune system. Neurosci. Biobehav. Rev. 2016, 68, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Regen, F.; Hellmann-Regen, J.; Costantini, E.; Reale, M. Neuroinflammation and Alzheimer’s Disease: Implications for Microglial Activation. Curr. Alzheimer Res. 2017, 14, 1140–1148. [Google Scholar] [CrossRef]
- Finneran, D.J.; Nash, K.R. Neuroinflammation and fractalkine signaling in Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 30. [Google Scholar] [CrossRef]
- Bulati, M.; Buffa, S.; Martorana Gervasi, F.; Camarda, C.; Azzarello, D.M.; Monastero, R.; Caruso, C.; Colonna-Romano, G. Double Negative (IgG+IgD−CD27−) B Cells are Increased in a Cohort of Moderate-Severe Alzheimer’s Disease Patients and Show a Pro-Inflammatory Trafficking Receptor Phenotype. J. Alzh. Dis. 2015, 14, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, R.; Severini, C.; Maftei, D.; Saso, L.; Badiani, A. The Role of Prokineticin 2 in Oxidative Stress and in Neuropathological Processes. Front. Pharmacol. 2021, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, R.; Maftei, D.; Fullone, M.R.; Miele, R. Identification and characterization of Prokineticin receptor 2 splicing variant and its modulation in an animal model of Alzheimer’s disease. Neuropeptides 2019, 73, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Caggiu, E.; Paulus, K.; Arru, G.; Pieredda, R.; Sechi, G.P.; Sechi, L.A. Humoral cross reactivity betweenα-synuclein and herpes simplex-1epitope in Parkinson’s disease, a triggering role in the disease. J. Neuroimmunol. 2016, 291, 110–114. [Google Scholar] [CrossRef]
- Nagatsu, T. Hypothesis: Neural mechanism of psychotherapy for the treatment of Parkinson’s disease: Cognitive behavioral therapy (CBT), acceptance and commitment therapy (ACT), and Morita therapy? J. Neural. Transm. 2020, 127, 273–276. [Google Scholar] [CrossRef]
- Gordon, R.; Neal, M.L.; Luo, J.; Langley, M.R.; Harischandra, D.S.; Panicker, N.; Charli, A.; Jin, H.; Anantharam, V.; Woodruff, T.M.; et al. Prokineticin-2 upregulation during neuronal injury mediates a compensatory protective response against dopaminergic neuronal degeneration. Nat. Comm. 2016, 1, 12932. [Google Scholar] [CrossRef]
- Schirinzi, T.; Maftei, D.; Pieri, M.; Bernardini, S.; Mercuri, N.B.; Lattanzi, R.; Severini, C. Increase of Prokineticin-2 in Serum of Patients with Parkinson’s Disease. Mov. Disord. 2021, 36, 1031–1036. [Google Scholar] [CrossRef]
- Booth, H.D.E.; Hirst, W.D.; Wade-Martins, R. The Role of Astrocyte Dysfunction in Parkinson’s Disease Pathogenesis. Trends Neurosci. 2017, 40, 358–370. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Zeydan, B.; Kantarci, O.H. Progressive Forms of Multiple Sclerosis: Distinct Entity or Age-Dependent Phenomena. Neurol. Clin. 2018, 36, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H.; Van Horssen, J.; Mahad, D. Progressive multiple sclerosis: Pathology and pathogenesis. Nat. Rev. Neurol. 2012, 8, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.F.; Miron, V.E. The pro-remyelination properties of microglia in the central nervous system. Nat. Rev. Neurol. 2019, 15, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Qu, X.; Tan, M.; Meng, Y.G.; Ferrara, N. Characterization and Regulation of Bv8 in Human Blood Cells during the priming phase. Clin. Cancer. Res. 2009, 15, 15. [Google Scholar] [CrossRef]
- Goverman, J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 2009, 9, 393–407. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Kroner, A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev Neurosci. 2011, 12, 388–399. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflammation 2019, 16, 142. [Google Scholar] [CrossRef]
- Cheng, M.Y.; Lee, A.G.; Culbertson, C.; Sun, G.; Talati, R.K.; Manley, N.C.; Li, X.; Zhao, H.; Lyons, D.M.; Zhou, Q.-Y.; et al. Prokineticin 2 is an endangering mediator of cerebral ischemic injury. Proc. Nat.l Acad. Sci. USA 2012, 109, 5475–5480. [Google Scholar] [CrossRef]
- Choke, E.; Cockerill, G.W.; Laing, K.; Dawson, J.; Wilson, W.R.; Loftus, I.M.; Thompson, M.M. Whole genome-expression profiling reveals a role for immune and inflammatory response in abdominal aortic aneurysm rupture. Eur. J. Vasc. Endo-Vasc. Surg. 2009, 37, 305–310. [Google Scholar] [CrossRef]
- Landucci, E.; Lattanzi, R.; Gerace, E.; Scartabelli, T.; Balboni, G.; Negri, L.; Pellegrini-Giampietro, D.E. Prokineticins are neuroprotective in models of cerebral ischemia and ischemic tolerance in vitro. Neuropharmacology 2016, 108, 39–48. [Google Scholar] [CrossRef]
- Bao, Z.; Liu, Y.; Chen, B.; Miao, Z.; Tu, Y.; Li, C.; Chao, H.; Ye, Y.; Xu, X.; Sun, G.; et al. Prokineticin-2 prevents neuronal cell deaths in a model of traumatic brain injury. Nat. Commun. 2021, 12, 4220. [Google Scholar] [CrossRef] [PubMed]
- Guillemot-Legris, O.; Muccioli, G.G. Obesity-Induced Neuroinflammation: Beyond the Hypothalamus. Trends Neurosci. 2017, 40, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, J.V.; Bataveljic, A.; Patel, N.A.; Bewick, G.A.; Roy, D.; Campbell, D.; Greenwood, H.C.; Murphy, K.G.; Hameed, S.; Jethwa, P.H.; et al. Prokineticin 2 is a hypothalamic neuropeptide that potently inhibits food intake. Diabetes 2010, 59, 397–406. [Google Scholar] [CrossRef]
- Chaly, A.L.; Srisai, D.; Gardner, E.E.; Sebag, J.A. The Melanocortin Receptor Accessory Protein 2 promotes food intake through inhibition of the Prokineticin Receptor-1. Elife 2016, 5, e12397. [Google Scholar] [CrossRef] [PubMed]
- Rouault, A.A.J.; Lee, A.A.; Sebag, J.A. Regions of MRAP2 required for the inhibition of orexin and prokineticin receptor signaling. Biochim. Biophys. Acta Mol. Cell. Res. 2017, 1864, 2322–2329. [Google Scholar] [CrossRef]
- Maftei, D.; Lattanzi, R.; Vincenzi, M.; Squillace, S.; Fullone, M.R.; Miele, R. The balance of concentration between Prokineticin 2β and Prokineticin 2 modulates the food intake by STAT3 signaling. BBA Advances 2021, 1, 100028. [Google Scholar] [CrossRef]
- Iwasa, T.; Matsuzaki, T.; Tungalagsuvd, A.; Munkhzaya, M.; Kawami, T.; Yamasaki, M.; Murakami, M.; Kato, T.; Kuwahara, A.; Yasui, T.; et al. Changes in the responsiveness of hypothalamic PK2 and PKR1 gene expression to fasting in developing male rats. Int. J. Dev. Neurosci. 2014, 38, 87–90. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; Ma, H.; Lu, L.; Zhang, R. Prokineticin-2 is associated with metabolic syndrome in a middle-aged and elderly Chinese population. Lipids Health. Dis. 2016, 15, 1. [Google Scholar] [CrossRef]
- Wang, H.; Jia, Y.; Yu, X.; Peng, L.; Mou, C.; Song, Z.; Chen, D.; Li, X. Circulating Prokineticin 2 Levels Are Increased in Children with Obesity and Correlated with Insulin Resistance. Int. J. Endocrinol. 2021, 2021, 6630102. [Google Scholar] [CrossRef]
- Doulamis, I.P.; Konstantopoulos, P.; Tzani, A.; Antoranz, A.; Minia, A.; Daskalopoulou, A.; Charalampopoulos, A.; Alexopoulos, L.; Perrea, D.N.; Menenakos, E. Visceral white adipose tissue and serum proteomic alternations in metabolically healthy obese patients undergoing bariatric surgery. Cytokine 2019, 115, 76–83. [Google Scholar] [CrossRef]
- Szatkowski, C.; Vallet, J.; Dormishian, M.; Messaddeq, N.; Valet, P.; Boulberdaa, M.; Metzger, D.; Chambon, P.; Nebigil, C.G. Prokineticin receptor 1 as a novel suppressor of preadipocyte proliferation and differentiation to control obesity. PLoS ONE 2013, 8, e81175. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, R.; Kindo, M.; Arora, H.; Boulberdaa, M.; Steenman, M.; Nebigil, C.G. Prokineticin receptor-1-dependent paracrine and autocrine pathways control cardiac tcf21(+) fibroblast progenitor cell transformation into adipocytes and vascular cells. Sci. Rep. 2017, 7, 12804. [Google Scholar] [CrossRef] [PubMed]
- Elias, I.; Franckhauser, S.; Ferre, T.; Vilà, L.; Tafuro, S.; Muñoz, S.; Roca, C.; Ramos, D.; Pujol, A.; Riu, E.; et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes 2012, 61, 1801–1813. [Google Scholar] [CrossRef] [PubMed]
- Nebigil, C.G. Prokineticin Is a New Linker between Obesity and Cardiovascular Diseases. Front. Cardiovasc. Med. 2017, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Van Dyken, P.; Lacoste, B. Impact of Metabolic Syndrome on Neuroinflammation and the Blood–Brain Barrier. Front. Neurosci. 2018, 12, 930. [Google Scholar] [CrossRef] [PubMed]
- Mortreux, M.; Foppen, E.; Denis, R.G.; Montaner, M.; Kassis, N.; Denom, J.; Vincent, M.; Fumeron, F.; Kujawski-Lafourcade, M.; Andréelli, F.; et al. New roles for prokineticin 2 in feeding behavior, insulin resistance and type 2 diabetes: Studies in mice and humans. Mol. Metab. 2019, 29, 182–196. [Google Scholar] [CrossRef]
- Dormishian, M.; Turkeri, G.; Urayama, K.; Nguyen, T.L.; Boulberdaa, M.; Messaddeq, N.; Renault, G.; Henrion, D.; Nebigil, C.G. Prokineticin receptor-1 is a new regulator of endothelial insulin uptake and capillary formation to control insulin sensitivity and cardiovascular and kidney functions. J. Am. Heart Assoc. 2013, 2, e000411. [Google Scholar] [CrossRef]
- Mok, J.; Park, T.S.; Kim, S.; Kim, D.; Choi, C.S.; Park, J. Prokineticin receptor 1 ameliorates insulin resistance in skeletal muscle. FASEB J. 2021, 35, e21179. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, M.; Zhang, Y.; Cai, F.; Jiang, B.; Zha, W.; Yu, W. Metformin Ameliorates Diabetic Cardiomyopathy by Activating the PK2/PKR Pathway. Front. Physiol. 2020, 11, 425. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Kong, D.; Zhang, Y.; Yu, W.; Zha, W. Metformin Ameliorates Testicular Damage in Male Mice with Streptozotocin-Induced Type 1 Diabetes through the PK2/PKR Pathway. Oxid. Med. Cell Longev. 2019, 2019, 5681701. [Google Scholar] [CrossRef]
- Ujvari, D.; Graells Brugalla, C.; Hirschberg, A.L. Dihydrotestosterone potentiates insulin to up-regulate prokineticin-1 in decidualizing human endometrial stromal cells. J. Cell Mol. Med. 2020, 24, 3242–3245. [Google Scholar] [CrossRef] [PubMed]
- Castelli, M.; Amodeo, G.; Negri, L.; Lattanzi, R.; Maftei, D.; Gotti, C.; Pistillo, F.; Onnis, V.; Congiu, C.; Panerai, A.E.; et al. Antagonism of the Prokineticin System Prevents and Reverses Allodynia and Inflammation in a Mouse Model of Diabetes. PLoS ONE 2016, 11, e0146259. [Google Scholar] [CrossRef] [PubMed]
- Ngan, E.S.; Shum, C.K.; Poon, H.C.; Sham, M.H.; Garcia-Barcelo, M.M.; Lui, V.C.; Tam, P.K. Prokineticin-1 (Prok-1) works coordinately with glial cell line-derived neurotrophic factor (GDNF) to mediate proliferation and differentiation of enteric neural crest cells. Biochim. Biophys. Acta. 2008, 1783, 467–478. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arighi, E.; Borrello, G.; Sariola, H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 2005, 16, 441–467. [Google Scholar] [CrossRef]
- Wade, P.R.; Palmer, J.M.; Mabus, J.; Saunders, P.R.; Prouty, S.; Chevalier, K.; Gareau, M.G.; McKenney, S.; Hornby, P.J. Prokineticin-1 evokes secretory and contractile activity in rat small intestine. Neurogastroenterol. Motil. 2010, 22, e152–e161. [Google Scholar] [CrossRef]
- Watson, R.P.; Lilley, E.; Panesar, M.; Bhalay, G.; Langridge, S.; Tian, S.S.; McClenaghan, C.; Ropenga, A.; Zeng, F.; Nash, M.S. Increased prokineticin 2 expression in gut inflammation: Role in visceral pain and intestinal ion transport. Neurogastroenterol. Motil. 2012, 24, 65–75. [Google Scholar] [CrossRef]
- Zinni, M.; Zuena, A.R.; Marconi, V.; Petrella, C.; Fusco, I.; Giuli, C.; Canu, N.; Severini, C.; Broccardo, M.; Theodorou, V.; et al. Maternal exposure to low levels of corticosterone during lactation protects adult rat progeny against TNBS-induced colitis: A study on GR-mediated anti-inflammatory effect and prokineticin system. PLoS ONE 2017, 7, e0173484. [Google Scholar] [CrossRef]
- Petrella, C.; Giuli, C.; Agostini, S.; Bacquie, V.; Zinni, M.; Theodorou, V.; Broccardo, M.; Casolini, P.; Improta, G. Maternal Exposure to Low Levels of Corticosterone during Lactation Protects against Experimental Inflammatory Colitis-Induced Damage in Adult Rat Offspring. PLoS ONE 2014, 9, e113389. [Google Scholar] [CrossRef]
- Marsango, S.; Bonaccorsi di Patti, M.C.; Barra, D.; Miele, R. The Bv8 gene from Bombina orientalis: Molecular cloning, genomic organization and functional characterization of the promoter. Peptides 2009, 30, 2182–2190. [Google Scholar] [CrossRef]
- Pugazhenthi, S.; Limei, Q.L.; Reddy, P.H. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 517–552. [Google Scholar]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lattanzi, R.; Miele, R. Versatile Role of Prokineticins and Prokineticin Receptors in Neuroinflammation. Biomedicines 2021, 9, 1648. https://doi.org/10.3390/biomedicines9111648
Lattanzi R, Miele R. Versatile Role of Prokineticins and Prokineticin Receptors in Neuroinflammation. Biomedicines. 2021; 9(11):1648. https://doi.org/10.3390/biomedicines9111648
Chicago/Turabian StyleLattanzi, Roberta, and Rossella Miele. 2021. "Versatile Role of Prokineticins and Prokineticin Receptors in Neuroinflammation" Biomedicines 9, no. 11: 1648. https://doi.org/10.3390/biomedicines9111648
APA StyleLattanzi, R., & Miele, R. (2021). Versatile Role of Prokineticins and Prokineticin Receptors in Neuroinflammation. Biomedicines, 9(11), 1648. https://doi.org/10.3390/biomedicines9111648





