Improvement of Cell Culture Methods for the Successful Generation of Human Keratinocyte Primary Cell Cultures Using EGF-Loaded Nanostructured Lipid Carriers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
- (1)
- Cell isolation by enzymatic digestion. Tissue samples were incubated in trypsin-EDTA as previously reported [24]. Briefly, samples were placed in a commercial solution containing 0.05% trypsin and 0.02% EDTA (Merck) at 37 °C with gentle shaking. After 20 min, the solution was harvested and inactivated with culture medium, and detached epithelial cells were harvested from the dissociation solution by centrifugation. This procedure was repeated up to 10 times, and all harvested cells were cultured together in 6-well plates. In one of the study groups, harvested cells were cultured on a feeder layer previously cultured on the plates. For this group, 3T3 cells (Merck) were cultured on 6-well plates and a lethal dose of gamma irradiation was applied when cells reached semiconfluence. The cells were washed with PBS, and then dissociated cells were cultured directly on the surface of this cell layer.
- (2)
- Explant technique. The tissues were trimmed into small pieces with a surgical blade, and each piece was placed on the surface of a 6-well plate, with the epithelial layer in direct contact with the culture surface. These explants were allowed to attach to the surface for 30 min, and a very small amount of culture medium was carefully added to prevent explant detachment. Culture plates were placed in a cell incubator, and 5 mL of culture medium was added to each well 24 h later.
2.2. Preparation of NLC
2.3. Characterization of rhEGF-Loaded NLC
2.4. Study Groups
- (1)
- BM. In this group, cells were cultured in basic culture medium (BM) consisting of a 3:1 mixture of DMEM and Ham’s F-12 supplemented with 10% fetal bovine serum (FBS), 1% antibiotics and antimycotics, 24 µg/mL adenine, 0.4 µg/mL hydrocortisone, 5 µg/mL insulin, and 1.3 ng/mL triiodothyronine. Cells cultured in this medium were used as a control.
- (2)
- L-rhEGF. In this group, cells were cultured in BM supplemented with liquid recombinant human EGF (L-rhEGF) at a final concentration of 10 ng/mL. This medium corresponds to the epithelial medium used for keratinocyte culture and expansion in the UGRSKIN model of tissue-engineered skin.
- (3)
- NLC-rhEGF. In this group, cells were cultured with BM supplemented with rhEGF-loaded NLC (NLC-rhEGF) at a final concentration of 10 ng/mL.
- (4)
- NLC-blank. NLC alone were added to BM at the same concentration used in group 3 (10 ng/mL). This group was also considered a control, since no EGF was used.
2.5. Biosafety Analysis of NLC on Epithelial Cells
2.6. Proliferation Potential of Each Culture Medium
2.7. Gene Expression Analysis
2.8. Statistical Analysis
3. Results
3.1. rhEGF-Loaded NLC Characterization
3.2. Biosafety Analysis of NLC in Epithelial Cell Lines
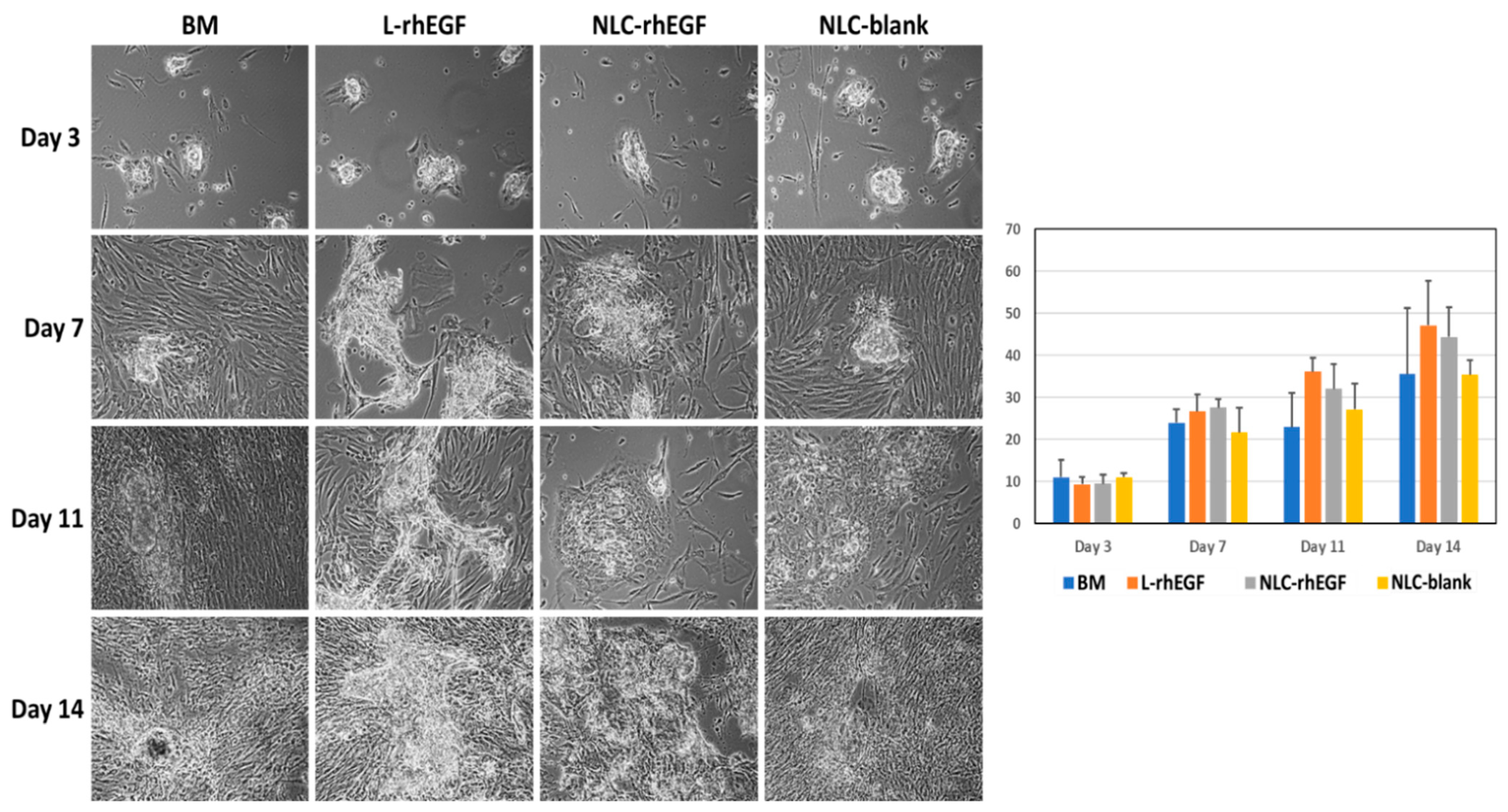
3.3. Establishment of Primary Cell Cultures of HKC with the Cell Isolation Technique
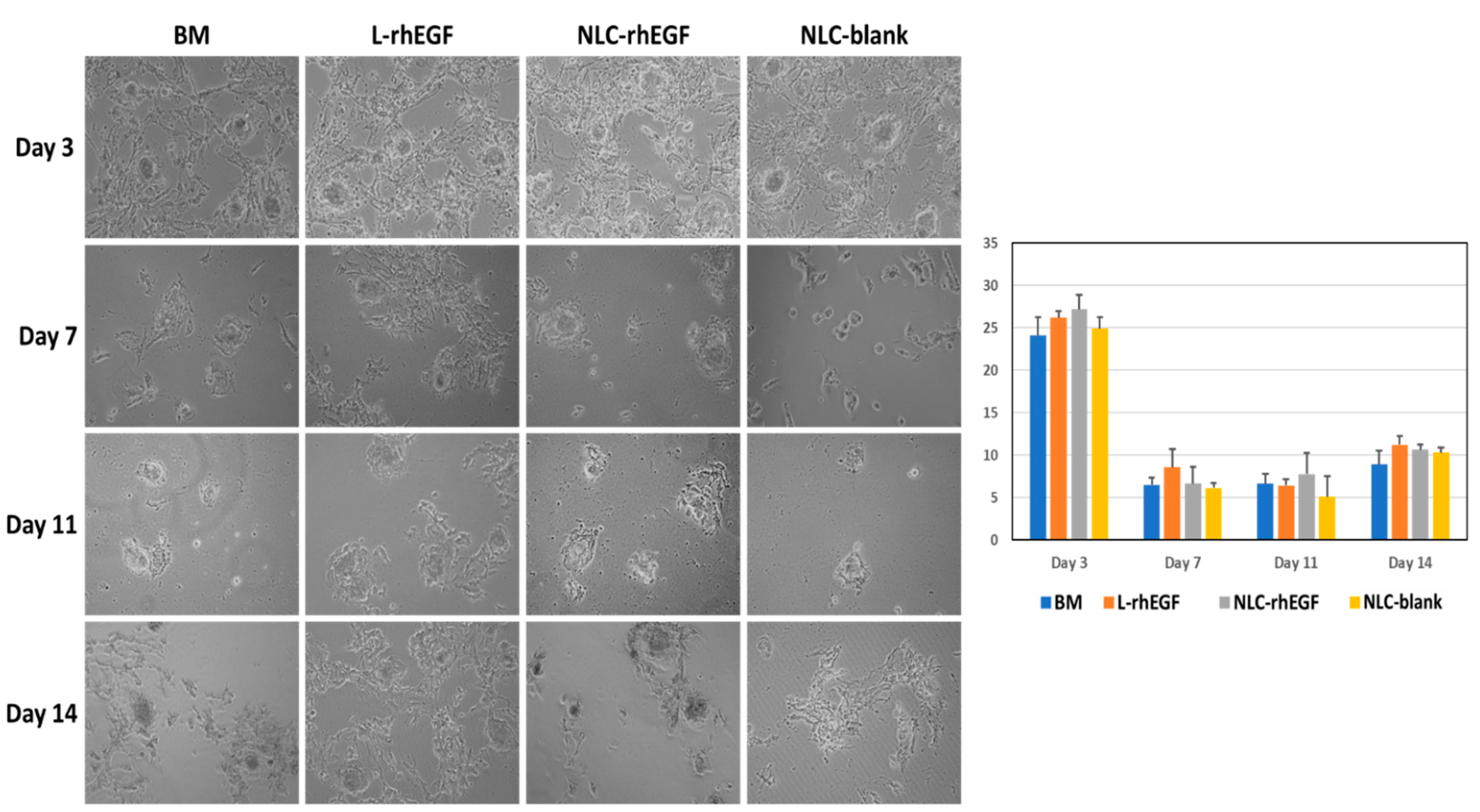
3.4. Establishment of Primary Cell Cultures of HKC with the Explant Technique
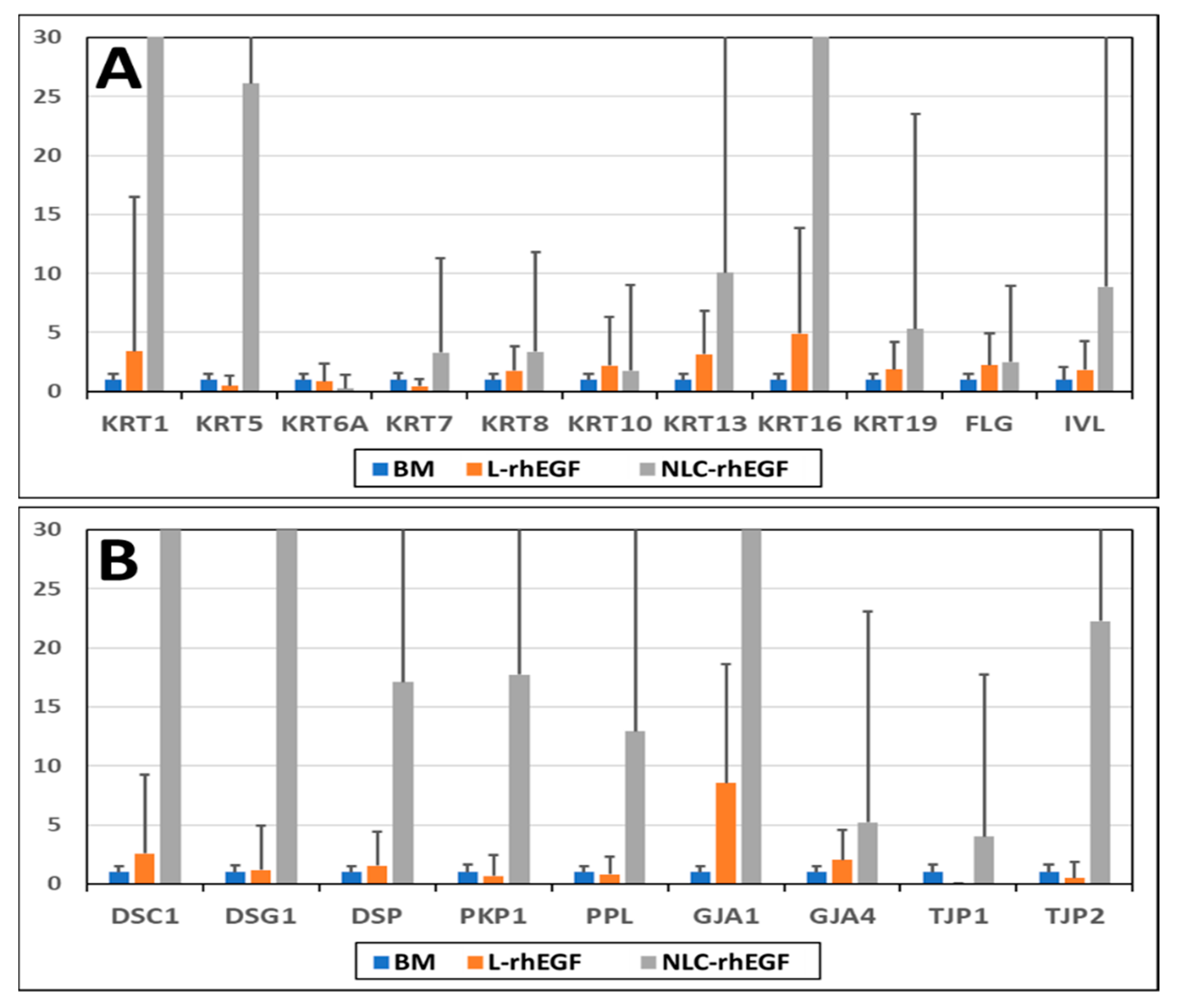
3.5. Gene Expression Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuende, N.; Rasko, J.E.J.; Koh, M.B.C.; Dominici, M.; Ikonomou, L. Cell, Tissue and Gene Products with Marketing Authorization in 2018 Worldwide. Cytotherapy 2018, 20, 1401–1413. [Google Scholar] [CrossRef]
- Egea-Guerrero, J.J.; Carmona, G.; Correa, E.; Mata, R.; Arias-Santiago, S.; Alaminos, M.; Gacto, P.; Cuende, N. Transplant of Tissue-Engineered Artificial Autologous Human Skin in Andalusia: An Example of Coordination and Institutional Collaboration. Transplant. Proc. 2019, 51, 3047–3050. [Google Scholar] [CrossRef]
- Goodarzi, P.; Falahzadeh, K.; Nematizadeh, M.; Farazandeh, P.; Payab, M.; Larijani, B.; Tayanloo Beik, A.; Arjmand, B. Tissue Engineered Skin Substitutes. Adv. Exp. Med. Biol. 2018, 1107, 143–188. [Google Scholar] [CrossRef] [PubMed]
- Carriel, V.; Garzón, I.; Jiménez, J.-M.; Oliveira, A.-C.-X.; Arias-Santiago, S.; Campos, A.; Sánchez-Quevedo, M.-C.; Alaminos, M. Epithelial and stromal developmental patterns in a novel substitute of the human skin generated with fibrin-agarose biomaterials. Cells Tissues Organs 2012, 196, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Breidahl, A.F.; Judson, R.T.; Clunie, G.J. Review of keratinocyte culture techniques: Problems of growing skin. Aust. N. Z. J. Surg. 1989, 59, 485–497. [Google Scholar] [CrossRef]
- Wang, J.; Mongan, M.; Zhang, X.; Xia, Y. Isolation and long-term expansion of murine epidermal stem-like cells. PLoS ONE 2021, 16, e0254731. [Google Scholar] [CrossRef]
- Dearman, B.L.; Boyce, S.T.; Greenwood, J.E. Advances in Skin Tissue Bioengineering and the Challenges of Clinical Translation. Front. Surg. 2021, 8, 640879. [Google Scholar] [CrossRef] [PubMed]
- Chato-Astrain, J.; Chato-Astrain, I.; Sánchez-Porras, D.; García-García, Ó.-D.; Bermejo-Casares, F.; Vairo, C.; Villar-Vidal, M.; Gainza, G.; Villullas, S.; Oruezabal, R.-I.; et al. Generation of a novel human dermal substitute functionalized with antibiotic-loaded nanostructured lipid carriers (NLCs) with antimicrobial properties for tissue engineering. J. Nanobiotechnol. 2020, 18, 174. [Google Scholar] [CrossRef]
- Lachiewicz, A.M.; Hauck, C.G.; Weber, D.J.; Cairns, B.A.; van Duin, D. Bacterial Infections After Burn Injuries: Impact of Multidrug Resistance. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 65, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Shirakata, Y. Regulation of epidermal keratinocytes by growth factors. J. Dermatol. Sci. 2010, 59, 73–80. [Google Scholar] [CrossRef]
- Glatzer, F.; Gschwandtner, M.; Ehling, S.; Rossbach, K.; Janik, K.; Klos, A.; Bäumer, W.; Kietzmann, M.; Werfel, T.; Gutzmer, R. Histamine induces proliferation in keratinocytes from atopic dermatitis patients. J. Allergy Clin. Immunol. 2013, 132, 1358–1367. [Google Scholar] [CrossRef] [Green Version]
- Sakai, Y.; Demay, M.B. Evaluation of Keratinocyte Proliferation and Differentiation in Vitamin D Receptor Knockout Mice. Endocrinology 2000, 141, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Blumenberg, M. Profiling and metaanalysis of epidermal keratinocytes responses to epidermal growth factor. BMC Genom. 2013, 14, 85. [Google Scholar] [CrossRef] [Green Version]
- Bodnar, R.J. Epidermal Growth Factor and Epidermal Growth Factor Receptor: The Yin and Yang in the Treatment of Cutaneous Wounds and Cancer. Adv. Wound Care 2013, 2, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gainza, G.; Pastor, M.; Aguirre, J.J.; Villullas, S.; Pedraz, J.L.; Hernandez, R.M.; Igartua, M. A novel strategy for the treatment of chronic wounds based on the topical administration of rhEGF-loaded lipid nanoparticles: In vitro bioactivity and in vivo effectiveness in healing-impaired db/db mice. J. Control. Release 2014, 185, 51–61. [Google Scholar] [CrossRef]
- Garcia-Orue, I.; Gainza, G.; Gutierrez, F.B.; Aguirre, J.J.; Evora, C.; Pedraz, J.L.; Hernandez, R.M.; Delgado, A.; Igartua, M. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int. J. Pharm. 2017, 523, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Gainza, G.; Bonafonte, D.C.; Moreno, B.; Aguirre, J.J.; Gutierrez, F.B.; Villullas, S.; Pedraz, J.L.; Igartua, M.; Hernandez, R.M. The topical administration of rhEGF-loaded nanostructured lipid carriers (rhEGF-NLC) improves healing in a porcine full-thickness excisional wound model. J. Control. Release Off. J. Control. Release Soc. 2015, 197, 41–47. [Google Scholar] [CrossRef]
- Vairo, C.; Collantes, M.; Quincoces, G.; Villullas, S.; Peñuelas, I.; Pastor, M.; Gil, A.G.; Gainza, E.; Hernandez, R.M.; Igartua, M.; et al. Preclinical safety of topically administered nanostructured lipid carriers (NLC) for wound healing application: Biodistribution and toxicity studies. Int. J. Pharm. 2019, 569, 118484. [Google Scholar] [CrossRef]
- Gainza, G.; Villullas, S.; Pedraz, J.L.; Hernandez, R.M.; Igartua, M. Advances in drug delivery systems (DDSs) to release growth factors for wound healing and skin regeneration. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1551–1573. [Google Scholar] [CrossRef]
- Kurakula, M.; Ahmed, O.A.A.; Fahmy, U.A.; Ahmed, T.A. Solid lipid nanoparticles for transdermal delivery of avanafil: Optimization, formulation, in-vitro and ex-vivo studies. J. Liposome Res. 2016, 26, 288–296. [Google Scholar] [CrossRef]
- Lee, H.-J.; Jeong, M.; Na, Y.-G.; Kim, S.-J.; Lee, H.-K.; Cho, C.-W. An EGF- and Curcumin-Co-Encapsulated Nanostructured Lipid Carrier Accelerates Chronic-Wound Healing in Diabetic Rats. Molecules 2020, 25, 4610. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ng, W.K.; Tan, R.B.H. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2012, 47, 139–151. [Google Scholar] [CrossRef]
- Pathak, P.; Nagarsenker, M. Formulation and evaluation of lidocaine lipid nanosystems for dermal delivery. AAPS PharmSciTech 2009, 10, 985–992. [Google Scholar] [CrossRef]
- Llames, S.G.; Del Rio, M.; Larcher, F.; García, E.; García, M.; Escamez, M.J.; Jorcano, J.L.; Holguín, P.; Meana, A. Human plasma as a dermal scaffold for the generation of a completely autologous bioengineered skin. Transplantation 2004, 77, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Naveen, N.R.; Patel, B.; Manne, R.; Patel, D.B. Preparation, Optimization and Evaluation of Chitosan-Based Avanafil Nanocomplex Utilizing Antioxidants for Enhanced Neuroprotective Effect on PC12 Cells. Gels 2021, 7, 96. [Google Scholar] [CrossRef]
- Shi, W.; Weng, D.; Niu, W. Nanoparticle drug delivery systems and three-dimensional cell cultures in cancer treatments and research. Cancer Transl. Med. 2016, 2, 154–161. [Google Scholar] [CrossRef]
- Abdel Fadeel, D.A.; Kamel, R.; Fadel, M. PEGylated lipid nanocarrier for enhancing photodynamic therapy of skin carcinoma using curcumin: In-vitro/in-vivo studies and histopathological examination. Sci. Rep. 2020, 10, 10435. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.A.; Lopez-Gonzalez, G.; Rodríguez, M.A.; Campos-Sanchez, F.; Alaminos, M. Biological evaluation of 2-hydroxyethylmethacrylate (HEMA) toxicity in human gingival fibroblasts with histochemical X-ray microanalysis. J. Adhes. Dent. 2011, 13, 375–381. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Green, H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef]
- Chua, A.W.C.; Khoo, Y.C.; Tan, B.K.; Tan, K.C.; Foo, C.L.; Chong, S.J. Skin tissue engineering advances in severe burns: Review and therapeutic applications. Burns Trauma 2016, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Gainza, G.; Chu, W.S.; Guy, R.H.; Pedraz, J.L.; Hernandez, R.M.; Delgado-Charro, B.; Igartua, M. Development and in vitro evaluation of lipid nanoparticle-based dressings for topical treatment of chronic wounds. Int. J. Pharm. 2015, 490, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [Green Version]
- Sangsen, Y.; Laochai, P.; Chotsathidchai, P.; Wiwattanapatapee, R. Effect of Solid Lipid and Liquid Oil Ratios on Properties of Nanostructured Lipid Carriers for Oral Curcumin Delivery. Adv. Mater. Res. 2015, 1060, 62–65. [Google Scholar] [CrossRef]
- Daniels, J.T.; Kearney, J.N.; Ingham, E. Human keratinocyte isolation and cell culture: A survey of current practices in the UK. Burns J. Int. Soc. Burn Inj. 1996, 22, 35–39. [Google Scholar] [CrossRef]
- Wang, X.; Shen, C.; Li, Z.; Xu, S.; Li, D. Efficient isolation and high yield of epidermal cells from foreskin biopsies by dynamic trypsinization. Burns J. Int. Soc. Burn Inj. 2018, 44, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Hybbinette, S.; Boström, M.; Lindberg, K. Enzymatic dissociation of keratinocytes from human skin biopsies for in vitro cell propagation. Exp. Dermatol. 1999, 8, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Meana, A.; Iglesias, J.; Del Rio, M.; Larcher, F.; Madrigal, B.; Fresno, M.F.; Martin, C.; San Roman, F.; Tevar, F. Large surface of cultured human epithelium obtained on a dermal matrix based on live fibroblast-containing fibrin gels. Burns 1998, 24, 621–630. [Google Scholar] [CrossRef]
- He, Y.; Zhang, W.-Y.; Gong, M.; Huang, J.-Y.; Tang, N.; Feng, T.; Wei, G.-H.; He, T.-C.; Bi, Y. Low serum concentration facilitates the differentiation of hepatic progenitor cells. Saudi Med. J. 2011, 32, 128–134. [Google Scholar] [PubMed]
- Pastore, S.; Mascia, F.; Mariani, V.; Girolomoni, G. The epidermal growth factor receptor system in skin repair and inflammation. J. Investig. Dermatol. 2008, 128, 1365–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shwetha, H.R.; Kotrashetti, V.S.; Babu, N.C.; Kumbar, V.; Bhat, K.; Reddy, R. Ex vivo culture of oral keratinocytes using direct explant cell culture technique. J. Oral Maxillofac. Pathol. JOMFP 2019, 23, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Bayar, G.R.; Gulses, A. Ex-vivo production of oral mucosa keratinocytes by the direct explant method. Int. J. Oral Maxillofac. Surg. 2013, 42, 417–418. [Google Scholar] [CrossRef] [PubMed]
- Kedjarune, U.; Pongprerachok, S.; Arpornmaeklong, P.; Ungkusonmongkhon, K. Culturing primary human gingival epithelial cells: Comparison of two isolation techniques. J. Cranio-Maxillo-fac. Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-fac. Surg. 2001, 29, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Bayar, G.R.; Aydıntuğ, Y.S.; Günhan, O.; Oztürk, K.; Gülses, A. Ex vivo produced oral mucosa equivalent by using the direct explant cell culture technique. Balk. Med. J. 2012, 29, 295–300. [Google Scholar] [CrossRef]

| Formulation | Size (nm) | Span | Zeta Potential (mV) |
|---|---|---|---|
| rhEGF-NLC | 137 ± 3.4 | 0.7 | −32 ± 0.31 |
| NLC-blank | 112 ± 5.2 | 0.9 | −33 ± 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chato-Astrain, J.; Sánchez-Porras, D.; García-García, Ó.D.; Vairo, C.; Villar-Vidal, M.; Villullas, S.; Sánchez-Montesinos, I.; Campos, F.; Garzón, I.; Alaminos, M. Improvement of Cell Culture Methods for the Successful Generation of Human Keratinocyte Primary Cell Cultures Using EGF-Loaded Nanostructured Lipid Carriers. Biomedicines 2021, 9, 1634. https://doi.org/10.3390/biomedicines9111634
Chato-Astrain J, Sánchez-Porras D, García-García ÓD, Vairo C, Villar-Vidal M, Villullas S, Sánchez-Montesinos I, Campos F, Garzón I, Alaminos M. Improvement of Cell Culture Methods for the Successful Generation of Human Keratinocyte Primary Cell Cultures Using EGF-Loaded Nanostructured Lipid Carriers. Biomedicines. 2021; 9(11):1634. https://doi.org/10.3390/biomedicines9111634
Chicago/Turabian StyleChato-Astrain, Jesús, David Sánchez-Porras, Óscar Darío García-García, Claudia Vairo, María Villar-Vidal, Silvia Villullas, Indalecio Sánchez-Montesinos, Fernando Campos, Ingrid Garzón, and Miguel Alaminos. 2021. "Improvement of Cell Culture Methods for the Successful Generation of Human Keratinocyte Primary Cell Cultures Using EGF-Loaded Nanostructured Lipid Carriers" Biomedicines 9, no. 11: 1634. https://doi.org/10.3390/biomedicines9111634
APA StyleChato-Astrain, J., Sánchez-Porras, D., García-García, Ó. D., Vairo, C., Villar-Vidal, M., Villullas, S., Sánchez-Montesinos, I., Campos, F., Garzón, I., & Alaminos, M. (2021). Improvement of Cell Culture Methods for the Successful Generation of Human Keratinocyte Primary Cell Cultures Using EGF-Loaded Nanostructured Lipid Carriers. Biomedicines, 9(11), 1634. https://doi.org/10.3390/biomedicines9111634








