Osteopontin Gene Polymorphisms Are Associated with Cardiovascular Risk Factors in Patients with Premature Coronary Artery Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Individuals
2.2. Clinical, Demographic, Biochemical Variables Assessment
2.3. Tomographic Evaluation
2.4. Estimation of Ancestry
2.5. SNPs Selection and Genetic Characterization
2.6. Bioinformatic Analysis
2.7. Statistical Analysis
3. Results
3.1. Evaluation of Anthropometric, Clinical and Metabolic Parameters and Prevalence of Cardiovascular Risk Factors
3.2. Association of rs2728127 and rs11730582 Polymorphisms with pCAD
3.3. Association of rs2728127 and rs11730582 Polymorphisms with Cardiovascular Risk Factors and Metabolic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carresi, C.; Mollace, R.; Macrì, R.; Scicchitano, M.; Bosco, F.; Scarano, F.; Coppoletta, A.R.; Guarnieri, L.; Ruga, S.; Zito, M.C.; et al. Oxidative Stress Triggers Defective Autophagy in Endothelial Cells: Role in Atherothrombosis Development. Antioxidants 2021, 10, 387. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Reviews. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Yuvaraj, J.; Cheng, K.; Lin, A.; Psaltis, P.J.; Nicholls, S.J.; Wong, D.T.L. The Emerging Role of CT-Based Imaging in Adipose Tissue and Coronary Inflammation. Cells 2021, 10, 1196. [Google Scholar] [CrossRef]
- Hutcheson, J.D.; Maldonado, N.; Aikawa, E. Small entities with large impact: Microcalcifications and atherosclerotic plaque vulnerability. Curr. Opin. Lipidol. 2014, 25, 327–332. [Google Scholar] [CrossRef]
- Chua, A.; Blankstein, R.; Ko, B. Coronary artery calcium in primary prevention. Aust. J. Gen. Pract. 2020, 49, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Faggiano, A.; Santangelo, G.; Carugo, S.; Pressman, G.; Picano, E.; Faggiano, P. Cardiovascular Calcification as a Marker of Increased Cardiovascular Risk and a Surrogate for Subclinical Atherosclerosis: Role of Echocardiography. J. Clin. Med. 2021, 10, 1668. [Google Scholar] [CrossRef]
- Reinhold, S.; Blankesteijn, W.M.; Foulquier, S. The Interplay of WNT and PPARγ Signaling in Vascular Calcification. Cells 2020, 9, 658. [Google Scholar] [CrossRef]
- Berezin, A.E.; Kremzer, A.A. Circulating osteopontin as a marker of early coronary vascular calcification in type two diabetes mellitus patients with known asymptomatic coronary artery disease. Atherosclerosis 2013, 229, 475–481. [Google Scholar] [CrossRef]
- Kosmopoulos, M.; Paschou, S.A.; Grapsa, J.; Anagnostis, P.; Vryonidou, A.; Goulis, D.G.; Siasos, G. The Emerging Role of Bone Markers in Diagnosis and Risk Stratification of Patients With Coronary Artery Disease. Angiology 2019, 70, 690–700. [Google Scholar] [CrossRef]
- Mohamadpour, A.H.; Abdolrahmani, L.; Mirzaei, H.; Sahebkar, A.; Moohebati, M.; Ghorbani, M.; Ferns, G.A.; Ghayour-Mobarhan, M. Serum osteopontin concentrations in relation to coronary artery disease. Arch. Med. Res. 2015, 46, 112–117. [Google Scholar] [CrossRef]
- Lok, Z.S.Y.; Lyle, A.N. Osteopontin in Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 613–622. [Google Scholar] [CrossRef]
- Shirakawa, K.; Sano, M. Osteopontin in Cardiovascular Diseases. Biomolecules 2021, 11, 1047. [Google Scholar] [CrossRef]
- Subraman, V.; Thiyagarajan, M.; Malathi, N.; Rajan, S.T. OPN -Revisited. J. Clin. Diagn. Res. JCDR 2015, 9, Ze10–Ze13. [Google Scholar] [CrossRef] [PubMed]
- Vianello, E.; Kalousová, M.; Dozio, E.; Tacchini, L.; Zima, T.; Corsi Romanelli, M.M. Osteopontin: The Molecular Bridge between Fat and Cardiac-Renal Disorders. Int. J. Mol. Sci. 2020, 21, 5568. [Google Scholar] [CrossRef] [PubMed]
- Kahles, F.; Findeisen, H.M.; Bruemmer, D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol. Metab. 2014, 3, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Icer, M.A.; Gezmen-Karadag, M. The multiple functions and mechanisms of osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar] [CrossRef]
- Eleftheriadou, I.; Tsilingiris, D.; Tentolouris, A.; Mourouzis, I.; Grigoropoulou, P.; Kapelios, C.; Pantos, C.; Makrilakis, K.; Tentolouris, N. Association of Circulating Osteopontin Levels With Lower Extremity Arterial Disease in Subjects With Type 2 Diabetes Mellitus: A Cross-Sectional Observational Study. Int. J. Low. Extrem. Wounds 2020, 19, 180–189. [Google Scholar] [CrossRef]
- Mack, P.C.; Redman, M.W.; Chansky, K.; Williamson, S.K.; Farneth, N.C.; Lara, P.N., Jr.; Franklin, W.A.; Le, Q.T.; Crowley, J.J.; Gandara, D.R. Lower osteopontin plasma levels are associated with superior outcomes in advanced non-small-cell lung cancer patients receiving platinum-based chemotherapy: SWOG Study S0003. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 4771–4776. [Google Scholar] [CrossRef]
- Carbone, F.; Rigamonti, F.; Burger, F.; Roth, A.; Bertolotto, M.; Spinella, G.; Pane, B.; Palombo, D.; Pende, A.; Bonaventura, A.; et al. Serum levels of osteopontin predict major adverse cardiovascular events in patients with severe carotid artery stenosis. Int. J. Cardiol. 2018, 255, 195–199. [Google Scholar] [CrossRef]
- Gürses, K.M.; Yalçın, M.U.; Koçyiğit, D.; Beşler, M.S.; Canpınar, H.; Evranos, B.; Yorgun, H.; Şahiner, M.L.; Kaya, E.B.; Özer, N.; et al. The association between serum angiogenin and osteopontin levels and coronary collateral circulation in patients with chronic total occlusion. Anatol. J. Cardiol. 2019, 22, 77–84. [Google Scholar] [CrossRef]
- Higgins, C.L.; Isbilir, S.; Basto, P.; Chen, I.Y.; Vaduganathan, M.; Vaduganathan, P.; Reardon, M.J.; Lawrie, G.; Peterson, L.; Morrisett, J.D. Distribution of alkaline phosphatase, osteopontin, RANK ligand and osteoprotegerin in calcified human carotid atheroma. Protein J. 2015, 34, 315–328. [Google Scholar] [CrossRef]
- Wolak, T. Osteopontin—A multi-modal marker and mediator in atherosclerotic vascular disease. Atherosclerosis 2014, 236, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, C. Haplotype analysis revealed a genetic influence of osteopontin on large artery atherosclerosis. J. Biomed. Sci. 2008, 15, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.F.; Wu, S.; Juang, J.J.; Chiang, F.T.; Hsu, L.A.; Teng, M.S.; Cheng, S.T.; Huang, H.L.; Ko, Y.L. Osteoprotegerin and osteopontin levels, but not gene polymorphisms, predict mortality in cardiovascular diseases. Biomark. Med. 2019, 13, 751–760. [Google Scholar] [CrossRef]
- Taylor, B.C.; Schreiner, P.J.; Doherty, T.M.; Fornage, M.; Carr, J.J.; Sidney, S. Matrix Gla protein and osteopontin genetic associations with coronary artery calcification and bone density: The CARDIA study. Hum. Genet. 2005, 116, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; Mcevoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef]
- Mahtta, D.; Ramsey, D.J.; Al Rifai, M.; Nasir, K.; Samad, Z.; Aguilar, D.; Jneid, H.; Ballantyne, C.M.; Petersen, L.A.; Virani, S.S. Evaluation of Aspirin and Statin Therapy Use and Adherence in Patients With Premature Atherosclerotic Cardiovascular Disease. JAMA Netw. Open 2020, 3, e2011051. [Google Scholar] [CrossRef]
- Acuña-Valerio, J.; Rodas-Díaz, M.A.; Macias-Garrido, E.; Posadas-Sánchez, R.; Juárez-Rojas, J.G.; Medina-Urrutia, A.X.; Cardoso-Saldaña, G.C.; Joge-Galarza, E.; Torres-Tamayo, M.; Vargas-Alarcón, G.; et al. Aortic valve calcification prevalence and association with coronary risk factors and atherosclerosis in Mexican population. Arch. Cardiol. Mex. 2017, 87, 108–115. [Google Scholar] [CrossRef]
- Cardoso-Saldaña, G.C.; Medina-Urrutia, A.X.; Posadas-Romero, C.; Juárez-Rojas, J.G.; Jorge-Galarza, E.; Vargas-Alarcón, G.; Posadas-Sánchez, R. Fatty liver and abdominal fat relationships with high C-reactive protein in adults without coronary heart disease. Ann. Hepatol. 2015, 14, 658–665. [Google Scholar] [CrossRef]
- López-Bautista, F.; Posadas-Sánchez, R.; Vázquez-Vázquez, C.; Fragoso, J.M.; Rodríguez-Pérez, J.M.; Vargas-Alarcón, G. IL-37 Gene and Cholesterol Metabolism: Association of Polymorphisms with the Presence of Hypercholesterolemia and Cardiovascular Risk Factors. The GEA Mexican Study. Biomolecules 2020, 10, 1409. [Google Scholar] [CrossRef]
- Posadas-Romero, C.; López-Bautista, F.; Rodas-Díaz, M.A.; Posadas-Sánchez, R.; Kimura-Hayama, E.; Juárez-Rojas, J.G.; Medina-Urrutia, A.X.; Cardoso-Saldaña, G.C.; Vargas-Alarcón, G.; Jorge-Galarza, E. Prevalence and extent of coronary artery calcification in an asymptomatic cardiovascular Mexican population: Genetics of Atherosclerotic Disease study. Arch. Cardiol. Mex. 2017, 87, 292–301. [Google Scholar] [CrossRef]
- Posadas-Sánchez, R.; Pérez-Hernández, N.; Angeles-Martínez, J.; López-Bautista, F.; Villarreal-Molina, T.; Rodríguez-Pérez, J.M.; Fragoso, J.M.; Posadas-Romero, C.; Vargas-Alarcón, G. Interleukin 35 Polymorphisms Are Associated with Decreased Risk of Premature Coronary Artery Disease, Metabolic Parameters, and IL-35 Levels: The Genetics of Atherosclerotic Disease (GEA) Study. Mediat. Inflamm. 2017, 2017, 6012795. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, J.M.; Posadas-Sánchez, R.; Blachman-Braun, R.; Vargas-Alarcón, G.; Posadas-Romero, C.; Rodríguez-Cortés, A.A.; López-Bautista, F.; Tovilla-Zárate, C.A.; Rojas-Toledo, E.X.; Borgonio-Cuadra, V.M.; et al. HHIPL-1 (rs2895811) gene polymorphism is associated with cardiovascular risk factors and cardiometabolic parameters in Mexicans patients with myocardial infarction. Gene 2018, 663, 34–40. [Google Scholar] [CrossRef] [PubMed]
- López-Bautista, F.; Posadas-Romero, C.; Ruiz-Vargas, L.Y.; Cardoso-Saldaña, G.; Juárez-Rojas, J.G.; Medina-Urrutia, A.; Pérez-Hernández, N.; Rodríguez-Pérez, J.M.; Vargas-Alarcón, G.; Posadas-Sánchez, R. Vitamin D Deficiency is not Associated with Fatty Liver in a Mexican Population. Ann. Hepatol. 2018, 17, 419–425. [Google Scholar] [CrossRef]
- Zamarrón-Licona, E.; Rodríguez-Pérez, J.M.; Posadas-Sánchez, R.; Vargas-Alarcón, G.; Baños-González, M.A.; Borgonio-Cuadra, V.M.; Pérez-Hernández, N. Variants of PCSK9 Gene Are Associated with Subclinical Atherosclerosis and Cardiometabolic Parameters in Mexicans. The GEA Project. Diagnostics 2021, 11, 774. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano,, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Kvist, H.; Chowdhury, B.; Grangård, U.; Tylén, U.; Sjöström, L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: Predictive equations. Am. J. Clin. Nutr. 1988, 48, 1351–1361. [Google Scholar] [CrossRef]
- Longo, R.; Ricci, C.; Masutti, F.; Vidimari, R.; Crocé, L.S.; Bercich, L.; Tiribelli, C.; Dalla Palma, L. Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Investig. Radiol. 1993, 28, 297–302. [Google Scholar] [CrossRef]
- Silva-Zolezzi, I.; Hidalgo-Miranda, A.; Estrada-Gil, J.; Fernandez-Lopez, J.C.; Uribe-Figueroa, L.; Contreras, A.; Balam-Ortiz, E.; Del Bosque-Plata, L.; Velazquez-Fernandez, D.; Lara, C.; et al. Analysis of genomic diversity in Mexican Mestizo populations to develop genomic medicine in Mexico. Proc. Natl. Acad. Sci. USA 2009, 106, 8611–8616. [Google Scholar] [CrossRef]
- Posadas-Sánchez, R.; Pérez-Hernández, N.; Rodríguez-Pérez, J.M.; Coral-Vázquez, R.M.; Roque-Ramírez, B.; Llorente, L.; Lima, G.; Flores-Dominguez, C.; Villarreal-Molina, T.; Posadas-Romero, C.; et al. Interleukin-27 polymorphisms are associated with premature coronary artery disease and metabolic parameters in the Mexican population: The genetics of atherosclerotic disease (GEA) Mexican study. Oncotarget 2017, 8, 64459–64470. [Google Scholar] [CrossRef]
- Hou, X.; Hu, Z.; Huang, X.; Chen, Y.; He, X.; Xu, H.; Wang, N. Serum osteopontin, but not OPN gene polymorphism, is associated with LVH in essential hypertensive patients. J. Mol. Med. 2014, 92, 487–495. [Google Scholar] [CrossRef]
- Jing, M.; Li, B.; Hou, X.; Shoba, J.; Li, C.; Liang, H.; Zhang, X.; Liu, E.; Yang, B.; Meng, X. OPN gene polymorphism and the serum OPN levels confer the susceptibility and prognosis of ischemic stroke in Chinese patients. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2013, 32, 1798–1807. [Google Scholar] [CrossRef]
- Juárez-Cedillo, T.; Zuñiga, J.; Acuña-Alonzo, V.; Pérez-Hernández, N.; Rodríguez-Pérez, J.M.; Barquera, R.; Gallardo, G.J.; Sánchez-Arenas, R.; García-Peña Mdel, C.; Granados, J.; et al. Genetic admixture and diversity estimations in the Mexican Mestizo population from Mexico City using 15 STR polymorphic markers. Forensic Sci. Int. Genet. 2008, 2, e37–e39. [Google Scholar] [CrossRef] [PubMed]
- Lisker, R.; Ramírez, E.; Babinsky, V. Genetic structure of autochthonous populations of Meso-America: Mexico. Hum. Biol. 1996, 68, 395–404. [Google Scholar] [PubMed]
- Rubi-Castellanos, R.; Martínez-Cortés, G.; Muñoz-Valle, J.F.; González-Martín, A.; Cerda-Flores, R.M.; Anaya-Palafox, M.; Rangel-Villalobos, H. Pre-Hispanic Mesoamerican demography approximates the present-day ancestry of Mestizos throughout the territory of Mexico. Am. J. Phys. Anthropol. 2009, 139, 284–294. [Google Scholar] [CrossRef]
- Glas, J.; Seiderer, J.; Bayrle, C.; Wetzke, M.; Fries, C.; Tillack, C.; Olszak, T.; Beigel, F.; Steib, C.; Friedrich, M.; et al. The role of osteopontin (OPN/SPP1) haplotypes in the susceptibility to Crohn’s disease. PLoS ONE 2011, 6, e29309. [Google Scholar] [CrossRef]
- Amar, A.; Afzal, A.; Hameed, A.; Ahmad, M.; Khan, A.R.; Najma, H.; Abid, A.; Khaliq, S. Osteopontin promoter polymorphisms and risk of urolithiasis: A candidate gene association and meta-analysis study. BMC Med. Genet. 2020, 21, 172. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R.; Shafiei, N.; Safarinejad, S. Association between polymorphisms in osteopontin gene (SPP1) and first episode calcium oxalate urolithiasis. Urolithiasis 2013, 41, 303–313. [Google Scholar] [CrossRef]
- Shang, H.; Hao, Y.; Hu, W.; Hu, X.; Jin, Q. OPN gene locus is associated with the risk of knee osteoarthritis: A case-control study. Biosci. Rep. 2019, 39, BSR20182023. [Google Scholar] [CrossRef]
- Liang, L.; Lu, G.; Pan, G.; Deng, Y.; Liang, J.; Liang, L.; Liu, J.; Tang, Y.; Wei, G. A Case-Control Study of the Association Between the SPP1 Gene SNPs and the Susceptibility to Breast Cancer in Guangxi, China. Front. Oncol. 2019, 9, 1415. [Google Scholar] [CrossRef]
- Cheema, B.S.; Iyengar, S.; Sharma, R.; Kohli, H.S.; Bhansali, A.; Khullar, M. Association between Osteopontin Promoter Gene Polymorphisms and Haplotypes with Risk of Diabetic Nephropathy. J. Clin. Med. 2015, 4, 1281–1292. [Google Scholar] [CrossRef]
- Lim, S.; Quon, M.J.; Koh, K.K. Modulation of adiponectin as a potential therapeutic strategy. Atherosclerosis 2014, 233, 721–728. [Google Scholar] [CrossRef]
- Ramakrishnan, N.; Auger, K.; Jialal, I. Biochemistry, Adiponectin; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Hui, E.; Xu, A.; Chow, W.S.; Lee, P.C.; Fong, C.H.; Cheung, S.C.; Tse, H.F.; Chau, M.T.; Cheung, B.M.; Lam, K.S. Hypoadiponectinemia as an independent predictor for the progression of carotid atherosclerosis: A 5-year prospective study. Metab. Syndr. Relat. Disord. 2014, 12, 517–522. [Google Scholar] [CrossRef]
- Di Chiara, T.; Argano, C.; Scaglione, A.; Duro, G.; Corrao, S.; Scaglione, R.; Licata, G. Hypoadiponectinemia, cardiometabolic comorbidities and left ventricular hypertrophy. Intern. Emerg. Med. 2015, 10, 33–40. [Google Scholar] [CrossRef][Green Version]
- Brea, A.; Hernández-Mijares, A.; Millán, J.; Ascaso, J.F.; Blasco, M.; Díaz, A.; Mantilla, T.; Pedro-Botet, J.C.; Pintó, X. Non-HDL cholesterol as a therapeutic goal. Clínica E Investig. Arterioscler. 31, (Suppl. 2). 28–33. [CrossRef]
- Feingold, K.R. Cholesterol Lowering Drugs; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., De Herder, W.W., Dhatariya, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Hsu, H.Y.; Lin, C.J.; Lee, Y.S.; Wu, T.H.; Chien, K.L. Efficacy of more intensive lipid-lowering therapy on cardiovascular diseases: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2020, 20, 334. [Google Scholar] [CrossRef]
- Masson, W.; Lobo, M.; Siniawski, D.; Molinero, G.; Huerín, M.; Nogueira, J.P. Impact of Lipid-Lowering Therapy on Mortality According to the Baseline Non-HDL Cholesterol Level: A Meta-Analysis. High Blood Press. Cardiovasc. Prev. Off. J. Ital. Soc. Hypertens. 2019, 26, 263–272. [Google Scholar] [CrossRef]
- Bruha, R.; Vitek, L.; Smid, V. Osteopontin—A potential biomarker of advanced liver disease. Ann. Hepatol. 2020, 19, 344–352. [Google Scholar] [CrossRef]
- Wen, Y.; Jeong, S.; Xia, Q.; Kong, X. Role of Osteopontin in Liver Diseases. Int. J. Biol. Sci. 2016, 12, 1121–1128. [Google Scholar] [CrossRef]
- Park, J.H.; Koo, B.K.; Kim, W.; Kim, W.H. Histological severity of nonalcoholic fatty liver disease is associated with 10-year risk for atherosclerotic cardiovascular disease. Hepatol. Int. 2021, 15, 1148–1159. [Google Scholar] [CrossRef]
- Hsiao, C.C.; Teng, P.H.; Wu, Y.J.; Shen, Y.W.; Mar, G.Y.; Wu, F.Z. Severe, but not mild to moderate, non-alcoholic fatty liver disease associated with increased risk of subclinical coronary atherosclerosis. BMC Cardiovasc. Disord. 2021, 21, 244. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Zhou, F.; Li, H.; Zhang, X.J.; She, Z.G.; Lu, Z.; Cai, J.; Li, H. Nonalcoholic Fatty Liver Disease: An Emerging Driver of Cardiac Arrhythmia. Circ. Res. 2021, 128, 1747–1765. [Google Scholar] [CrossRef]
- Im, J.A.; Kim, S.H.; Lee, J.W.; Shim, J.Y.; Lee, H.R.; Lee, D.C. Association between hypoadiponectinemia and cardiovascular risk factors in nonobese healthy adults. Metab. Clin. Exp. 2006, 55, 1546–1550. [Google Scholar] [CrossRef] [PubMed]
- Kazumi, T.; Kawaguchi, A.; Hirano, T.; Yoshino, G. Serum adiponectin is associated with high-density lipoprotein cholesterol, triglycerides, and low-density lipoprotein particle size in young healthy men. Metab. Clin. Exp. 2004, 53, 589–593. [Google Scholar] [CrossRef]
- Komatsu, M.; Ohfusa, H.; Aizawa, T.; Hashizume, K. Adiponectin inversely correlates with high sensitive C-reactive protein and triglycerides, but not with insulin sensitivity, in apparently healthy Japanese men. Endocr. J. 2007, 54, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Vlad, C.E.; Foia, L.; Popescu, R.; Ivanov, I.; Luca, M.C.; Delianu, C.; Toma, V.; Statescu, C.; Rezus, C.; Florea, L. Apolipoproteins A and B and PCSK9: Nontraditional Cardiovascular Risk Factors in Chronic Kidney Disease and in End-Stage Renal Disease. J. Diabetes Res. 2019, 2019, 6906278. [Google Scholar] [CrossRef] [PubMed]
- Climent, E.; Benaiges, D.; Pedro-Botet, J. Hydrophilic or Lipophilic Statins? Front. Cardiovasc. Med. 2021, 8, 687585. [Google Scholar] [CrossRef] [PubMed]
- Lisker-Yourkowitzky, R.; Ramírez-Arroyo, E.; Pérez-Rendon, G.; Díaz-Barrigo Pardo, R.; Siperstein-Blumovicz, M.; Mutchinick-Baringoltz, O. Genotypes of alcohol-metabolizing enzymes in Mexicans with alcoholic liver cirrhosis. Arch. Med. Res. 1995, 26, S63–S67. [Google Scholar]
- Lisker, R.; Pérez-Briceno, R.; Granados, J.; Babinsky, V. Gene frequencies and admixture estimates in the state of Puebla, Mexico. Am. J. Phys. Anthropol. 1988, 76, 331–335. [Google Scholar] [CrossRef]
- Rosenberg, N.A.; Huang, L.; Jewett, E.M.; Szpiech, Z.A.; Jankovic, I.; Boehnke, M. Genome-wide association studies in diverse populations. Nat. Rev. Genet. 2010, 11, 356–366. [Google Scholar] [CrossRef]
- Metzler, B.; Abia, R.; Ahmad, M.; Wernig, F.; Pachinger, O.; Hu, Y.; Xu, Q. Activation of heat shock transcription factor 1 in atherosclerosis. Am. J. Pathol. 2003, 162, 1669–1676. [Google Scholar] [CrossRef]
- Haybar, H.; Shahrabi, S.; Rezaeeyan, H.; Shirzad, R.; Saki, N. Protective role of heat shock transcription factor 1 in heart failure: A diagnostic approach. J. Cell. Physiol. 2019, 234, 7764–7770. [Google Scholar] [CrossRef] [PubMed]
- De Luca, F. Regulatory role of NF-κB in growth plate chondrogenesis and its functional interaction with Growth Hormone. Mol. Cell. Endocrinol. 2020, 514, 110916. [Google Scholar] [CrossRef] [PubMed]
- Fiordelisi, A.; Iaccarino, G.; Morisco, C.; Coscioni, E.; Sorriento, D. NFkappaB is a Key Player in the Crosstalk between Inflammation and Cardiovascular Diseases. Int. J. Mol. Sci. 2019, 20, 1599. [Google Scholar] [CrossRef]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef]
- Men, H.; Cai, H.; Cheng, Q.; Zhou, W.; Wang, X.; Huang, S.; Zheng, Y.; Cai, L. The regulatory roles of p53 in cardiovascular health and disease. Cell. Mol. Life Sci. CMLS 2021, 78, 2001–2018. [Google Scholar] [CrossRef] [PubMed]
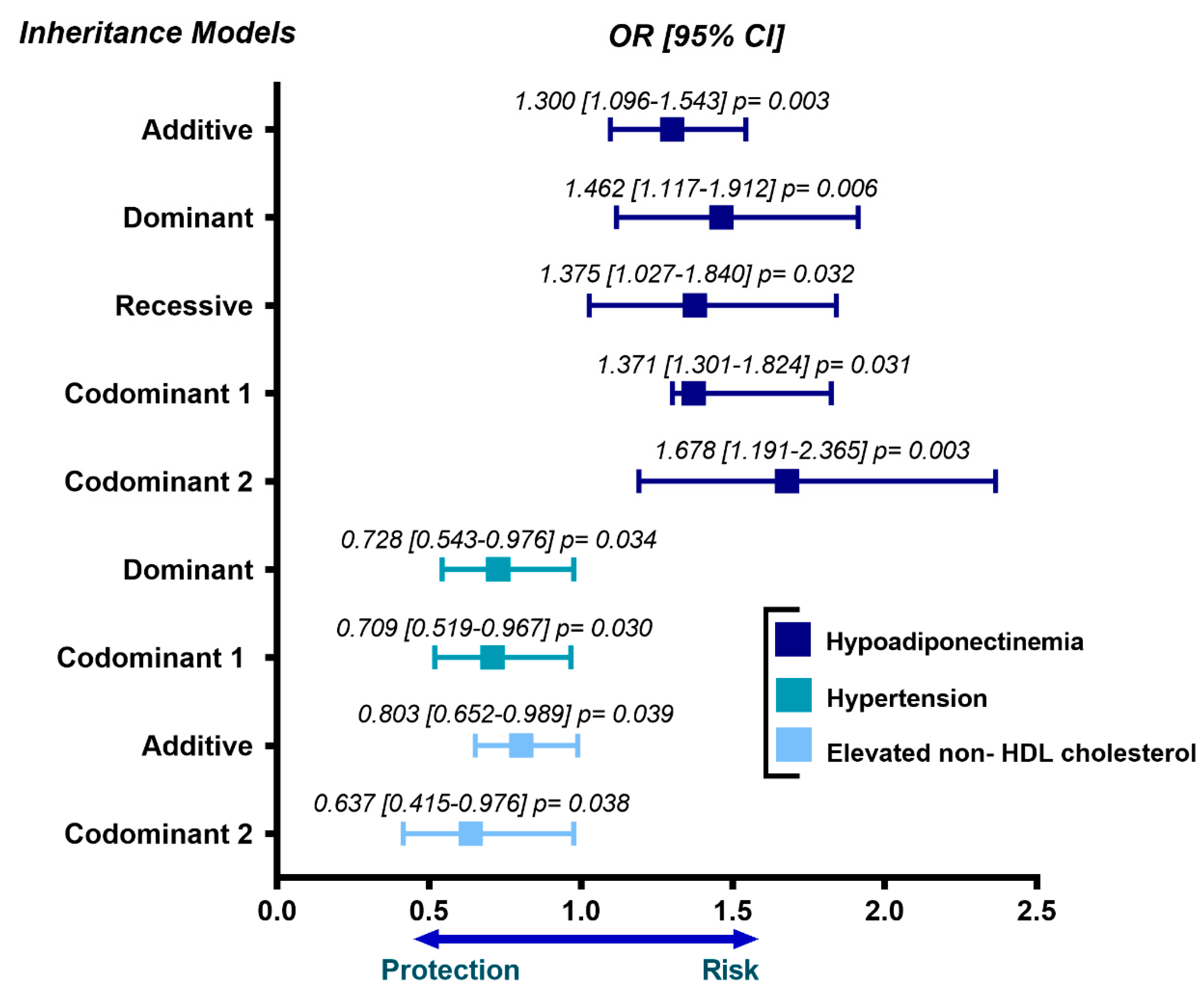
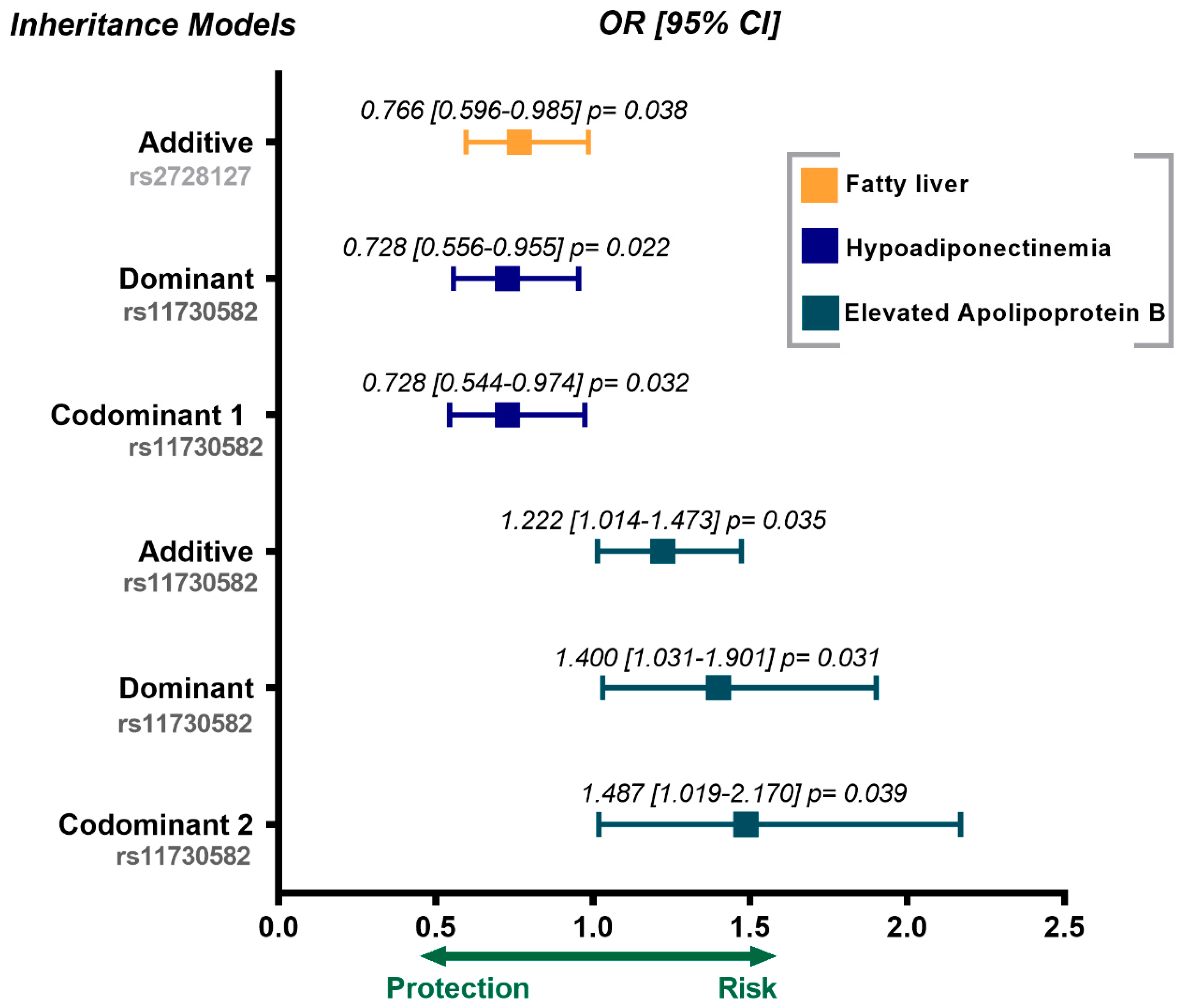
| Characteristics | Control (n = 1073) | pCAD (n = 1142) | * p |
|---|---|---|---|
| Age (years) | 51 ± 9 | 54 ± 8 | <0.001 |
| Sex (male %) | 42.3 | 80.8 | <0.001 |
| Body mass index (kg/m2) | 27.9 [25.5–30.9] | 28.3 [25.9–31.1] | 0.007 |
| Waist circumference (cm) | 94 ± 11 | 98 ± 10 | <0.001 |
| Systolic blood pressure (mmHg) | 113 [104–123] | 116 [106–127] | <0.001 |
| Diastolic blood pressure (mmHg) | 71 [65–77] | 71 [66–78] | 0.046 |
| Visceral abdominal fat (cm2) | 141 [106–181] | 168 [129–218] | <0.001 |
| High-density lipoprotein cholesterol (mg/dL) | 45 [36–55] | 37 [32–44] | <0.001 |
| Low-density lipoprotein cholesterol (mg/dL) | 116 [95–134] | 91 [68–116] | <0.001 |
| Triglycerides (mg/dL) | 145 [108–202] | 162 [119–219] | <0.001 |
| Apolipoprotein B (mg/dL) | 94 [76–113] | 79 [63–102] | <0.001 |
| Apolipoprotein A (mg/dL) | 134 [115–156] | 120 [101–138] | <0.001 |
| Lipoprotein (a) (mg/dL) | 5.2 [2.3–11.5] | 4.8 [2.4–14.1] | 0.478 |
| Glucose (mg/dL) | 90 [84–97] | 95 [87–117] | <0.001 |
| High-sensitivity C-reactive protein (mg/L) | 1.5 [0.8–3.1] | 1.2 [0.6–2.6] | <0.001 |
| Adiponectin (µg/mL) | 8.1 [5.0–12.8] | 5.2 [3.2–8.1] | <0.001 |
| Alkaline phosphatase (IU/L) | 81 [68–96] | 76 [63–95] | <0.001 |
| Serum calcium (mg/dL) | 9.7 ± 0.6 | 9.7 ± 0.7 | 0.278 |
| Characteristics | Control (n = 1073) | pCAD (n = 1142) | * p |
|---|---|---|---|
| LDL-cholesterol ≥ 130 mg/dL (%) | 29.6 | 16.1 | <0.001 |
| Hypoalphalipoproteinemia (%) | 52.1 | 66.9 | <0.001 |
| Hypertriglyceridemia (%) | 47.5 | 56.2 | <0.001 |
| Non-HDL cholesterol > 160 mg/dL (%) | 28.0 | 19.6 | <0.001 |
| Obesity (%) | 30.5 | 35.0 | 0.024 |
| Hypertension (%) | 19.2 | 68.0 | <0.001 |
| High visceral abdominal adipose tissue (%) | 54.8 | 64.5 | <0.001 |
| Current smoking (%) | 22.4 | 11.6 | <0.001 |
| Hypoadiponectinemia (%) | 42.5 | 57.5 | <0.001 |
| Alkaline phosphatase > p75 (%) | 37.6 | 38.9 | 0.569 |
| Polymorphism | Genotype Frequency | MAF | Model | OR [95% CI] | p | ||
|---|---|---|---|---|---|---|---|
| rs2728127 | AA | AG | GG | ||||
| Additive | 0.967 [0.804–1.164] | 0.726 | |||||
| Control (n = 1073) | 0.679 | 0.281 | 0.039 | 0.160 | Dominant | 1.027 [0.829–1.271] | 0.807 |
| Recessive | 0.595 [0.333–1.064] | 0.080 | |||||
| pCAD (n = 1142) | 0.681 | 0.293 | 0.025 | 0.194 | Heterozygous | 1.108 [0.890–1.381] | 0.359 |
| Co-dominant 1 | 1.085 [0.869–1.354] | 0.471 | |||||
| Co-dominant 2 | 0.610 [0.339–1.095] | 0.098 | |||||
| rs11730582 | CC | CT | TT | ||||
| Additive | 0.990 [0.863–1.137] | 0.891 | |||||
| Control (n = 1073) | 0.301 | 0.469 | 0.247 | 0.464 | Dominant | 1.046 [0.841–1.303] | 0.684 |
| Recessive | 0.923 [0.729–1.168] | 0.503 | |||||
| pCAD (n = 1142) | 0.282 | 0.486 | 0.232 | 0.475 | Heterozygous | 1.100 [0.901–1.342] | 0.350 |
| Co-dominant 1 | 1.159 [0.828–1.623] | 0.389 | |||||
| Co-dominant 2 | 1.288 [0.875–1.896] | 0.199 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Hernández, N.; Posadas-Sánchez, R.; Vargas-Alarcón, G.; Hernández-Germán, L.P.; Borgonio-Cuadra, V.M.; Rodríguez-Pérez, J.M. Osteopontin Gene Polymorphisms Are Associated with Cardiovascular Risk Factors in Patients with Premature Coronary Artery Disease. Biomedicines 2021, 9, 1600. https://doi.org/10.3390/biomedicines9111600
Pérez-Hernández N, Posadas-Sánchez R, Vargas-Alarcón G, Hernández-Germán LP, Borgonio-Cuadra VM, Rodríguez-Pérez JM. Osteopontin Gene Polymorphisms Are Associated with Cardiovascular Risk Factors in Patients with Premature Coronary Artery Disease. Biomedicines. 2021; 9(11):1600. https://doi.org/10.3390/biomedicines9111600
Chicago/Turabian StylePérez-Hernández, Nonanzit, Rosalinda Posadas-Sánchez, Gilberto Vargas-Alarcón, Lizet Paola Hernández-Germán, Verónica Marusa Borgonio-Cuadra, and José Manuel Rodríguez-Pérez. 2021. "Osteopontin Gene Polymorphisms Are Associated with Cardiovascular Risk Factors in Patients with Premature Coronary Artery Disease" Biomedicines 9, no. 11: 1600. https://doi.org/10.3390/biomedicines9111600
APA StylePérez-Hernández, N., Posadas-Sánchez, R., Vargas-Alarcón, G., Hernández-Germán, L. P., Borgonio-Cuadra, V. M., & Rodríguez-Pérez, J. M. (2021). Osteopontin Gene Polymorphisms Are Associated with Cardiovascular Risk Factors in Patients with Premature Coronary Artery Disease. Biomedicines, 9(11), 1600. https://doi.org/10.3390/biomedicines9111600







