Mesenchymal Stem Cells Influence Activation of Hepatic Stellate Cells, and Constitute a Promising Therapy for Liver Fibrosis
Abstract
1. Introduction
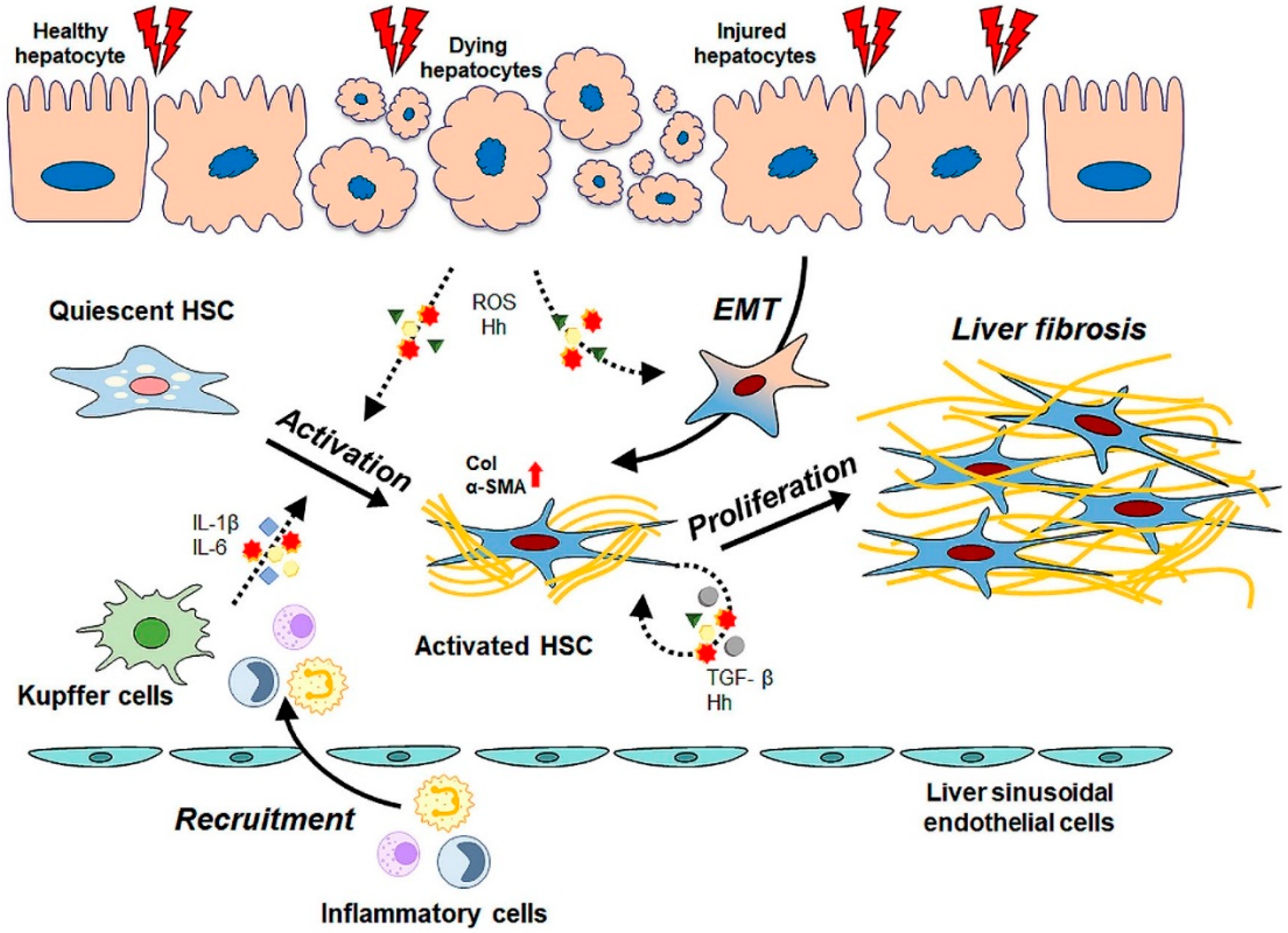
2. Pathogenesis of Liver Fibrosis and Activation of HSCs
3. Basic Information of MSCs Focusing on Therapeutic Potential
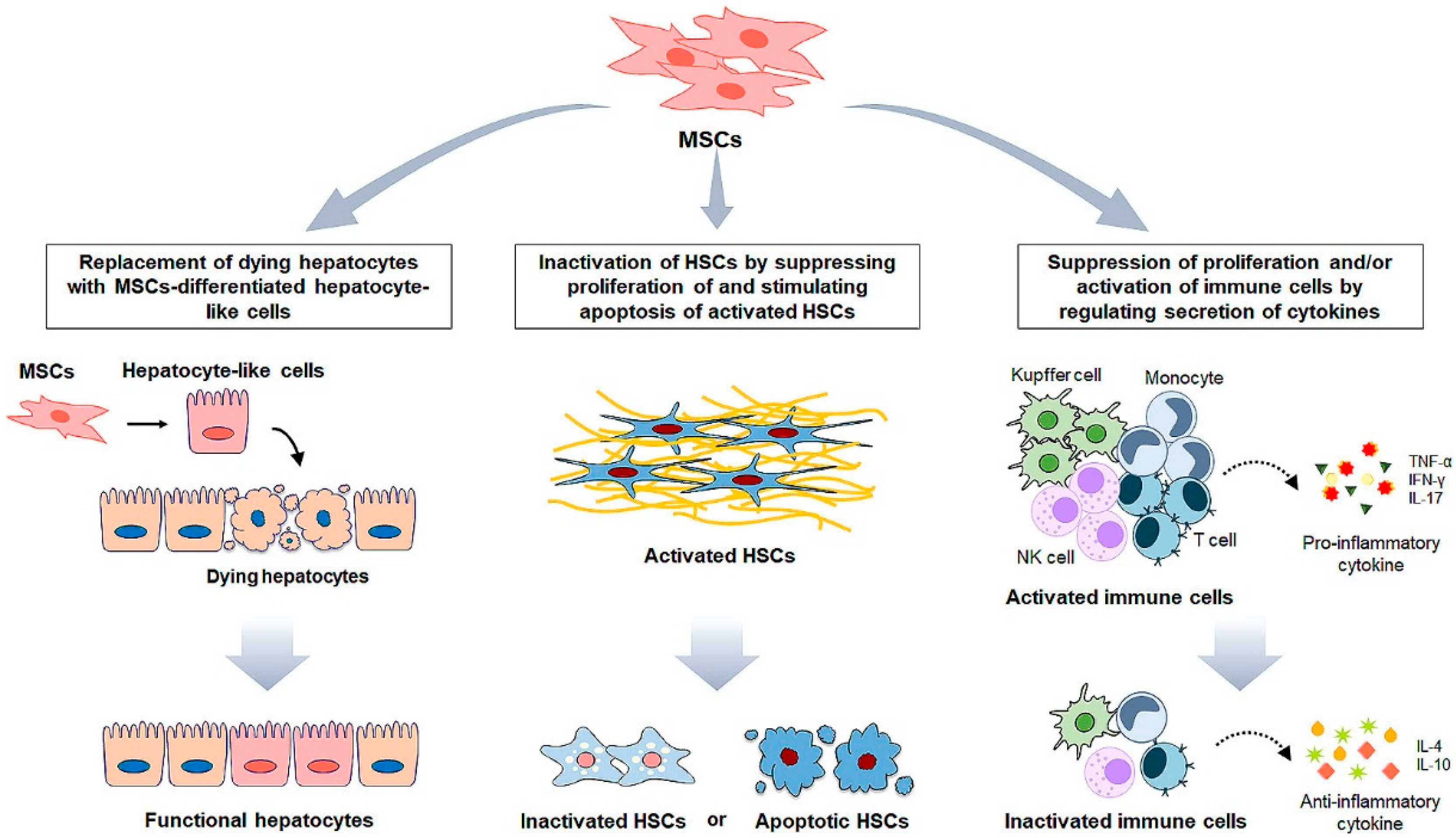
4. MSC Administration for Liver Fibrosis
5. MSCs-Based Clinical Application for Liver Disease
6. MSC Cell-Free Therapy for Liver Fibrosis
| Type | Treatment | Target/Experimental Model | Mechanism | Outcome | Ref |
|---|---|---|---|---|---|
| In vitro | MSC-CM | HSCs | Reducing expressions of pro-fibrotic genes | Inhibition of HSCs activation | [131] |
| Cytokine, TSG-6 | HSCs | Decreasing HSC activation Promoting the transdifferentiation of activated HSCs into stem-like cells | Amelioration of liver fibrosis | [136] | |
| MSC-exosomes | HSCs | Inhibiting Wnt/β-catenin signaling | Inhibition of HSC activation | [144] | |
| Co-culture with miR-125b manipulated MSCs | LX2 | Targeting Smo; suppression of Hh signaling | Inhibition of HSC activation | [149] | |
| In vivo | MSC-CM | CCl4-induced liver fibrosis in mice | Reducing collagen accumulation and inflammation Elevating hepatocyte survival | Amelioration of liver fibrosis | [132] |
| Cytokine, TSG-6 | CCl4-induced liver fibrosis in mice | Inducing M2 polarization Upregulating MMP12 expression in macrophages | Amelioration of liver fibrosis | [137] | |
| Cytokine, MFGE 8 | TAA or CCl4-induced liver fibrosis in mice | Downregulating the expression of TGFβR1 of HSC | Amelioration of liver fibrosis | [138] | |
| MSC-exosomes | CCl4-induced liver fibrosis in mice | Reducing hepatocyte death Decreasing oxidative stress | Amelioration of liver fibrosis | [142] | |
| CCl4-induced liver fibrosis in mice | Inactivating TGF-β/SMAD signaling pathway | Amelioration of liver fibrosis | [143] | ||
| CCl4-induced liver fibrosis in rats | Suppressing Wnt/β-catenin signaling | Improvement of liver function | [144] | ||
| GPX1 delivered by the MSC-exosomes | CCl4-induced liver fibrosis in mice | Decreasing oxidative stress Increasing hepatocyte proliferation | Amelioration of liver fibrosis | [145] | |
| miR-150-5p contained in MSC-exosomes | CCl4-induced liver fibrosis in mice | Reducing the expression of CXCL-1 | Amelioration of liver fibrosis | [14] | |
| miR-1246 contained in MSC-exosomes | ischemia/reperfusion-induced liver injury in mice | Modulating the balance of regulatory T cells and Th17 cells | Protection of hepatocytes Suppression of inflammation | [147] | |
| miR-486-5p contained in MSC-exosomes | CCl4-induced liver fibrosis in mice | Suppressing Hh signaling | Amelioration of liver fibrosis | [148,149] | |
| miR-125b contained in MSC-exosomes | CCl4-induced liver fibrosis in rats |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Thrift, A.P.; El-Serag, H.B.; Kanwal, F. Global epidemiology and burden of HCV infection and HCV-related disease. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Shield, K.D. Global Burden of Alcohol Use Disorders and Alcohol Liver Disease. Biomedicines 2019, 7, 99. [Google Scholar] [CrossRef]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Marcellin, P.; Kutala, B.K. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018, 38, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Mokdad, A.A.; Lopez, A.D.; Shahraz, S.; Lozano, R.; Mokdad, A.H.; Stanaway, J.; Murray, C.J.; Naghavi, M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: A systematic analysis. BMC Med. 2014, 12, 145. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Pinzani, M.; Rombouts, K. Liver fibrosis: From the bench to clinical targets. Dig. Liver Dis. 2004, 36, 231–242. [Google Scholar] [CrossRef]
- Iredale, J.P. Models of liver fibrosis: Exploring the dynamic nature of inflammation and repair in a solid organ. J. Clin. Investig. 2007, 117, 539–548. [Google Scholar] [CrossRef]
- Ishibashi, H.; Nakamura, M.; Komori, A.; Migita, K.; Shimoda, S. Liver architecture, cell function, and disease. Semin. Immunopathol. 2009, 31, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D.; Afdhal, N.H. Liver cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef]
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2016, 2, 16018. [Google Scholar] [CrossRef] [PubMed]
- Affo, S.; Yu, L.X.; Schwabe, R.F. The Role of Cancer-Associated Fibroblasts and Fibrosis in Liver Cancer. Annu. Rev. Pathol. 2017, 12, 153–186. [Google Scholar] [CrossRef] [PubMed]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Friedman, S.L. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008, 134, 1655–1669. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef] [PubMed]
- da Meirelles, L.S.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef]
- Kolf, C.M.; Cho, E.; Tuan, R.S. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: Regulation of niche, self-renewal and differentiation. Arthritis Res. Ther. 2007, 9, 204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ankrum, J.; Karp, J.M. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol. Med. 2010, 16, 203–209. [Google Scholar] [CrossRef]
- He, A.; Jiang, Y.; Gui, C.; Sun, Y.; Li, J.; Wang, J.A. The antiapoptotic effect of mesenchymal stem cell transplantation on ischemic myocardium is enhanced by anoxic preconditioning. Can. J. Cardiol. 2009, 25, 353–358. [Google Scholar] [CrossRef]
- Nauta, A.J.; Fibbe, W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007, 110, 3499–3506. [Google Scholar] [CrossRef]
- Staff, N.P.; Jones, D.T.; Singer, W. Mesenchymal Stromal Cell Therapies for Neurodegenerative Diseases. Mayo Clin. Proc. 2019, 94, 892–905. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Hao, H.; Liu, J.; Li, Y.; Han, W.; Mu, Y. Mesenchymal stem cell therapy in type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2017, 9, 36. [Google Scholar] [CrossRef]
- Vija, L.; Farge, D.; Gautier, J.F.; Vexiau, P.; Dumitrache, C.; Bourgarit, A.; Verrecchia, F.; Larghero, J. Mesenchymal stem cells: Stem cell therapy perspectives for type 1 diabetes. Diabetes Metab. 2009, 35, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Morimatsu, M.; Feng, T.; Lan, F.; Chang, D.; Wan, F.; Ling, Y. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res. Ther. 2020, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.O.; Kim, Y.J.; Baik, S.K.; Kim, M.Y.; Eom, Y.W.; Cho, M.Y.; Park, H.J.; Park, S.Y.; Kim, B.R.; Kim, J.W.; et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: A pilot study. Liver Int. 2014, 34, 33–41. [Google Scholar] [CrossRef]
- Yao, Y.; Xia, Z.; Cheng, F.; Jang, Q.; He, J.; Pan, C.; Zhang, L.; Ye, Y.; Wang, Y.; Chen, S.; et al. Human placental mesenchymal stem cells ameliorate liver fibrosis in mice by upregulation of Caveolin1 in hepatic stellate cells. Stem Cell Res. Ther. 2021, 12, 294. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, M.J.; Gao, B. Hepatocytes: A key cell type for innate immunity. Cell. Mol. Immunol. 2016, 13, 301–315. [Google Scholar] [CrossRef]
- Tu, T.; Calabro, S.R.; Lee, A.; Maczurek, A.E.; Budzinska, M.A.; Warner, F.J.; McLennan, S.V.; Shackel, N.A. Hepatocytes in liver injury: Victim, bystander, or accomplice in progressive fibrosis? J. Gastroenterol. Hepatol. 2015, 30, 1696–1704. [Google Scholar] [CrossRef]
- Malhi, H.; Guicciardi, M.E.; Gores, G.J. Hepatocyte death: A clear and present danger. Physiol. Rev. 2010, 90, 1165–1194. [Google Scholar] [CrossRef]
- Atzori, L.; Poli, G.; Perra, A. Hepatic stellate cell: A star cell in the liver. Int. J. Biochem. Cell Biol. 2009, 41, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Blaner, W.S.; O’Byrne, S.M.; Wongsiriroj, N.; Kluwe, J.; D’Ambrosio, D.M.; Jiang, H.; Schwabe, R.F.; Hillman, E.M.; Piantedosi, R.; Libien, J. Hepatic stellate cell lipid droplets: A specialized lipid droplet for retinoid storage. Biochim. Biophys. Acta 2009, 1791, 467–473. [Google Scholar] [CrossRef]
- Neubauer, K.; Knittel, T.; Aurisch, S.; Fellmer, P.; Ramadori, G. Glial fibrillary acidic protein--a cell type specific marker for Ito cells in vivo and in vitro. J. Hepatol. 1996, 24, 719–730. [Google Scholar] [CrossRef]
- Reeves, H.L.; Friedman, S.L. Activation of hepatic stellate cells—A key issue in liver fibrosis. Front. Biosci. 2002, 7, d808–d826. [Google Scholar] [CrossRef]
- Sanchez-Valle, V.; Chavez-Tapia, N.C.; Uribe, M.; Mendez-Sanchez, N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef]
- Li, J.; Fan, R.; Zhao, S.; Liu, L.; Guo, S.; Wu, N.; Zhang, W.; Chen, P. Reactive oxygen species released from hypoxic hepatocytes regulates MMP-2 expression in hepatic stellate cells. Int. J. Mol. Sci. 2011, 12, 2434–2447. [Google Scholar] [CrossRef]
- Friedman, S.L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 2000, 275, 2247–2250. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, C.; Zhou, D.; Zhang, L. TGF-beta/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J. Histochem. Cytochem. 2016, 64, 157–167. [Google Scholar] [CrossRef]
- Machado, M.V.; Diehl, A.M. Hedgehog signalling in liver pathophysiology. J. Hepatol. 2018, 68, 550–562. [Google Scholar] [CrossRef]
- Borkham-Kamphorst, E.; Weiskirchen, R. The PDGF system and its antagonists in liver fibrosis. Cytokine Growth Factor Rev. 2016, 28, 53–61. [Google Scholar] [CrossRef]
- Hellerbrand, C.; Stefanovic, B.; Giordano, F.; Burchardt, E.R.; Brenner, D.A. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. J. Hepatol. 1999, 30, 77–87. [Google Scholar] [CrossRef]
- Kondou, H.; Mushiake, S.; Etani, Y.; Miyoshi, Y.; Michigami, T.; Ozono, K. A blocking peptide for transforming growth factor-beta1 activation prevents hepatic fibrosis in vivo. J. Hepatol. 2003, 39, 742–748. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, N.; Ping, J.; Xu, L. TGFbeta1induced autophagy activates hepatic stellate cells via the ERK and JNK signaling pathways. Int. J. Mol. Med. 2021, 47, 256–266. [Google Scholar] [CrossRef]
- Syn, W.K.; Witek, R.P.; Curbishley, S.M.; Jung, Y.; Choi, S.S.; Enrich, B.; Omenetti, A.; Agboola, K.M.; Fearing, C.M.; Tilg, H.; et al. Role for hedgehog pathway in regulating growth and function of invariant NKT cells. Eur. J. Immunol. 2009, 39, 1879–1892. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Song, K.; Han, C.; Chen, W.; Wang, Y.; Dash, S.; Lim, K.; Wu, T. Inhibition of hedgehog signaling ameliorates hepatic inflammation in mice with nonalcoholic fatty liver disease. Hepatology 2016, 63, 1155–1169. [Google Scholar] [CrossRef] [PubMed]
- Sicklick, J.K.; Li, Y.X.; Choi, S.S.; Qi, Y.; Chen, W.; Bustamante, M.; Huang, J.; Zdanowicz, M.; Camp, T.; Torbenson, M.S.; et al. Role for hedgehog signaling in hepatic stellate cell activation and viability. Lab. Investig. 2005, 85, 1368–1380. [Google Scholar] [CrossRef]
- Jung, Y.; McCall, S.J.; Li, Y.X.; Diehl, A.M. Bile ductules and stromal cells express hedgehog ligands and/or hedgehog target genes in primary biliary cirrhosis. Hepatology 2007, 45, 1091–1096. [Google Scholar] [CrossRef]
- Carballo, G.B.; Honorato, J.R.; de Lopes, G.P.F.; Spohr, T. A highlight on Sonic hedgehog pathway. Cell Commun. Signal. 2018, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hyun, J.; Wang, S.; Lee, C.; Jung, Y. MicroRNA-378 is involved in hedgehog-driven epithelial-to-mesenchymal transition in hepatocytes of regenerating liver. Cell Death Dis. 2018, 9, 721. [Google Scholar] [CrossRef]
- Zeisberg, M.; Yang, C.; Martino, M.; Duncan, M.B.; Rieder, F.; Tanjore, H.; Kalluri, R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J. Biol. Chem. 2007, 282, 23337–23347. [Google Scholar] [CrossRef]
- Jung, Y.; Brown, K.D.; Witek, R.P.; Omenetti, A.; Yang, L.; Vandongen, M.; Milton, R.J.; Hines, I.N.; Rippe, R.A.; Spahr, L.; et al. Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology 2008, 134, 1532–1543. [Google Scholar] [CrossRef]
- Jung, Y.; Witek, R.P.; Syn, W.K.; Choi, S.S.; Omenetti, A.; Premont, R.; Guy, C.D.; Diehl, A.M. Signals from dying hepatocytes trigger growth of liver progenitors. Gut 2010, 59, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Omenetti, A.; Choi, S.; Michelotti, G.; Diehl, A.M. Hedgehog signaling in the liver. J. Hepatol. 2011, 54, 366–373. [Google Scholar] [CrossRef]
- Chung, S.I.; Moon, H.; Ju, H.L.; Cho, K.J.; Kim, D.Y.; Han, K.H.; Eun, J.W.; Nam, S.W.; Ribback, S.; Dombrowski, F.; et al. Hepatic expression of Sonic Hedgehog induces liver fibrosis and promotes hepatocarcinogenesis in a transgenic mouse model. J. Hepatol. 2016, 64, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Macrin, D.; Joseph, J.P.; Pillai, A.A.; Devi, A. Eminent Sources of Adult Mesenchymal Stem Cells and Their Therapeutic Imminence. Stem Cell Rev. Rep. 2017, 13, 741–756. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Jeong, J.A.; Gang, E.J.; Hong, S.H.; Hwang, S.H.; Kim, S.W.; Yang, I.H.; Ahn, C.; Han, H.; Kim, H. Rapid neural differentiation of human cord blood-derived mesenchymal stem cells. Neuroreport 2004, 15, 1731–1734. [Google Scholar] [CrossRef] [PubMed]
- Beier, J.P.; Bitto, F.F.; Lange, C.; Klumpp, D.; Arkudas, A.; Bleiziffer, O.; Boos, A.M.; Horch, R.E.; Kneser, U. Myogenic differentiation of mesenchymal stem cells co-cultured with primary myoblasts. Cell Biol. Int. 2011, 35, 397–406. [Google Scholar] [CrossRef]
- Hong, S.H.; Gang, E.J.; Jeong, J.A.; Ahn, C.; Hwang, S.H.; Yang, I.H.; Park, H.K.; Han, H.; Kim, H. In Vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem. Biophys. Res. Commun. 2005, 330, 1153–1161. [Google Scholar] [CrossRef]
- Kingham, E.; Oreffo, R.O. Embryonic and induced pluripotent stem cells: Understanding, creating, and exploiting the nano-niche for regenerative medicine. ACS Nano 2013, 7, 1867–1881. [Google Scholar] [CrossRef] [PubMed]
- de Machado, C.V.; Telles, P.D.; Nascimento, I.L. Immunological characteristics of mesenchymal stem cells. Rev. Bras. Hematol. Hemoter. 2013, 35, 62–67. [Google Scholar] [CrossRef]
- Ding, H.R.; Wang, J.L.; Tang, Z.T.; Wang, Y.; Zhou, G.; Liu, Y.; Ren, H.Z.; Shi, X.L. Mesenchymal Stem Cells Improve Glycometabolism and Liver Regeneration in the Treatment of Post-hepatectomy Liver Failure. Front. Physiol. 2019, 10, 412. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Li, J.; Wang, J.; Wang, X.; Zhang, Y.; Yuan, X.; Zhu, W.; Shi, X. Mesenchymal Stem Cells Enhance Liver Regeneration via Improving Lipid Accumulation and Hippo Signaling. Stem Cells Int. 2018, 2018, 7652359. [Google Scholar] [CrossRef]
- Saidi, R.F.; Rajeshkumar, B.; Shariftabrizi, A.; Bogdanov, A.A.; Zheng, S.; Dresser, K.; Walter, O. Human adipose-derived mesenchymal stem cells attenuate liver ischemia-reperfusion injury and promote liver regeneration. Surgery 2014, 156, 1225–1231. [Google Scholar] [CrossRef]
- Li, B.; Cheng, Y.; Yu, S.; Zang, L.; Yin, Y.; Liu, J.; Zhang, L.; Mu, Y. Human Umbilical Cord-Derived Mesenchymal Stem Cell Therapy Ameliorates Nonalcoholic Fatty Liver Disease in Obese Type 2 Diabetic Mice. Stem Cells Int. 2019, 2019, 8628027. [Google Scholar] [CrossRef]
- Park, M.; Kim, Y.H.; Woo, S.Y.; Lee, H.J.; Yu, Y.; Kim, H.S.; Park, Y.S.; Jo, I.; Park, J.W.; Jung, S.C.; et al. Tonsil-derived mesenchymal stem cells ameliorate CCl4-induced liver fibrosis in mice via autophagy activation. Sci. Rep. 2015, 5, 8616. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, L.; Lin, N.; Pan, W.; Hu, K.; Xu, R. Activation of Notch1 signaling by marrow-derived mesenchymal stem cells through cell-cell contact inhibits proliferation of hepatic stellate cells. Life Sci. 2011, 89, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bian, C.; Liao, L.; Zhu, Y.; Li, J.; Zeng, L.; Zhao, R.C. Inhibition of hepatic stellate cells proliferation by mesenchymal stem cells and the possible mechanisms. Hepatol. Res. 2009, 39, 1219–1228. [Google Scholar] [CrossRef]
- Qiao, H.; Zhou, Y.; Qin, X.; Cheng, J.; He, Y.; Jiang, Y. NADPH Oxidase Signaling Pathway Mediates Mesenchymal Stem Cell-Induced Inhibition of Hepatic Stellate Cell Activation. Stem Cells Int. 2018, 2018, 1239143. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Hu, K.; Chen, S.; Xie, S.; Tang, Z.; Lin, J.; Xu, R. Nerve growth factor-mediated paracrine regulation of hepatic stellate cells by multipotent mesenchymal stromal cells. Life Sci. 2009, 85, 291–295. [Google Scholar] [CrossRef]
- Wang, P.P.; Xie, D.Y.; Liang, X.J.; Peng, L.; Zhang, G.L.; Ye, Y.N.; Xie, C.; Gao, Z.L. HGF and direct mesenchymal stem cells contact synergize to inhibit hepatic stellate cells activation through TLR4/NF-kB pathway. PLoS ONE 2012, 7, e43408. [Google Scholar] [CrossRef]
- Yu, F.; Ji, S.; Su, L.; Wan, L.; Zhang, S.; Dai, C.; Wang, Y.; Fu, J.; Zhang, Q. Adipose-derived mesenchymal stem cells inhibit activation of hepatic stellate cells in vitro and ameliorate rat liver fibrosis In Vivo. J. Formos. Med. Assoc. 2015, 114, 130–138. [Google Scholar] [CrossRef]
- Abdi, R.; Fiorina, P.; Adra, C.N.; Atkinson, M.; Sayegh, M.H. Immunomodulation by mesenchymal stem cells: A potential therapeutic strategy for type 1 diabetes. Diabetes 2008, 57, 1759–1767. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Z.F.; Zhang, J.Y. Immunomodulatory role of mesenchymal stem cells in Alzheimer’s disease. Life Sci. 2020, 246, 117405. [Google Scholar] [CrossRef]
- Nagaishi, K.; Arimura, Y.; Fujimiya, M. Stem cell therapy for inflammatory bowel disease. J. Gastroenterol. 2015, 50, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, J.; Davies, J.E.; Stanford, W.L. Concise Review: Musculoskeletal Stem Cells to Treat Age-Related Osteoporosis. Stem Cells Transl. Med. 2017, 6, 1930–1939. [Google Scholar] [CrossRef]
- Kang, S.H.; Kim, M.Y.; Eom, Y.W.; Baik, S.K. Mesenchymal Stem Cells for the Treatment of Liver Disease: Present and Perspectives. Gut Liver 2020, 14, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Chai, N.L.; Zhang, X.B.; Chen, S.W.; Fan, K.X.; Linghu, E.Q. Umbilical cord-derived mesenchymal stem cells alleviate liver fibrosis in rats. World J. Gastroenterol. 2016, 22, 6036–6048. [Google Scholar] [CrossRef]
- Kyurkchiev, D.; Bochev, I.; Ivanova-Todorova, E.; Mourdjeva, M.; Oreshkova, T.; Belemezova, K.; Kyurkchiev, S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells 2014, 6, 552–570. [Google Scholar] [CrossRef]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Gazdic, M.; Arsenijevic, A.; Markovic, B.S.; Volarevic, A.; Dimova, I.; Djonov, V.; Arsenijevic, N.; Stojkovic, M.; Volarevic, V. Mesenchymal Stem Cell-Dependent Modulation of Liver Diseases. Int. J. Biol. Sci. 2017, 13, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Milosavljevic, N.; Gazdic, M.; Markovic, B.S.; Arsenijevic, A.; Nurkovic, J.; Dolicanin, Z.; Jovicic, N.; Jeftic, I.; Djonov, V.; Arsenijevic, N.; et al. Mesenchymal stem cells attenuate liver fibrosis by suppressing Th17 cells—An experimental study. Transpl. Int. 2018, 31, 102–115. [Google Scholar] [CrossRef]
- Minakuchi, R.; Wacholtz, M.C.; Davis, L.S.; Lipsky, P.E. Delineation of the mechanism of inhibition of human T cell activation by PGE2. J. Immunol. 1990, 145, 2616–2625. [Google Scholar]
- Qiu, G.; Zheng, G.; Ge, M.; Huang, L.; Tong, H.; Chen, P.; Lai, D.; Hu, Y.; Cheng, B.; Shu, Q.; et al. Adipose-derived mesenchymal stem cells modulate CD14(++)CD16(+) expression on monocytes from sepsis patients in vitro via prostaglandin E2. Stem Cell Res. Ther. 2017, 8, 97. [Google Scholar] [CrossRef]
- Spaggiari, G.M.; Capobianco, A.; Abdelrazik, H.; Becchetti, F.; Mingari, M.C.; Moretta, L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 2008, 111, 1327–1333. [Google Scholar] [CrossRef]
- Goss, J.A.; Mangino, M.J.; Callery, M.P.; Flye, M.W. Prostaglandin E2 downregulates Kupffer cell production of IL-1 and IL-6 during hepatic regeneration. Am. J. Physiol. 1993, 264, G601–G608. [Google Scholar] [CrossRef]
- Milosavljevic, N.; Gazdic, M.; Markovic, B.S.; Arsenijevic, A.; Nurkovic, J.; Dolicanin, Z.; Djonov, V.; Lukic, M.L.; Volarevic, V. Mesenchymal stem cells attenuate acute liver injury by altering ratio between interleukin 17 producing and regulatory natural killer T cells. Liver Transpl. 2017, 23, 1040–1050. [Google Scholar] [CrossRef]
- Chivu, M.; Dima, S.O.; Stancu, C.I.; Dobrea, C.; Uscatescu, V.; Necula, L.G.; Bleotu, C.; Tanase, C.; Albulescu, R.; Ardeleanu, C.; et al. In vitro hepatic differentiation of human bone marrow mesenchymal stem cells under differential exposure to liver-specific factors. Transl. Res. 2009, 154, 122–132. [Google Scholar] [CrossRef]
- Kang, X.Q.; Zang, W.J.; Song, T.S.; Xu, X.L.; Yu, X.J.; Li, D.L.; Meng, K.W.; Wu, S.L.; Zhao, Z.Y. Rat bone marrow mesenchymal stem cells differentiate into hepatocytes in vitro. World J. Gastroenterol. 2005, 11, 3479–3484. [Google Scholar] [CrossRef]
- Lee, K.D.; Kuo, T.K.; Whang-Peng, J.; Chung, Y.F.; Lin, C.T.; Chou, S.H.; Chen, J.R.; Chen, Y.P.; Lee, O.K. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology 2004, 40, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Sgodda, M.; Aurich, H.; Kleist, S.; Aurich, I.; Konig, S.; Dollinger, M.M.; Fleig, W.E.; Christ, B. Hepatocyte differentiation of mesenchymal stem cells from rat peritoneal adipose tissue In Vitro and In Vivo. Exp. Cell Res. 2007, 313, 2875–2886. [Google Scholar] [CrossRef] [PubMed]
- Ewida, S.F.; Abdou, A.G.; Elhosary, A.A.E.-R.; Metawe, S.A.E.-G. Hepatocyte-like Versus Mesenchymal Stem Cells in CCl4-induced Liver Fibrosis. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Boucher, S.; Koh, S.; Sastry, K.S.; Chase, L.; Lakshmipathy, U.; Choong, C.; Yang, Z.; Vemuri, M.C.; Rao, M.S.; et al. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): Transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 2008, 112, 295–307. [Google Scholar] [CrossRef]
- Kim, Y.I.; Ryu, J.S.; Yeo, J.E.; Choi, Y.J.; Kim, Y.S.; Ko, K.; Koh, Y.G. Overexpression of TGF-beta1 enhances chondrogenic differentiation and proliferation of human synovium-derived stem cells. Biochem. Biophys. Res. Commun. 2014, 450, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Shen, X.; Liu, I.; Semenov, M.; He, X.; Wang, X.F. Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 2006, 20, 666–674. [Google Scholar] [CrossRef]
- Kim, W.; Barron, D.A.; Martin, R.S.; Chan, K.S.; Tran, L.L.; Yang, F.; Ressler, S.J.; Rowley, D.R. RUNX1 is essential for mesenchymal stem cell proliferation and myofibroblast differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 16389–16394. [Google Scholar] [CrossRef]
- Daneshmandi, S.; Karimi, M.H.; Pourfathollah, A.A. TGF-beta engineered mesenchymal stem cells (TGF-beta/MSCs) for treatment of Type 1 diabetes (T1D) mice model. Int. Immunopharmacol. 2017, 44, 191–196. [Google Scholar] [CrossRef]
- Liu, F.; Xie, J.; Zhang, X.; Wu, Z.; Zhang, S.; Xue, M.; Chen, J.; Yang, Y.; Qiu, H. Overexpressing TGF-beta1 in mesenchymal stem cells attenuates organ dysfunction during CLP-induced septic mice by reducing macrophage-driven inflammation. Stem Cell Res. Ther. 2020, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Jiao, X.; Zhao, S.; Liang, Y.; Ning, Y.; Shi, Y.; Fang, Y.; Ding, X.; Yu, X. Transforming growth factor-beta1-overexpressing mesenchymal stromal cells induced local tolerance in rat renal ischemia/reperfusion injury. Cytotherapy 2019, 21, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Regmi, S.; Raut, P.K.; Pathak, S.; Shrestha, P.; Park, P.H.; Jeong, J.H. Enhanced viability and function of mesenchymal stromal cell spheroids is mediated via autophagy induction. Autophagy 2021, 17, 2991–3010. [Google Scholar] [CrossRef]
- Regmi, S.; Pathak, S.; Thanh, T.P.; Nguyen, T.T.; Sung, J.H.; Yook, S.; Kim, J.O.; Yong, C.S.; Choi, I.; Doh, K.O.; et al. Intraportally delivered stem cell spheroids localize in the liver and protect hepatocytes against GalN/LPS-induced fulminant hepatic toxicity. Stem Cell Res. Ther. 2019, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Cen, S.; Wang, P.; Xie, Z.; Liu, Z.; Deng, W.; Su, H.; Wu, X.; Wang, S.; Li, J.; et al. Autophagy Improves the Immunosuppression of CD4+ T Cells by Mesenchymal Stem Cells Through Transforming Growth Factor-beta1. Stem Cells Transl. Med. 2016, 5, 1496–1505. [Google Scholar] [CrossRef]
- Wang, H.Y.; Li, C.; Liu, W.H.; Deng, F.M.; Ma, Y.; Guo, L.N.; Kong, H.; Hu, K.A.; Liu, Q.; Wu, J.; et al. Autophagy inhibition via Becn1 downregulation improves the mesenchymal stem cells antifibrotic potential in experimental liver fibrosis. J. Cell. Physiol. 2020, 235, 2722–2737. [Google Scholar] [CrossRef]
- Bonab, M.M.; Alimoghaddam, K.; Talebian, F.; Ghaffari, S.H.; Ghavamzadeh, A.; Nikbin, B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006, 7, 14. [Google Scholar] [CrossRef]
- Veriter, S.; Andre, W.; Aouassar, N.; Poirel, H.A.; Lafosse, A.; Docquier, P.L.; Dufrane, D. Human Adipose-Derived Mesenchymal Stem Cells in Cell Therapy: Safety and Feasibility in Different “Hospital Exemption” Clinical Applications. PLoS ONE 2015, 10, e0139566. [Google Scholar] [CrossRef]
- Lechanteur, C.; Briquet, A.; Giet, O.; Delloye, O.; Baudoux, E.; Beguin, Y. Clinical-scale expansion of mesenchymal stromal cells: A large banking experience. J. Transl. Med. 2016, 14, 145. [Google Scholar] [CrossRef]
- Furlani, D.; Ugurlucan, M.; Ong, L.; Bieback, K.; Pittermann, E.; Westien, I.; Wang, W.; Yerebakan, C.; Li, W.; Gaebel, R.; et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc. Res. 2009, 77, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhao, L.; Zhang, L.; Bao, Q.; Li, L. Mesenchymal stem cell-based cell-free strategies: Safe and effective treatments for liver injury. Stem Cell Res. Ther. 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- von Bahr, L.; Batsis, I.; Moll, G.; Hagg, M.; Szakos, A.; Sundberg, B.; Uzunel, M.; Ringden, O.; Blanc, K.L. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 2012, 30, 1575–1578. [Google Scholar] [CrossRef]
- Russo, F.P.; Alison, M.R.; Bigger, B.W.; Amofah, E.; Florou, A.; Amin, F.; Bou-Gharios, G.; Jeffery, R.; Iredale, J.P.; Forbes, S.J. The bone marrow functionally contributes to liver fibrosis. Gastroenterology 2006, 130, 1807–1821. [Google Scholar] [CrossRef]
- Quante, M.; Tu, S.P.; Tomita, H.; Gonda, T.; Wang, S.S.; Takashi, S.; Baik, G.H.; Shibata, W.; Diprete, B.; Betz, K.S.; et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 2011, 19, 257–272. [Google Scholar] [CrossRef]
- Yu, J.M.; Jun, E.S.; Bae, Y.C.; Jung, J.S. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth In Vivo. Stem Cells Dev. 2008, 17, 463–473. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tsuchiya, A.; Terai, S. The development of mesenchymal stem cell therapy in the present, and the perspective of cell-free therapy in the future. Clin. Mol. Hepatol. 2021, 27, 70–80. [Google Scholar] [CrossRef]
- Zhu, M.; Hua, T.; Ouyang, T.; Qian, H.; Yu, B. Applications of Mesenchymal Stem Cells in Liver Fibrosis: Novel Strategies, Mechanisms, and Clinical Practice. Stem Cells Int. 2021, 2021, 6546780. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Gong, Y.; Wang, B.; Shi, K.; Hou, Y.; Wang, L.; Lin, Z.; Han, Y.; Lu, L.; Chen, D.; et al. Randomized trial of autologous bone marrow mesenchymal stem cells transplantation for hepatitis B virus cirrhosis: Regulation of Treg/Th17 cells. J. Gastroenterol. Hepatol. 2014, 29, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Mohamadnejad, M.; Alimoghaddam, K.; Bagheri, M.; Ashrafi, M.; Abdollahzadeh, L.; Akhlaghpoor, S.; Bashtar, M.; Ghavamzadeh, A.; Malekzadeh, R. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013, 33, 1490–1496. [Google Scholar] [CrossRef]
- Yang, X.; Meng, Y.; Han, Z.; Ye, F.; Wei, L.; Zong, C. Mesenchymal stem cell therapy for liver disease: Full of chances and challenges. Cell Biosci. 2020, 10, 123. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.D.; Wang, S.Z.; Sarkar, D.; Lee, H.M.; Khan, A.; Bhati, C.; Sharma, A.; Kumaran, V.; Bruno, D.; et al. Effect of mesenchymal stem cell in liver regeneration and clinical applications. Hepatoma Res. 2021, 7, 53. [Google Scholar] [CrossRef]
- Suk, K.T.; Yoon, J.H.; Kim, M.Y.; Kim, C.W.; Kim, J.K.; Park, H.; Hwang, S.G.; Kim, D.J.; Lee, B.S.; Lee, S.H.; et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology 2016, 64, 2185–2197. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, H.; Shi, M.; Xu, R.; Fu, J.; Lv, J.; Chen, L.; Lv, S.; Li, Y.; Yu, S.; et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J. Gastroenterol. Hepatol. 2012, 27, 112–120. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, Z.; Xu, R.; Lin, H.; Fu, J.; Zou, Z.; Zhang, A.; Shi, J.; Chen, L.; Lv, S.; et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl. Med. 2012, 1, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Takamura, M.; Seki, A.; Sunagozaka, H.; Terashima, T.; Komura, T.; Yamato, M.; Miyazawa, M.; Kawaguchi, K.; Nasti, A.; et al. Phase I clinical study of liver regenerative therapy for cirrhosis by intrahepatic arterial infusion of freshly isolated autologous adipose tissue-derived stromal/stem (regenerative) cell. Regen. Ther. 2017, 6, 52–64. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, M.; Abdel-Aziz, I.; Mogawer, S.; Abdel-Hamid, S.; Hammam, O.; Teaema, S.; Wahdan, M. Phase II trial: Undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev. Rep. 2012, 8, 972–981. [Google Scholar] [CrossRef]
- Kharaziha, P.; Hellstrom, P.M.; Noorinayer, B.; Farzaneh, F.; Aghajani, K.; Jafari, F.; Telkabadi, M.; Atashi, A.; Honardoost, M.; Zali, M.R.; et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: A phase I-II clinical trial. Eur. J. Gastroenterol. Hepatol. 2009, 21, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.E.; El-Sayed, S.Z.; El-Kheir, W.A.; Gabr, H.; Gomaa, A.A.; El-Noomani, N.; Hegazy, M. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur. J. Gastroenterol. Hepatol. 2011, 23, 936–941. [Google Scholar] [CrossRef]
- Kantarcioglu, M.; Demirci, H.; Avcu, F.; Karslioglu, Y.; Babayigit, M.A.; Karaman, B.; Ozturk, K.; Gurel, H.; Kayhan, M.A.; Kacar, S.; et al. Efficacy of autologous mesenchymal stem cell transplantation in patients with liver cirrhosis. Turk. J. Gastroenterol. 2015, 26, 244–250. [Google Scholar] [CrossRef]
- Fu, Q.; Ohnishi, S.; Sakamoto, N. Conditioned Medium from Human Amnion-Derived Mesenchymal Stem Cells Regulates Activation of Primary Hepatic Stellate Cells. Stem Cells Int. 2018, 2018, 4898152. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lee, J.S.; Hyun, J.; Kim, J.; Kim, S.U.; Cha, H.J.; Jung, Y. Tumor necrosis factor-inducible gene 6 promotes liver regeneration in mice with acute liver injury. Stem Cell Res. Ther. 2015, 6, 20. [Google Scholar] [CrossRef]
- Kumar, P.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Milner, C.M. TSG-6: A multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2019, 78, 60–83. [Google Scholar] [CrossRef]
- Wang, S.; Kim, J.; Lee, C.; Oh, D.; Han, J.; Kim, T.J.; Kim, S.W.; Seo, Y.S.; Oh, S.H.; Jung, Y. Tumor necrosis factor-inducible gene 6 reprograms hepatic stellate cells into stem-like cells, which ameliorates liver damage in mouse. Biomaterials 2019, 219, 119375. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, M.; Fu, L.; Lin, J.; Zhou, X.; Zhou, P.; Huang, P.; Hu, H.; Han, Y. Liver-targeted delivery of TSG-6 by calcium phosphate nanoparticles for the management of liver fibrosis. Theranostics 2020, 10, 36–49. [Google Scholar] [CrossRef]
- An, S.Y.; Jang, Y.J.; Lim, H.J.; Han, J.; Lee, J.; Lee, G.; Park, J.Y.; Park, S.Y.; Kim, J.H.; Do, B.R.; et al. Milk Fat Globule-EGF Factor 8, Secreted by Mesenchymal Stem Cells, Protects Against Liver Fibrosis in Mice. Gastroenterology 2017, 152, 1174–1186. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Tkach, M.; Thery, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Tan, Y.; Cai, M.; Zhao, T.; Mao, F.; Zhang, X.; Xu, W.; Yan, Z.; Qian, H.; Yan, Y. Human Umbilical Cord MSC-Derived Exosomes Suppress the Development of CCl4-Induced Liver Injury through Antioxidant Effect. Stem Cells Int. 2018, 2018, 6079642. [Google Scholar] [CrossRef]
- Li, T.; Yan, Y.; Wang, B.; Qian, H.; Zhang, X.; Shen, L.; Wang, M.; Zhou, Y.; Zhu, W.; Li, W.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013, 22, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Liu, J.; Yao, X.; Jiang, T.; Wang, Y.; Xie, F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/beta-catenin pathway. Stem Cell Res. Ther. 2019, 10, 98. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Tan, Y.; Zou, S.; Zhang, H.; Mao, F.; Gong, A.; Qian, H.; Xu, W. hucMSC Exosome-Derived GPX1 Is Required for the Recovery of Hepatic Oxidant Injury. Mol. Ther. 2017, 25, 465–479. [Google Scholar] [CrossRef]
- Du, Z.; Wu, T.; Liu, L.; Luo, B.; Wei, C. Extracellular vesicles-derived miR-150-5p secreted by adipose-derived mesenchymal stem cells inhibits CXCL1 expression to attenuate hepatic fibrosis. J. Cell. Mol. Med. 2021, 25, 701–715. [Google Scholar] [CrossRef]
- Xie, K.; Liu, L.; Chen, J.; Liu, F. Exosomal miR-1246 derived from human umbilical cord blood mesenchymal stem cells attenuates hepatic ischemia reperfusion injury by modulating T helper 17/regulatory T balance. IUBMB Life 2019, 71, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, C.; Shin, Y.; Wang, S.; Han, J.; Kim, M.; Kim, J.M.; Shin, S.C.; Lee, B.J.; Kim, T.J.; et al. sEVs from tonsil-derived mesenchymal stromal cells alleviate activation of hepatic stellate cells and liver fibrosis through miR-486-5p. Mol. Ther. 2021, 29, 1471–1486. [Google Scholar] [CrossRef]
- Hyun, J.; Wang, S.; Kim, J.; Kim, G.J.; Jung, Y. MicroRNA125b-mediated Hedgehog signaling influences liver regeneration by chorionic plate-derived mesenchymal stem cells. Sci. Rep. 2015, 5, 14135. [Google Scholar] [CrossRef]
- Willms, E.; Cabanas, C.; Mager, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef]
- Vagner, T.; Chin, A.; Mariscal, J.; Bannykh, S.; Engman, D.M.; Di Vizio, D. Protein Composition Reflects Extracellular Vesicle Heterogeneity. Proteomics 2019, 19, e1800167. [Google Scholar] [CrossRef]
- Carnino, J.M.; Lee, H.; Jin, Y. Isolation and characterization of extracellular vesicles from Broncho-alveolar lavage fluid: A review and comparison of different methods. Respir. Res. 2019, 20, 240. [Google Scholar] [CrossRef]
- Wiklander, O.P.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mager, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle In Vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef] [PubMed]
- Gimona, M.; Pachler, K.; Laner-Plamberger, S.; Schallmoser, K.; Rohde, E. Manufacturing of Human Extracellular Vesicle-Based Therapeutics for Clinical Use. Int. J. Mol. Sci. 2017, 18, 1190. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.T.; Witwer, K.W.; van Balkom, B.W.M.; de Beer, J.; Brodie, C.; Corteling, R.L.; Gabrielsson, S.; Gimona, M.; Ibrahim, A.G.; de Kleijn, D.; et al. Concise Review: Developing Best-Practice Models for the Therapeutic Use of Extracellular Vesicles. Stem Cells Transl. Med. 2017, 6, 1730–1739. [Google Scholar] [CrossRef]
- Busatto, S.; Vilanilam, G.; Ticer, T.; Lin, W.L.; Dickson, D.W.; Shapiro, S.; Bergese, P.; Wolfram, J. Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells 2018, 7, 273. [Google Scholar] [CrossRef] [PubMed]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol. Ther. 2018, 26, 2838–2847. [Google Scholar] [CrossRef]
- Lou, G.; Yang, Y.; Liu, F.; Ye, B.; Chen, Z.; Zheng, M.; Liu, Y. MiR-122 modification enhances the therapeutic efficacy of adipose tissue-derived mesenchymal stem cells against liver fibrosis. J. Cell. Mol. Med. 2017, 21, 2963–2973. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, Q.; Cai, X.; Li, F.; Ma, Z.; Xu, M.; Lu, L. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J. Cell. Mol. Med. 2017, 21, 2491–2502. [Google Scholar] [CrossRef]
- Fiore, E.; Dominguez, L.M.; Bayo, J.; Malvicini, M.; Atorrasagasti, C.; Rodriguez, M.; Cantero, M.J.; Garcia, M.; Yannarelli, G.; Mazzolini, G. Human umbilical cord perivascular cells-derived extracellular vesicles mediate the transfer of IGF-I to the liver and ameliorate hepatic fibrogenesis in mice. Gene Ther. 2020, 27, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Mardpour, S.; Ghanian, M.H.; Sadeghi-Abandansari, H.; Mardpour, S.; Nazari, A.; Shekari, F.; Baharvand, H. Hydrogel-Mediated Sustained Systemic Delivery of Mesenchymal Stem Cell-Derived Extracellular Vesicles Improves Hepatic Regeneration in Chronic Liver Failure. ACS Appl. Mater. Interfaces 2019, 11, 37421–37433. [Google Scholar] [CrossRef]
- Yang, Y.; Hong, Y.; Nam, G.H.; Chung, J.H.; Koh, E.; Kim, I.S. Virus-Mimetic Fusogenic Exosomes for Direct Delivery of Integral Membrane Proteins to Target Cell Membranes. Adv. Mater. 2017, 29, 1605604. [Google Scholar] [CrossRef]
- Tamura, R.; Uemoto, S.; Tabata, Y. Augmented liver targeting of exosomes by surface modification with cationized pullulan. Acta Biomater. 2017, 57, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Bodzin, A.S.; Baker, T.B. Liver Transplantation Today: Where We Are Now and Where We Are Going. Liver Transpl. 2018, 24, 1470–1475. [Google Scholar] [CrossRef]
- Jadlowiec, C.C.; Taner, T. Liver transplantation: Current status and challenges. World J. Gastroenterol. 2016, 22, 4438–4445. [Google Scholar] [CrossRef] [PubMed]

| Type | Treatment | Target/Experimental Model | Mechanism | Outcome | Ref |
|---|---|---|---|---|---|
| In vitro | Direct contact with MSCs | HSCs | Upregulating Notch1 expression Downregulating PI3K/Akt pathway | Suppression of HSC proliferation | [71] |
| Co-culture with MSCs | HSCs | Upregulating p27kip1 and p21cip1 Downregulating cyclin D and p-ERK | [72] | ||
| Co-culture with MSCs | HSCs | Increasing pro-apoptotic proteins (Bax and cleaved caspase-3) | Increase of apoptosis of activated HSCs | [73] | |
| Co-culture with MSCs | HSCs | Secreting NGF; inhibition of NF-κB Decrease of Bcl-xl expression | [74] | ||
| Direct contact or co-culture with MSCs | LX2 | Releasing HGF; inhibition of NF-κB | [75] | ||
| Co-culture with MSCs | KCs | Promoting secretion of anti-inflammatory cytokines | Alleviation of immune response | [82] | |
| Co-culture with MSCs | NK cells | Producing PGE2; impairment of proliferation and activation of NK cells | [89] | ||
| Hepatocyte differentiating factors | Hepatocytes | Inducing expression of hepatocyte markers (albumin, α-fetoprotein) | Differentiation of MSCs into functional hepatocytes | [63,92,93,94,95] | |
| In vivo | MSC transplantation | CCl4-induced liver fibrosis in rats | Inhibiting proliferation and promoting apoptosis of activated HSCs | Amelioration of liver fibrosis | [76] |
| MSC transplantation | DMN-induced liver fibrosis in rats | Releasing IL-4 and IL-10 | Alleviation of immune response and liver fibrosis | [82] | |
| MSC transplantation | CCl4-induced liver fibrosis in mice | Decreasing IL-17 Increasing IL-10 | [86] | ||
| MSC transplantation | CCl4-induced liver fibrosis in mice | Increasing IDO level and decreasing IL-17; decrease of proliferation of Th17 cells | [91] | ||
| MSCs derived hepatocyte-like cells transplantation | CCl4-induced liver fibrosis in mice | Mimicking hepatocyte functions | Amelioration of liver fibrosis Improvement of liver function | [96] |
| Clinical Trials | |||||
|---|---|---|---|---|---|
| Patients | Dose /Frequency | Administration Route | Outcome | Ref | |
| Therapeutic effects | MSC injection | ||||
| 11 patients with alcoholic cirrhosis | 5 × 107 MSCs Two times | Hepatic artery | Decrease of MELD and Child-Pugh score Downregulation of collagen accumulation | [28] | |
| 55 patients with alcoholic cirrhosis | 5 × 107 MSCs One or two times | Hepatic artery | Decrease of Child-Pugh score Decrease of ALP Downregulation of collagen accumulation | [123] | |
| 45 patients with HBV liver cirrhosis | 0.5 × 106 MSCs/kg Three times | Peripheral vein | Increase of serum albumin Decrease of total bilirubin Decrease of MELD Na score Downregulation of serum laminin | [124] | |
| 43 patients with HBV-induced acute-on-chronic liver failure | 0.5 × 106 MSCs/kg Three times | Peripheral vein | Increase of serum albumin, cholinesterase Decrease of total bilirubin and ALT Increase of survival rate Decrease of MELD score | [125] | |
| 39 patients with HBV liver cirrhosis | Unknown | Hepatic artery | Increase of serum albumin Decrease of total bilirubin Decrease of MELD score Ameliorating imbalance of Treg/Th17 cells | [119] | |
| 4 patients with liver cirrhosis | 3.3 or 6.6 × 105 MSCs/kg One time | Hepatic artery | Increase of serum albumin Elevating immunomodulatory factors | [126] | |
| Administration of hepatocyte-like differentiated MSCs | |||||
| 25 patients with HCV liver cirrhosis | 1 × 106 cells/kg One time | Peripheral vein | Increase of serum albumin Decrease of serum creatinine, total bilirubin Decrease of MELD score | [127] | |
| 8 patients with end-stage of liver disease | 3–5 × 107 cells One time | Peripheral vein | Decrease of serum creatinine Decrease of MELD score | [128] | |
| 40 patients with HCV-induced end-stage liver disease | 2 × 107 cells One time | Intrasplenic or intrahepatic | Increase of serum albumin Decrease MELD and Child-Pugh score | [129] | |
| No effect | 27 patients with liver cirrhosis | 1.2–2.95 × 108 MSCs One time | Peripheral vein | No changes in serum albumin, ALT and MELD scores | [120] |
| 25 patients with liver cirrhosis | 1 × 106 MSCs/kg One time | Peripheral vein | No change in serum ALT, ALP, total bilirubin MELD, and Child-Pugh scores | [130] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Kim, M.; Han, J.; Yoon, M.; Jung, Y. Mesenchymal Stem Cells Influence Activation of Hepatic Stellate Cells, and Constitute a Promising Therapy for Liver Fibrosis. Biomedicines 2021, 9, 1598. https://doi.org/10.3390/biomedicines9111598
Lee C, Kim M, Han J, Yoon M, Jung Y. Mesenchymal Stem Cells Influence Activation of Hepatic Stellate Cells, and Constitute a Promising Therapy for Liver Fibrosis. Biomedicines. 2021; 9(11):1598. https://doi.org/10.3390/biomedicines9111598
Chicago/Turabian StyleLee, Chanbin, Minju Kim, Jinsol Han, Myunghee Yoon, and Youngmi Jung. 2021. "Mesenchymal Stem Cells Influence Activation of Hepatic Stellate Cells, and Constitute a Promising Therapy for Liver Fibrosis" Biomedicines 9, no. 11: 1598. https://doi.org/10.3390/biomedicines9111598
APA StyleLee, C., Kim, M., Han, J., Yoon, M., & Jung, Y. (2021). Mesenchymal Stem Cells Influence Activation of Hepatic Stellate Cells, and Constitute a Promising Therapy for Liver Fibrosis. Biomedicines, 9(11), 1598. https://doi.org/10.3390/biomedicines9111598






