Renal Hypouricemia 1: Rare Disorder as Common Disease in Eastern Slovakia Roma Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Subjects
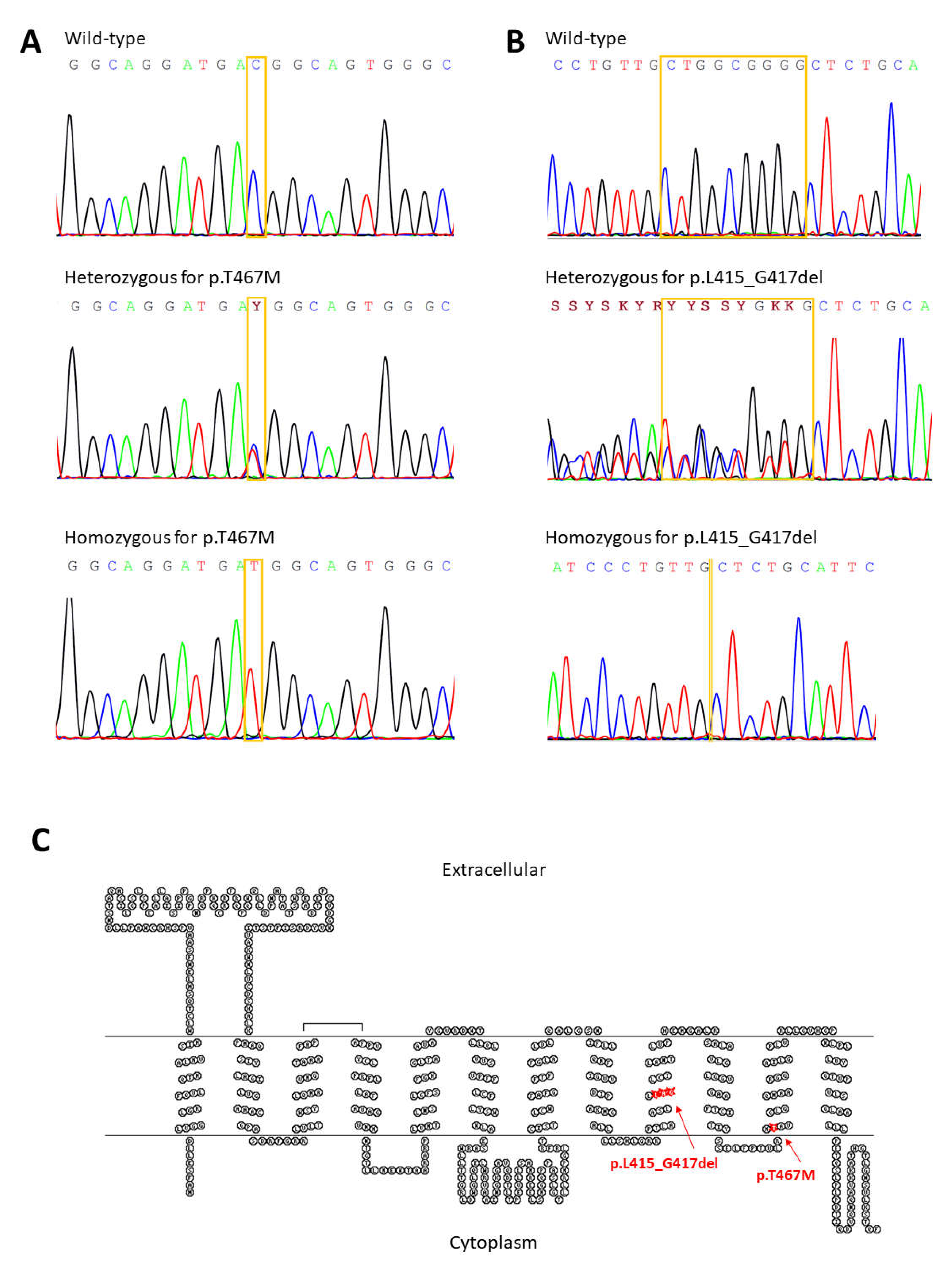
2.2. Clinical Investigations and Sequence Analyses
3. Results
3.1. Clinical Subjects
3.2. Clinical Investigations and Sequence Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bugdayci, G.; Balaban, Y.; Sahin, O. Causes of Hypouricemia among Outpatients. Lab. Med. 2008, 39, 550–552. [Google Scholar] [CrossRef] [Green Version]
- Koo, B.S.; Jeong, H.J.; Son, C.N.; Kim, S.H.; Kim, H.J.; Kim, G.H. Distribution of serum uric acid levels and prevalence of hyper- and hypouricemia in a Korean general population of 172,970. Korean J. Intern. Med. 2021, 36 (Suppl. S1), S264–S272. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Endou, H. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef]
- Matsuo, H.; Chiba, T.; Nagamori, S.; Nakayama, A.; Domoto, H.; Phetdee, K.; Shinomiya, N. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am. J. Hum. Genet. 2008, 83, 744–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugihara, S.; Hisatome, I.; Kuwabara, M.; Niwa, K.; Maharani, N.; Kato, M.; Yamamoto, K. Depletion of Uric Acid Due to SLC22A12 (URAT1) Loss-of-Function Mutation Causes Endothelial Dysfunction in Hypouricemia. Circ. J. 2015, 79, 1125–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancikova, A.; Krylov, V.; Hurba, O.; Sebesta, I.; Nakamura, M.; Ichida, K.; Stiburkova, B. Functional analysis of novel allelic variants in URAT1 and GLUT9 causing renal hypouricemia type 1 and 2. Clin. Exp. Nephrol. 2016, 20, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Dinour, D.; Gray, N.K.; Campbell, S.; Shu, X.; Sawyer, L.; Richardson, W.; Holtzman, E.J. Homozygous SLC2A9 mutations cause severe renal hypouricemia. J. Am. Soc. Nephrol. 2010, 21, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiburkova, B.; Taylor, J.; Marinaki, A.M.; Sebesta, I. Acute kidney injury in two children caused by renal hypouricaemia type 2. Pediatric Nephrol. 2012, 27, 1411–1415. [Google Scholar] [CrossRef]
- Bhasin, B.; Stiburkova, B.; De Castro-Pretelt, M.; Beck, N.; Bodurtha, J.N.; Atta, M.G. Hereditary renal hypouricemia: A new role for allopurinol? Am. J. Med. 2014, 127, e3–e4. [Google Scholar] [CrossRef] [PubMed]
- Ichida, K.; Hosoyamada, M.; Kamatani, N.; Kamitsuji, S.; Hisatome, I.; Shibasaki, T.; Hosoya, T. Age and origin of the G774A mutation in SLC22A12 causing renal hypouricemia in Japanese. Clin. Genet. 2008, 74, 243–251. [Google Scholar] [CrossRef]
- Stiburkova, B.; Gabrikova, D.; Cepek, P.; Simek, P.; Kristian, P.; Cordoba-Lanus, E.; Claverie-Martin, F. Prevalence of URAT1 allelic variants in the Roma population. Nucleosides Nucleotides Nucleic Acids 2016, 35, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Gabrikova, D.; Bernasovska, J.; Sokolova, J.; Stiburkova, B. High frequency of SLC22A12 variants causing renal hypouricemia 1 in the Czech and Slovak Roma population; simple and rapid detection method by allele-specific polymerase chain reaction. Urolithiasis 2015, 43, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Stiburkova, B.; Ichida, K.; Sebesta, I. Novel homozygous insertion in SLC2A9 gene caused renal hypouricemia. Mol. Genet. Metab. 2011, 102, 430–435. [Google Scholar] [CrossRef]
- Stiburkova, B.; Sebesta, I.; Ichida, K.; Nakamura, M.; Hulkova, H.; Krylov, V.; Jahnova, H. Novel allelic variants and evidence for a prevalent mutation in URAT1 causing renal hypouricemia: Biochemical, genetics and functional analysis. Eur. J. Hum. Genet. 2013, 21, 1067–1073. [Google Scholar] [CrossRef] [Green Version]
- Cha, D.H.; Gee, H.Y.; Cachau, R.; Choi, J.M.; Park, D.; Jee, S.H.; Ryu, S.; Cho, S.K. Contribution of SLC22A12 on hypouricemia and its clinical significance for screening purposes. Sci. Rep. 2019, 9, 14360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, A.; Matsuo, H.; Abhishek, A.; Ichida, K.; Shinomiya, N.; Guideline Development Committee of Clinical Practice Guideline for Renal Hypouricaemia. First clinical practice guideline for renal hypouricemia: A rare disorder that aided the development of urate-lowering drugs for gout. Rheumatology 2021, 60, 3961–3963. [Google Scholar] [CrossRef] [PubMed]
- Lisyova, J.; Chandoga, J.; Jungova, P.; Repisky, M.; Knapkova, M.; Machkova, M.; Bohmer, D. An unusually high frequency of SCAD deficiency caused by two pathogenic variants in the ACADS gene and its relationship to the ethnic structure in Slovakia. BMC Med. Genet. 2018, 19, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebesta, I.; Stiburkova, B.; Bartl, J.; Ichida, K.; Hosoyamada, M.; Taylor, J.; Marinaki, A. Diagnostic tests for primary renal hypouricemia. Nucleosides Nucleotides Nucleic Acids 2011, 30, 1112–1116. [Google Scholar] [CrossRef]
- Stiburkova, B.; Sebesta, I. Hypouricemia and hyperuricosuria in a pubescent girl: Answers. Pediatric Nephrol. 2018, 33, 2277–2279. [Google Scholar] [CrossRef]
- Stiburkova, B.; Sebesta, I. Hypouricemia and hyperuricosuria in a pubescent girl: Questions. Pediatric Nephrol. 2018, 33, 2275. [Google Scholar] [CrossRef]
- Claverie-Martin, F.; Trujillo-Suarez, J.; Gonzalez-Acosta, H.; Aparicio, C.; Roldan, M.L.J.; Stiburkova, B.; Garcia-Nieto, V.M. URAT1 and GLUT9 mutations in Spanish patients with renal hypouricemia. Clin. Chim. Acta 2018, 481, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Morar, B.; Gresham, D.; Angelicheva, D.; Tournev, I.; Gooding, R.; Guergueltcheva, V.; Kalaydjieva, L. Mutation history of the roma/gypsies. Am. J. Hum. Genet. 2004, 75, 596–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurba, O.; Mancikova, A.; Krylov, V.; Pavlikova, M.; Pavelka, K.; Stiburkova, B. Complex analysis of urate transporters SLC2A9, SLC22A12 and functional characterization of non-synonymous allelic variants of GLUT9 in the Czech population: No evidence of effect on hyperuricemia and gout. PLoS ONE 2014, 9, e107902. [Google Scholar] [CrossRef] [PubMed]
- Stiburkova, B.; Bleyer, A.J. Changes in serum urate and urate excretion with age. Adv. Chronic Kidney Disease 2012, 19, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Kolvek, G.; Rosicova, K.; Rosenberger, J.; Podracka, L.; Stewart, R.E.; Nagyova, I.; van Dijk, J.P. End-stage renal disease among Roma and non-Roma: Roma are at risk. Int. J. Public Health 2012, 57, 751–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberger, J.; Majernikova, M.; Jarcuska, P.; Pella, D.; Marekova, M.; Geckova, A.M. Higher prevalence of nephropathy in young Roma females compared with non-Roma females. Cent. Eur. J. Public Health 2014, 22, S28–S31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallayova, M.; Brenisin, M.; Putrya, A.; Vrsko, M.; Drazilova, S.; Janicko, M.; Team, H. Roma Ethnicity and Sex-Specific Associations of Serum Uric Acid with Cardiometabolic and Hepatorenal Health Factors in Eastern Slovakian Population: The HepaMeta Study. Int. J. Environ. Res. Public Health 2020, 17, 7673. [Google Scholar] [CrossRef]
- Ichida, K.; Hosoyamada, M.; Hisatome, I.; Enomoto, A.; Hikita, M.; Endou, H.; Hosoya, T. Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J. Am. Soc. Nephrol. 2004, 15, 164–173. [Google Scholar] [CrossRef] [PubMed]
| Family | Patient | Sex | Year of Birth | Year of Onset | sUA µmol/L | FE-UA % | Associated Diseases |
|---|---|---|---|---|---|---|---|
| A | 1 | F | 1962 | N/A | 263 | 7.6 | PKU heterozygote |
| 2 | F | 1991 | N/A | 137 | 9.7 | PKU | |
| B | 3 | M | 2014 | 4 | 101 (2018), 102 (2021) | 13.1 (2018), 16.2 (2021) | autism, ADHD |
| 4 | M | 1985 | N/A | 326 | 7.3 | ||
| 5 | F | 1981 | 20 | 167 | 8.6 | ||
| C | 6 | M | 2006 | 12 | 94 (2018) | 23.4 (2018) | PKU |
| 7 | M | 2012 | 6 | 122 (2017), 150 (2018) | 15.8 (2018) | PKU | |
| 8 | F | 2011 | 7 | 118 (2018) | 12.4 (2018) | PKU | |
| D | 9 | F | 2008 | 10 | 194 (2018) | 7.3 (2018) | MTHFR homozygote, PAI heterozygote |
| 10 | F | 1999 | 20 | 141 (2019) | 16.2 (2019) | SCADD heterozygote | |
| E | 11 | F | 2018 | 10 mth | 95 (2018), 146 (2019), 151 (2021) | 17.1 (2018), 12.0 (2021) | SCADD |
| 12 | F | 1994 | 24 | N/A | N/A | SCADD heterozygote | |
| F | 13 | F | 1991 | N/A | 309 (2018) | 5.1 (2018) | xanthinuria heterozygote |
| 14 | M | 2017 | 18 mth | 72 (2018), 136 (2019), 141 (2021) | 10.3 (2021) | PKU | |
| 15 | M | 2016 | 3 | 135 (2016), 140 (2019) | 11.3 (2019) | SCADD | |
| G | 16 | M | 2018 | 6 mth | 140 (2018), 114 (2019), 235 (2020) | 16.5 (2018) | transitory hypertyrosinemia |
| 17 | F | 1981 | 38 | 247 (2018) | 8.4 (2018) | ||
| 18 | M | 1988 | 31 | 415 (2018) | 5.9 (2018) | ||
| H | 19 | M | 2008 | 11 | 55 (2019), 77 (2020) | 28.8 (2020) | hypogonadotropic hypogonadism, Gilbert syndrome, hepatopathy, cholecystolithiasis, obesity |
| 20 | F | 1986 | 34 | 125 | 11.6 | ||
| 21 | M | 1983 | 37 | 44 | 43 | ||
| I | 22 | F | 2016 | 27 mth | 118 (2017), 131 (2019), 152 (2021) | 10.6 (2019) | central hypocorticism, epilepsy, familiar hypercholesterolemia |
| 23 | F | 1982 | 37 | 161 | 8.2 | neurofibromatosis type 1 | |
| 24 | M | 1984 | 35 | 366 | 6.1 | familial hypercholesterolemia | |
| J | 25 | M | 2019 | 23 mth | 61 (2019), 43 (2020) | 51 (2019) | SCADD |
| 26 | F | 2002 | 18 | N/A | N/A | SCADD heterozygote | |
| K | 27 | M | 2010 | 10 | 108 (2012), 88 (2013), 84 (2018), 71 (2019), 49 (2021) | 56 (2012), 30 (2013), 40 (2018), 40 (2019), 50 (2021) | SCADD, prematurity, perinatal hypoxia, psychom. retardation, cardiomyopathy |
| Family | Patient | Gene SLC22A12, URAT1 | Gene SLC2A9, GLUT9 |
|---|---|---|---|
| A | 1 | reference | N/A |
| 2 | reference | p.G25R, p.V282I, p.P350L | |
| B | 3 | reference | p.G25R, p.V282I |
| 4 | reference | p.G25R, p.V282I, p.R294H | |
| 5 | p.T467M | N/A | |
| C | 6 | p.T467M | p.G25R, p.V282I, p.R294H |
| 7 | p.T467M | p.G25R, p.V282I, p.R294H | |
| 8 | p.T467M | p.G25R, p.R294H | |
| D | 9 | reference | p.G25R, p.R294H, p.P350L |
| 10 | p.T467M | p.G25R, p.R294H, p.P350L | |
| E | 11 | p.T467M | p.G25R, p.V282I |
| 12 | reference | p.G25R | |
| F | 13 | reference | p.G25R, p.V282I, p.P350L |
| 14 | p.T467M | p.G25R, p.V282I, p.P350L | |
| 15 | p.L415_G417del | p.G25R, p.P350L | |
| G | 16 | reference | N/A |
| 17 | reference | p.P350L | |
| 18 | reference | p.G25R, p.R294H, p.P350L | |
| H | 19 | p.L415_G417del, p.T467M | p.R294H, p.P350L |
| 20 | p.T467M | N/A | |
| 21 | p.L415_G417del | N/A | |
| I | 22 | reference | p.G25R, p.V282I, p.P350L |
| 23 | reference | p.G25R, p.V282I, p.P350L | |
| 24 | reference | p.G25R, p.R294H, p.V282I, p.P350L | |
| J | 25 | p.L415_G417del, p.T467M | p.G25R, p.R294H, p.P350L |
| 26 | p.T467M | N/A | |
| K | 27 | p.T467M | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stiburkova, B.; Bohatá, J.; Pavelcová, K.; Tasic, V.; Plaseska-Karanfilska, D.; Cho, S.-K.; Potočnaková, L.; Šaligová, J. Renal Hypouricemia 1: Rare Disorder as Common Disease in Eastern Slovakia Roma Population. Biomedicines 2021, 9, 1607. https://doi.org/10.3390/biomedicines9111607
Stiburkova B, Bohatá J, Pavelcová K, Tasic V, Plaseska-Karanfilska D, Cho S-K, Potočnaková L, Šaligová J. Renal Hypouricemia 1: Rare Disorder as Common Disease in Eastern Slovakia Roma Population. Biomedicines. 2021; 9(11):1607. https://doi.org/10.3390/biomedicines9111607
Chicago/Turabian StyleStiburkova, Blanka, Jana Bohatá, Kateřina Pavelcová, Velibor Tasic, Dijana Plaseska-Karanfilska, Sung-Kweon Cho, Ludmila Potočnaková, and Jana Šaligová. 2021. "Renal Hypouricemia 1: Rare Disorder as Common Disease in Eastern Slovakia Roma Population" Biomedicines 9, no. 11: 1607. https://doi.org/10.3390/biomedicines9111607
APA StyleStiburkova, B., Bohatá, J., Pavelcová, K., Tasic, V., Plaseska-Karanfilska, D., Cho, S.-K., Potočnaková, L., & Šaligová, J. (2021). Renal Hypouricemia 1: Rare Disorder as Common Disease in Eastern Slovakia Roma Population. Biomedicines, 9(11), 1607. https://doi.org/10.3390/biomedicines9111607








