The Role of Ultrasound as a Diagnostic and Therapeutic Tool in Experimental Animal Models of Stroke: A Review
Abstract
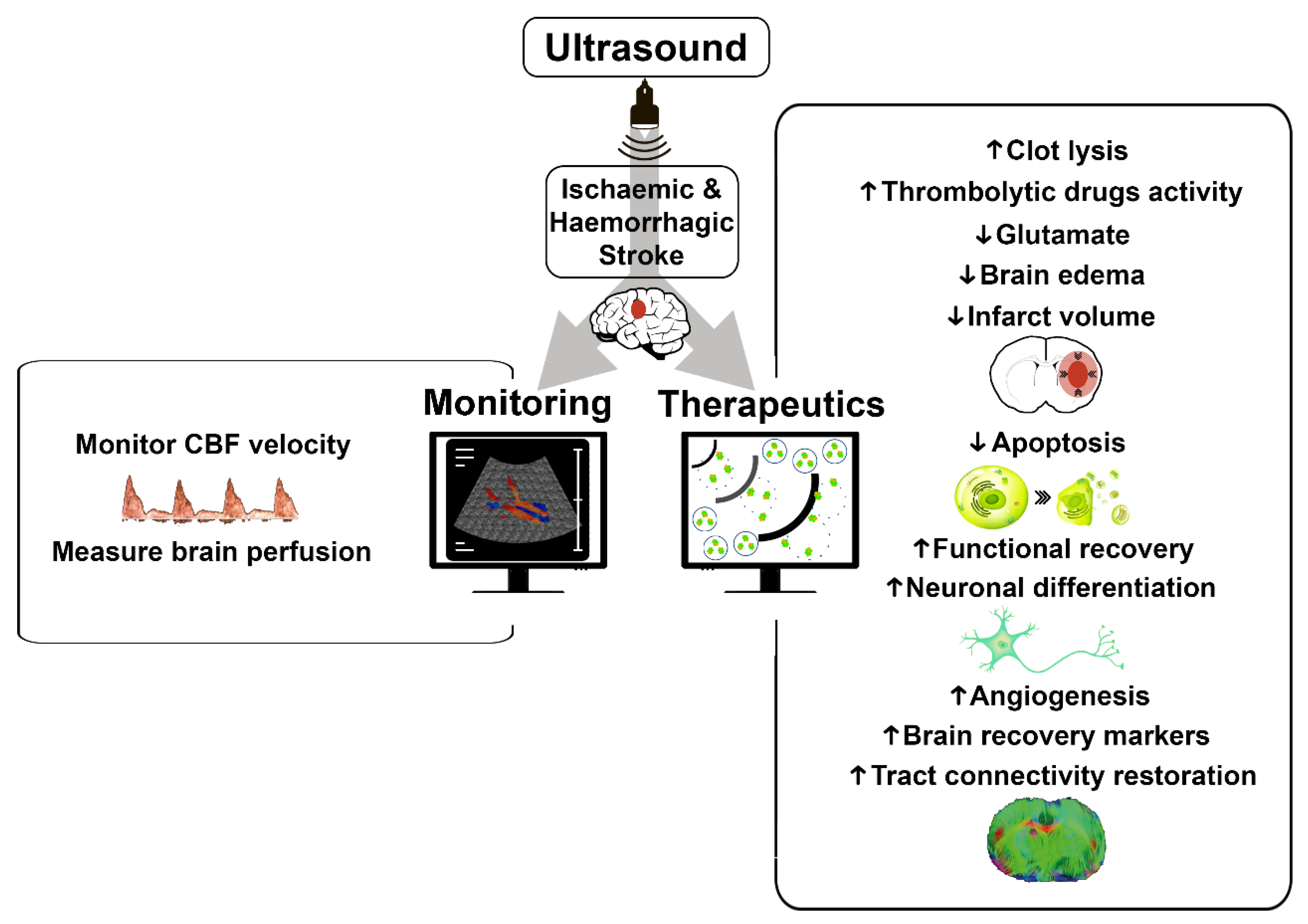
:1. Introduction
2. Ultrasound Applications in Ischemic Stroke
2.1. Ultrasound Monitoring in Ischemic Stroke
2.2. Therapeutic Ultrasound in Ischemic Stroke
3. Ultrasound Applications in Intracerebral Hemorrhage
3.1. Ultrasound Monitoring in Intracerebral Hemorrhage
3.2. Therapeutic Ultrasound in Intracerebral Hemorrhage
4. Safety of Ultrasound
4.1. Safety Data of Ultrasound in Monitoring Stroke
4.2. Safety Data of Therapeutic Ultrasound in Stroke
5. Limitations and Future of Ultrasound
5.1. Limitations of Ultrasound
5.2. Future of Ultrasound in Preclinical Models
5.2.1. New Approaches of Ultrasound
5.2.2. Imaging 3D/4D in Ultrasound
6. From Preclinical Models to Clinical Practice
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyer-Wiethe, K.; Sallustio, F.; Kern, R. Diagnosis of intracerebral hemorrhage with transcranial ultrasound. Cerebrovasc. Dis. 2009, 27, 40–47. [Google Scholar] [CrossRef]
- Greco, A.; Mancini, M.; Gargiulo, S.; Gramanzini, M.; Claudio, P.P.; Brunetti, A.; Salvatore, M. Ultrasound biomicroscopy in small animal research: Applications in molecular and preclinical imaging. J. Biomed. Biotechnol. 2012, 2012, 519238. [Google Scholar] [CrossRef]
- Neri, L.; Storti, E.; Lichtenstein, D. Toward an ultrasound curriculum for critical care medicine. Crit. Care Med. 2007, 35 (Suppl. S5), S290–S304. [Google Scholar] [CrossRef] [Green Version]
- Bobbia, X.; Zieleskiewicz, L.; Pradeilles, C.; Hudson, C.; Muller, L.; Claret, P.G.; Group, W.F. The clinical impact and prevalence of emergency point-of-care ultrasound: A prospective multicenter study. Anaesth. Crit. Care Pain Med. 2017, 36, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Weile, J.; Frederiksen, C.A.; Laursen, C.B.; Graumann, O.; Sloth, E.; Kirkegaard, H. Point-of-care ultrasound induced changes in management of unselected patients in the emergency department-a prospective single-blinded observational trial. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 47. [Google Scholar] [CrossRef]
- Puig, J.; Shankar, J.; Liebeskind, D.; Terceño, M.; Nael, K.; Demchuk, A.M.; Menon, B.; Dowlatshashi, D.; Leiva-Salinas, C.; Demchuk, A.M.; et al. From “Time is Brain” to “Imaging is Brain”: A Paradigm Shift in the Management of Acute Ischemic Stroke. J. Neuroimaging 2020, 30, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hua, Y.; Feng, W.; Ovbiagele, B. Multimodality ultrasound imaging in stroke: Current concepts and future focus. Expert Rev. Cardiovasc. Ther. 2016, 14, 1325–1333. [Google Scholar] [CrossRef]
- Ojaghihaghighi, S.; Vahdati, S.S.; Mikaeilpour, A.; Ramouz, A. Comparison of neurological clinical manifestation in patients with hemorrhagic and ischemic stroke. World J. Emerg. Med. 2017, 8, 34–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rink, C.; Khanna, S. Significance of brain tissue oxygenation and the arachidonic acid cascade in stroke. Antioxid. Redox Signal. 2011, 14, 1889–1903. [Google Scholar] [CrossRef] [Green Version]
- Prabhakaran, S.; Ruff, I.; Bernstein, R.A. Acute stroke intervention: A systematic review. JAMA 2015, 313, 1451–1462. [Google Scholar] [CrossRef]
- Els, T.; Daffertshofer, M.; Schroeck, H.; Kuschinsky, W.; Hennerici, M. Comparison of transcranial Doppler flow velocity and cerebral blood flow during focal ischemia in rabbits. Ultrasound Med. Biol. 1999, 25, 933–938. [Google Scholar] [CrossRef]
- Premilovac, D.; Blackwood, S.J.; Ramsay, C.J.; Keske, M.A.; Howells, D.W.; Sutherland, B.A. Transcranial contrast-enhanced ultrasound in the rat brain reveals substantial hyperperfusion acutely post-stroke. J. Cereb. Blood Flow Metab. 2020, 40, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Kreis, D.; Schulz, D.; Stein, M.; Preuss, M.; Nestler, U. Assessment of parameters influencing the blood flow velocities in cerebral arteries of the rat using ultrasonographic examination. Neurol. Res. 2011, 33, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Brunner, C.; Isabel, C.; Martin, A.; Dussaux, C.; Savoye, A.; Emmrich, J.; Montaldo, G.; Mas, J.L.; Baron, J.C.; Urban, A. Mapping the dynamics of brain perfusion using functional ultrasound in a rat model of transient middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 2017, 37, 263–276. [Google Scholar] [CrossRef]
- Dayton, P.A.; Rychak, J.J. Molecular ultrasound imaging using microbubble contrast agents. Front. Biosci. 2007, 12, 5124–5142. [Google Scholar] [CrossRef] [Green Version]
- Chong, W.K.; Papadopoulou, V.; Dayton, P.A. Imaging with ultrasound contrast agents: Current status and future. Abdom. Radiol. 2018, 43, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ke, Z.; Tong, K.Y.; Ying, M. Evaluation of Cerebral Blood Flow Changes in Focal Cerebral Ischemia Rats by Using Transcranial Doppler Ultrasonography. Ultrasound Med. Biol. 2010, 36, 595–603. [Google Scholar] [CrossRef]
- Bonnin, P.; Leger, P.L.; Deroide, N.; Fau, S.; Baud, O.; Pocard, M.; Charriaut-Marlangue, C.; Renolleau, S. Impact of intracranial blood-flow redistribution on stroke size during ischemia-reperfusion in 7-day-old rats. J. Neurosci. Methods 2011, 198, 103–109. [Google Scholar] [CrossRef]
- Brunner, C.; Korostelev, M.; Raja, S.; Montaldo, G.; Urban, A.; Baron, J.C. Evidence from functional ultrasound imaging of enhanced contralesional microvascular response to somatosensory stimulation in acute middle cerebral artery occlusion/reperfusion in rats: A marker of ultra-early network reorganization? J. Cereb. Blood Flow Metab. 2018, 38, 1690–1700. [Google Scholar] [CrossRef]
- Hingot, V.; Brodin, C.; Lebrun, F.; Heiles, B.; Chagnot, A.; Yetim, M.; Gauberti, M.; Orset, C.; Tanter, M.; Couture, O.; et al. Early Ultrafast Ultrasound Imaging of Cerebral Perfusion correlates with Ischemic Stroke outcomes and responses to treatment in Mice. Theranostics 2020, 10, 7480–7491. [Google Scholar] [CrossRef]
- Guo, T.; Li, H.; Lv, Y.; Lu, H.; Niu, J.; Sun, J.; Yang, G.Y.; Ren, C.; Tong, S. Pulsed transcranial ultrasound stimulation immediately after the ischemic brain injury is neuroprotective. IEEE Trans. Biomed. Engl. 2015, 62, 2352–2357. [Google Scholar] [CrossRef]
- Alexandrov, A.V.; Barlinn, K.; Strong, R.; Alexandrov, A.W.; Aronowski, J. Low-Power 2-MHz Pulsed-Wave Transcranial Ultrasound Reduces Ischemic Brain Damage in Rats. Transl. Stroke Res. 2011, 2, 376–381. [Google Scholar] [CrossRef]
- Chen, C.M.; Wu, C.T.; Yang, T.H.; Liu, S.H.; Yang, F.Y. Preventive Effect of Low Intensity Pulsed Ultrasound against Experimental Cerebral Ischemia/Reperfusion Injury via Apoptosis Reduction and Brain-derived Neurotrophic Factor Induction. Sci. Rep. 2018, 8, 5568. [Google Scholar] [CrossRef]
- Wu, C.T.; Yang, T.H.; Chen, M.C.; Chung, Y.P.; Guan, S.S.; Long, L.H.; Liu, S.H.; Chen, C.M. Low Intensity Pulsed Ultrasound Prevents Recurrent Ischemic Stroke in a Cerebral Ischemia/Reperfusion Injury Mouse Model via Brain-derived Neurotrophic Factor Induction. Int. J. Mol. Sci. 2019, 20, 5169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.E.; Kim, Y.M.; Jeong, J.S.; Seo, Y.K. The effect of ultrasound for increasing neural differentiation in hBM-MSCs and inducing neurogenesis in ischemic stroke model. Life Sci. 2016, 165, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Daffertshofer, M.; Huang, Z.; Fatar, M.; Popolo, M.; Schroeck, H.; Kuschinsky, W.; Moskowitz, M.A.; Hennerici, M.G. Efficacy of sonothrombolysis in a rat model of embolic ischemic stroke. Neurosci. Lett. 2004, 361, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.T.; Flores, R.; Hamilton, E.; Roberson, P.K.; Borrelli, M.J.; Culp, W.C. Microbubbles improve sonothrombolysis in vitro and decrease hemorrhage in vivo in a rabbit stroke model. Investig. Radiol. 2011, 46, 202–207. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Zhang, Y.; Wu, J.; Shi, W.T.; Lof, J.; Vignon, F.; Drvol, L.; Xie, F.; Muirhead, D.; Powers, J.E.; et al. Improvements in cerebral blood flow and recanalization rates with transcranial diagnostic ultrasound and intravenous microbubbles after acute cerebral emboli. Investig. Radiol. 2014, 49, 593–600. [Google Scholar] [CrossRef]
- Culp, W.C.; Flores, R.; Brown, A.T.; Lowery, J.D.; Roberson, P.K.; Hennings, L.J.; Woods, S.D.; Hatton, J.H.; Culp, B.C.; Skinner, R.D.; et al. Successful Microbubble Sonothrombolysis without Tissue Plasminogen Activator in a Rabbit Model of Acute Ischemic Stroke. Stroke 2011, 42, 2280–2285. [Google Scholar] [CrossRef] [PubMed]
- Fatar, M.; Stroick, M.; Griebe, M.; Alonso, A.; Kreisel, S.; Kern, R.; Hennerici, M.; Meairs, S. Effect of Combined Ultrasound and Microbubbles Treatment in an Experimental Model of Cerebral Ischemia. Ultrasound Med. Biol. 2008, 34, 1414–1420. [Google Scholar] [CrossRef]
- Schleicher, N.; Tomkins, A.J.; Kampschulte, M.; Hyvelin, J.M.; Botteron, C.; Juenemann, M.; Yeniguen, M.; Krombach, G.A.; Kaps, M.; Spratt, N.J.; et al. Sonothrombolysis with BR38 microbubbles improves microvascular patency in a rat model of stroke. PLoS ONE 2016, 11, e0152898. [Google Scholar] [CrossRef]
- Culp, W.C.; Porter, T.R.; Lowery, J.; Xie, F.; Roberson, P.K.; Marky, L. Intracranial clot lysis with intravenous microbubbles and transcranial ultrasound in swine. Stroke 2004, 35, 2407–2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Frutos, B.; Otero-Ortega, L.; Ramos-Cejudo, J.; Martínez-Sánchez, P.; Barahona-Sanz, I.; Navarro-Hernanz, T.; Gómez-de Frutos, M.C.; Díez-Tejedor, E.; Gutiérrez-Fernández, M. Enhanced brain-derived neurotrophic factor delivery by ultrasound and microbubbles promotes white matter repair after stroke. Biomaterials 2016, 100, 41–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.; Jiang, J.; Li, H.; Chen, M.; Liu, R.; Sun, S.; De, M.; Liang, X.; Wang, S. Phosphatidylserine-microbubble targeting-activated microglia/macrophage in inflammation combined with ultrasound for breaking through the blood-brain barrier. J. Neuroinflamm. 2018, 15, 334. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Yang, L.; Wu, J.; Sun, L.; Wu, J.; Tian, H.; Weisel, R.D.; Li, R.K. Reduced ischemic injury after stroke in mice by angiogenic gene delivery via ultrasound-targeted microbubble destruction. J. Neuropathol. Exp. Neurol. 2014, 73, 548–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, H.M.; Wang, L.Y. Effect of therapeutic ultrasound on brain angiogenesis following intracerebral hemorrhage in rats. Microvasc. Res. 2015, 102, 11–18. [Google Scholar] [CrossRef]
- Ke, Z.; Ying, M.; Li, L.; Zhang, S.; Tong, K.Y. Evaluation of transcranial Doppler flow velocity changes in intracerebral hemorrhage rats using ultrasonography. J. Neurosci. Methods 2012, 210, 272–280. [Google Scholar] [CrossRef]
- Stroick, M.; Alonso, A.; Fatar, M.; Griebe, M.; Kreisel, S.; Kern, R.; Gaud, E.; Arditi, M.; Hennerici, M.; Meairs, S. Effects of simultaneous application of ultrasound and microbubbles on intracerebral hemorrhage in an animal model. Ultrasound Med. Biol. 2006, 32, 1377–1382. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, L.; Feng, C.; Li, B.; Tang, J.; Liu, A.; Lv, F.; Li, T. Establishing an animal model of intracerebral hemorrhage under the guidance of ultrasound. Ultrasound Med. Biol. 2013, 39, 2116–2122. [Google Scholar] [CrossRef]
- Sobbe, A.; Stumpff, U.; Trübestein, G.; Figge, H.; Kozuschek, W. Die Ultraschall-Auflösung von Thromben. Klin. Wochenschr. 1974, 52, 1117–1121. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Alexandrov, A.V. Ultrasound-enhanced thrombolysis in acute ischemic stroke: Potential, failures, and safety. Neurotherapeutics 2007, 4, 420–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikulik, R.; Alexandrov, A.V. Acute stroke: Therapeutic transcranial Doppler sonography. Front. Neurol. Neurosci. 2006, 21, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.; Huang, Y.; Waspe, A.C.; Ganguly, M.; Goertz, D.E.; Hynynen, K. High-intensity focused ultrasound (HIFU) for dissolution of clots in a rabbit model of embolic stroke. PLoS ONE 2012, 7, e42311. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.A.; Alexandrov, A.V. Transcranial ultrasound in acute stroke: From diagnosis to therapy. Cerebrovasc. Dis. 2007, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Auboire, L.; Sennoga, C.A.; Hyvelin, J.M.; Ossant, F.; Escoffre, J.M.; Tranquart, F.; Bouakaz, A. Microbubbles combined with ultrasound therapy in ischemic stroke: A systematic review of in-vivo preclinical studies. PLoS ONE 2018, 13, e0191788. [Google Scholar] [CrossRef] [Green Version]
- Kiessling, F.; Fokong, S.; Koczera, P.; Lederle, W.; Lammers, T. Ultrasound microbubbles for molecular diagnosis, therapy, and theranostics. J. Nucl. Med. 2012, 53, 345–348. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Hwang, J.H. Ultrasound-targeted microbubble destruction for chemotherapeutic drug delivery to solid tumors. J. Ther. Ultrasound 2013, 1, 10. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.B.; Di Ianni, T.; Vyas, D.B.; Huang, Z.; Park, S.; Hosseini-Nassab, N.; Aryal, M.; Airan, R.D. Focused Ultrasound for Noninvasive, Focal Pharmacologic Neurointervention. Front. Neurosci. 2020, 14, 675. [Google Scholar] [CrossRef]
- Cammalleri, A.; Croce, P.; Lee, W.; Yoon, K.; Yoo, S.S. Therapeutic Potentials of Localized Blood–Brain Barrier Disruption by Noninvasive Transcranial Focused Ultrasound: A Technical Review. J. Clin. Neurophysiol. 2020, 37, 104–117. [Google Scholar] [CrossRef]
- Bakay, L.; Ballantine, J.R.; Hueter, T.F.; Sosa, D. Ultrasonically produced changes in the blood-brain barrier. AMA Arch. Neurol. Psychiatry 1956, 76, 457–467. [Google Scholar] [CrossRef]
- Pandit, R.; Koh, W.K.; Sullivan, R.K.; Palliyaguru, T.; Parton, R.G.; Götz, J. Role for caveolin-mediated transcytosis in facilitating transport of large cargoes into the brain via ultrasound. J. Control Release 2020, 327, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.Y.; Liu, Y.H.; Wang, P.; Xue, Y.X. Low-frequency ultrasound irradiation increases blood–tumor barrier permeability by transcellular pathway in a rat glioma model. J. Mol. Neurosci. 2012, 48, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A. Ultrasound-Induced Blood-Brain Barrier Opening for Drug Delivery. Front. Neurol. Neurosci. 2015, 36, 106–115. [Google Scholar] [CrossRef]
- Meairs, S. Facilitation of Drug Transport across the Blood–Brain Barrier with Ultrasound and Microbubbles Stephen Meairs. Pharmaceutics 2015, 7, 275–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escoffre, J.M.; de Senneville, B.D.; Sasaki, N.; Derieppe, M. Editorial: Bubbles, Droplets and Micelles for Acoustically-Mediated Drug/Gene Delivery. Front. Pharmacol. 2020, 11, 954. [Google Scholar] [CrossRef] [PubMed]
- Pérez, E.S.; Delgado-Mederos, R.; Rubiera, M.; Delgado, P.; Ribó, M.; Maisterra, O.; Ortega, G.; Alvarez-Sabin, J.; Molina, C.A. Transcranial duplex sonography for monitoring hyperacute intracerebral hemorrhage. Stroke 2009, 40, 987–990. [Google Scholar] [CrossRef] [Green Version]
- Saunders, N.R.; Dziegielewska, K.M.; Møllgård, K.; Habgood, M.D. Markers for blood-brain barrier integrity: How appropriate is Evans blue in the twenty-first century and what are the alternatives? Front. Neurosci. 2015, 9, 385. [Google Scholar] [CrossRef] [Green Version]
- Aryal, M.; Papademetriou, I.; Zhang, Y.Z.; Power, C.; McDannold, N.; Porter, T. MRI Monitoring and Quantification of Ultrasound-Mediated Delivery of Liposomes Dually Labeled with Gadolinium and Fluorophore through the Blood-Brain Barrier. Ultrasound Med. Biol. 2019, 45, 1733–1742. [Google Scholar] [CrossRef]
- Chen, D.; Nie, Z.B.; Chi, Z.H.; Wang, Z.Y.; Wei, X.T.; Guan, J.H. Neuroprotective Effect of ZnT3 Knockout on Subarachnoid Hemorrhage. Transl. Neurosci. 2018, 9, 26–32. [Google Scholar] [CrossRef]
- Lapergue, B.; Deroide, N.; Pocard, M.; Michel, J.-B.; Meilhac, O.; Bonnin, P. Transcranial duplex sonography for monitoring circle of Willis artery occlusion in a rat embolic stroke model. J. Neurosci. Methods 2011, 197, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Gómez-de Frutos, M.C.; García-Suárez, I.; Laso-García, F.; Diekhorst, L.; Otero-Ortega, L.; Alonso-López, E.; Díez-Tejedor, E.; Gutiérrez-Fernández, M.; Ruiz-Ares, G. Identification of brain structures and blood vessels by conventional ultrasound in rats. J. Neurosci. Methods 2020, 346, 108935. [Google Scholar] [CrossRef]
- Giustetto, P.; Filippi, M.; Castano, M.; Terreno, E. Non-invasive Parenchymal, Vascular and Metabolic High-frequency Ultrasound and Photoacoustic Rat Deep Brain Imaging. J. Vis. Exp. 2015, 2, 52162. [Google Scholar] [CrossRef] [Green Version]
- Riesz, P.; Kondo, T. Free radical formation induced by ultrasound and its biological implications. Free Radic. Biol. Med. 1992, 13, 247–270. [Google Scholar] [CrossRef]
- Karagöz, I.; Biri, A.; Babacan, F.; Kavutu, M. Evaluation of biological effects induced by diagnostic ultrasound in the rat foetal tissues. Mol. Cell Biochem. 2007, 294, 217–224. [Google Scholar] [CrossRef]
- Ter Haar, G. Ultrasonic imaging: Safety considerations. Interface Focus 2011, 1, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Dijkmans, P.A.; Juffermans, L.J.; Musters, R.J.; van Wamel, A.; ten Cate, F.J.; van Gilst, W.; Visser, C.A.; de Jong, N.; Kamp, O. Microbubbles and ultrasound: From diagnosis to therapy. Eur. J. Echocardiogr. 2004, 5, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Daffertshofer, M.; Hennerici, M. Ultrasound in the treatment of ischaemic stroke. Lancet Neurol 2003, 2, 283–290. [Google Scholar] [CrossRef]
- Vignon, F.; Shi, W.T.; Powers, J.E.; Everbach, E.C.; Liu, J.; Gao, S.; Xie, F.; Porter, T.R. Microbubble cavitation imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Burgess, A.; Shah, K.; Hough, O.; Hynynen, K. Focused ultrasound-mediated drug delivery through the blood-brain barrier. Rev. Neurother. 2015, 15, 477–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedelmann, M.; Ritschel, N.; Doenges, S.; Langheinrich, A.C.; Acker, T.; Reuter, P.; Yeniguen, M.; Pukropski, J.; Kaps, M.; Mueller, C.; et al. Combined contrast-enhanced ultrasound and rt-PA treatment is safe and improves impaired microcirculation after reperfusion of middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 2010, 30, 1712–1720. [Google Scholar] [CrossRef]
- Schneider, F.; Gerriets, T.; Walberer, M.; Mueller, C.; Rolke, R.; Eicke, B.M.; Bohl, J.; Kempski, O.; Kaps, M.; Bachmann, G.; et al. Brain edema and intracerebral necrosis caused by transcranial low-frequency 20-kHz ultrasound: A safety study in rats. Stroke 2006, 37, 1301–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuter, P.; Masomi, J.; Kuntze, H.; Fischer, I.; Helling, K.; Sommer, C.; Alessandri, B.; Heimann, A.; Gerriets, T.; Marx, J.; et al. Low-frequency therapeutic ultrasound with varied duty cycle: Effects on the ischemic brain and the inner ear. Ultrasound Med. Biol. 2010, 36, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.D.; Ullah, M.; Concepcion, W.; Dahl, J.J.; Thakor, A.S. The role of ultrasound in enhancing mesenchymal stromal cell-based therapies. Stem Cells Transl. Med. 2020, 9, 850–866. [Google Scholar] [CrossRef] [Green Version]
- Maresca, D.; Lakshmanan, A.; Abedi, M.; Bar-Zion, A.; Farhadi, A.; Lu, G.J.; Szablowski, J.O.; Wu, D.; Yoo, S.; Shapiro, M.G. Biomolecular Ultrasound and Sonogenetics. Annu. Rev. Chem. Biomol. Engl. 2018, 9, 229–252. [Google Scholar] [CrossRef] [Green Version]
- Macé, E.; Cohen, I.; Montaldo, G.; Miles, R.; Fink, M.; Tanter, M. In vivo mapping of brain elasticity in small animals using shear wave imaging. IEEE Trans. Med. Imaging 2011, 30, 550–558. [Google Scholar] [CrossRef]
- Macé, E.; Montaldo, G.; Cohen, I.; Baulac, M.; Fink, M.; Tanter, M. Functional ultrasound imaging of the brain. Nat. Methods 2011, 8, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.; Pham, B.; Zong, Y.; Perez, C.; Maris, D.O.; Hemphill, A.; Miao, C.H.; Matula, T.J.; Mourad, P.D.; Wei, H.; et al. Microbubbles and ultrasound increase intraventricular polyplex gene transfer to the brain. J. Control Release 2016, 231, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.; Sheng, Z.; Yan, F. Ultrasound-induced blood-brain-barrier opening enhances anticancer efficacy in the treatment of glioblastoma: Current status and future prospects. J. Oncol. 2019, 2019, 2345203. [Google Scholar] [CrossRef]
- Lee, J.; Chang, W.S.; Shin, J.; Seo, Y.; Kong, C.; Song, B.W.; Na, Y.C.; Kim, B.S.; Chang, J.W. Non-invasively enhanced intracranial transplantation of mesenchymal stem cells using focused ultrasound mediated by overexpression of cell-adhesion molecules. Stem Cell Res. 2020, 43, 101726. [Google Scholar] [CrossRef]
- Kwon, S.H.; Gopal, A.S. 3D and 4D Ultrasound: Current Progress and Future Perspectives. Curr. Cardiovasc. Imaging Rep. 2017, 10, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macé, É.; Montaldo, G.; Trenholm, S.; Cowan, C.; Brignall, A.; Urban, A.; Roska, B. Whole-Brain Functional Ultrasound Imaging Reveals Brain Modules for Visuomotor Integration. Neuron 2018, 100, 1241–1251.e7. [Google Scholar] [CrossRef] [Green Version]
- Gesnik, M.; Blaize, K.; Deffieux, T.; Gennisson, J.L.; Sahel, J.A.; Fink, M.; Picaud, S.; Tanter, M. 3D functional ultrasound imaging of the cerebral visual system in rodents. Neuroimage 2017, 149, 267–274. [Google Scholar] [CrossRef]
- Rau, R.; Kruizinga, P.; Mastik, F.; Belau, M.; de Jong, N.; Bosch, J.G.; Scheffer, W.; Maret, G. 3D functional ultrasound imaging of pigeons. Neuroimage 2018, 183, 469–477. [Google Scholar] [CrossRef]
- Bimbard, C.; Demene, C.; Girard, C.; Radtke-Chuller, S.; Shamma, S.; Tanter, M.; Boubenec, Y. Multi-scale mapping along the auditory hierarchy using high-resolution functional UltraSound in the awake ferret. eLife 2018, 7, e35028. [Google Scholar] [CrossRef] [PubMed]
- Demené, C.; Tiran, E.; Sieu, L.A.; Bergel, A.; Gennisson, J.L.; Pernot, M.; Deffieux, T.; Cohen, I.; Tanter, M. 4D microvascular imaging based on ultrafast Doppler tomography. Neuroimage 2016, 127, 472–483. [Google Scholar] [CrossRef]
- Rabut, C.; Correia, M.; Finel, V.; Pezet, S.; Pernot, M.; Deffieux, T.; Tanter, M. 4D functional ultrasound imaging of whole-brain activity in rodents. Nat. Methods 2019, 16, 994–997. [Google Scholar] [CrossRef]
- Vinciguerra, L.; Bösel, J. Noninvasive neuromonitoring: Current utility in subarachnoid hemorrhage, traumatic brain injury, and stroke. Neurocritical Care 2017, 27, 122–140. [Google Scholar] [CrossRef]
- Camps-Renom, P.; Méndez, J.; Granell, E.; Casoni, F.; Prats-Sánchez, L.; Martínez-Domeño, A.; Guisado-Alonso, D.; Martí-Fàbregas, J.; Delgado-Mederos, R. Transcranial duplex sonography predicts outcome following an intracerebral hemorrhage. AJNR Am. J. Neuroradiol. 2017, 38, 1543–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Du, H.; Song, Z.; Wang, H.; Tan, Z.; Xiao, M.; Zhang, F. Efficacy and safety of sonothrombolysis in patients with acute ischaemic stroke: A systematic review and meta-analysis. J. Neurol. Sci. 2020, 416, 116998. [Google Scholar] [CrossRef] [PubMed]
- Meairs, S.; Kern, R.; Alonso, A. Why and how do microbubbles enhance the effectiveness of diagnostic and therapeutic interventions in cerebrovascular disease? Curr. Pharm. Des. 2012, 18, 2223–2235. [Google Scholar] [CrossRef] [PubMed]
| ISCHEMIC STROKE | |||||||
|---|---|---|---|---|---|---|---|
| Reference | Sex/Species/n | Stroke type | Ultrasound | Frequency | Duration | Application | Findings |
| Els T. [11] | M/New Zealand rabbits/9 | MCAO | Transcranial Doppler | 4 MHz | Burst interval of 2 s | Monitoring: CBF velocity measurements | Decrease in CBF in the affected hemisphere |
| Premilova D. [12] | M/SD rats/16 | MCAO | Transcranial CEU | 15.2 Hz | 3 times, 60 s | Monitoring: CBF velocity measurements | Hyperemia poststroke |
| Brunner C. [14] | M/SD rats18 | MCAO | fUS | 15 MHz | 3 min | Monitoring: CBF velocity measurements | Mapping changes of CBF in stroke |
| Li L. [17] | M/SD rats/12 | MCAO | Transcranial Doppler ultrasonography | 6.3 MHz | -- | Monitoring: CBF velocity measurements | Redistribution of CBF after stroke |
| Bonnin P. [18] | M&F/Wistar rats/92 | MCAO | Colour Doppler | 12 MHz | 15 min | Monitoring: CBF velocity measurements | Collateral CBF protective role |
| Brunner C. [19] | M/SD rats/12 | MCAO | fUS | 1.43 MHz | 30 min (baseline), 90 min (occlusion period) and 90 min (after clip removal) | Monitoring: CBF velocity measurements | Contralesional CBF response after stroke |
| Hingot V. [20] | M/Swiss mice/19 | MCAO | Ultrafast | 500 Hz | -- | Monitoring: CBF measurements | Hypoperfusion in ischemic lesion after stroke |
| Guo T. [21] | M/SD rats/38 | MCAO | pTUS | 0.5 MHz | 60 min | Therapeutic: protective role of ultrasound | Promotion of CBF, decrease of ischemic lesion |
| Alexandrov AV. [22] | --/Long-Evans rats/32 | MCAO | Pulsed-wave transcranial | 2 MHz | 90 min | Therapeutic: effect on infarct volume | Reduction of ischemic brain damage and oedema Promotion of microcirculation |
| Chen CM. [23] | M/C57BL/6 mice/18 | MCAO | Low-intensity pulsed | 1 MHz | 15 min daily | Therapeutic: protective effect | Reduction of brain damage VEGF levels recovered Increase BDNF protein expression |
| WU CT. [24] | M/C57BL/6 mice/40 | MCAO | Low-intensity pulsed | 1 MHz | 15 min daily | Therapeutic: prevention of recurrent stroke | Reduction of lethality rate Prevent histopathological changes in brain |
| Cho SE. [25] | M/C57BL/6N mice/30 | Photothrombosis | -- | 0.04 MHz | 20 min/day | Therapeutic: evaluation of ultrasound effect in vitro and in vivo | Promotion of neuronal differentiation and neurogenesis |
| Daffertshofer M. [26] | --/Wistar & SD rats/47 | Embolic stroke | Low-intensity | 22.570 Hz | 1 h | Therapeutic: ultrasound with tPA | Reduction of infarct volume |
| Brown AT. [27] | --/New Zealand rabbits/-- | Emboli | -- | 1 MHz | 60 min | Therapeutic: ultrasound and MB with tPA | Significant clot lysis. Reduction of infarct volume and ICH |
| Gao S. [28] | --/Pigs/-- | Bilateral ICAO | Transcranial | 1.6 MHz | 5 and 20 µs | Therapeutic: ultrasound w/o MB | Improvement in CBF |
| Culp WC. [29] | --/New Zealand Rabbits/74 | Internal carotid embolization | Pulsed-wave | 1 MHz | 1 h | Therapeutic: ultrasound and MB or tPA | Reduction of infarct volume |
| Fatar M. [30] | M/Wistar rats/16 | MCAO | TCCD | 2 MHz | 30 min | Therapeutic: ultrasound and MB | Reduction of infarct volume |
| Schleicher N. [31] | M/Wistar rats/36 | MCAO | Transcranial | 3 MHz | 60 min | Therapeutic: ultrasound and MB with tPA | Improvement of microvascular patency |
| Culp WC. [32] | --/Pigs/15 | Autogenous thrombus | LFUS | 1 MHz | 24 min | Therapeutic: ultrasound and MB with glycoprotein | Augmented thrombolysis |
| Rodríguez-Frutos B. [33] | M/SD rats/143 | Subcortical stroke | UTMD | 7 MHz | 5 min | Therapeutic: ultrasound and MB with trophic factor | Functional recovery. Fiber connectivity restoration Increased brain marker expression |
| Zhao R. [34] | M/SD rats/30 | Intraluminal MCA blockage | UTMD | 1.03 MHz | 60 s | Therapeutic: ultrasound and MB with phosphatidylserine | Reduction in time of BBB opening Produced an early activated microglia/macrophage |
| Wang HB. [35] | F/CD1 mice/-- | Transient MCAO | UTMD | 1.6 MHz | 5 min | Therapeutic: ultrasound and MB with VEGF gene | Infarct areas and apoptosis reduction Increased vessel density |
| HEMORRHAGIC STROKE | |||||||
| Reference | Species | Stroke type | Ultrasound type | Frequency | Duration | Application | Findings |
| Mu HM. [36] | M/SD rats/280 | ICH | Therapeutic | 1 MHz | 15 min, once daily | Therapeutic: effects on brain angiogenesis | Increase expression of integrins and collagen Microvessels formation promotion Functional recovery |
| Ke Z. [37] | M/SD rats/18 | ICH | Transcranial Doppler | 4–13 MHz | -- | Monitoring: CBF velocity measurements | Flow velocity changes after stroke |
| Stroick M. [38] | M/Wistar rats/14 | ICH | Transcranial | 2 MHz | 30 min | Therapeutic: ultrasound and MB safety | Ultrasound + MB due not increase additional damage |
| Zhou X. [39] | M/Dogs/12 | ICH | Contrast-enhanced ultrasound | 7 MHz | Every 30 min until hematoma formed | Monitoring: hematoma visualization | Brain hemorrhage imaging |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-de Frutos, M.C.; Laso-García, F.; García-Suárez, I.; Diekhorst, L.; Otero-Ortega, L.; Alonso de Leciñana, M.; Fuentes, B.; Piniella, D.; Ruiz-Ares, G.; Díez-Tejedor, E.; et al. The Role of Ultrasound as a Diagnostic and Therapeutic Tool in Experimental Animal Models of Stroke: A Review. Biomedicines 2021, 9, 1609. https://doi.org/10.3390/biomedicines9111609
Gómez-de Frutos MC, Laso-García F, García-Suárez I, Diekhorst L, Otero-Ortega L, Alonso de Leciñana M, Fuentes B, Piniella D, Ruiz-Ares G, Díez-Tejedor E, et al. The Role of Ultrasound as a Diagnostic and Therapeutic Tool in Experimental Animal Models of Stroke: A Review. Biomedicines. 2021; 9(11):1609. https://doi.org/10.3390/biomedicines9111609
Chicago/Turabian StyleGómez-de Frutos, Mari Carmen, Fernando Laso-García, Iván García-Suárez, Luke Diekhorst, Laura Otero-Ortega, María Alonso de Leciñana, Blanca Fuentes, Dolores Piniella, Gerardo Ruiz-Ares, Exuperio Díez-Tejedor, and et al. 2021. "The Role of Ultrasound as a Diagnostic and Therapeutic Tool in Experimental Animal Models of Stroke: A Review" Biomedicines 9, no. 11: 1609. https://doi.org/10.3390/biomedicines9111609
APA StyleGómez-de Frutos, M. C., Laso-García, F., García-Suárez, I., Diekhorst, L., Otero-Ortega, L., Alonso de Leciñana, M., Fuentes, B., Piniella, D., Ruiz-Ares, G., Díez-Tejedor, E., & Gutiérrez-Fernández, M. (2021). The Role of Ultrasound as a Diagnostic and Therapeutic Tool in Experimental Animal Models of Stroke: A Review. Biomedicines, 9(11), 1609. https://doi.org/10.3390/biomedicines9111609









