Novel Nematode-Killing Protein-1 (Nkp-1) from a Marine Epiphytic Bacterium Pseudoalteromonas tunicata
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Maintenance and Synchronisation of Nematode C. elegans
2.3. Generation of HG8 Mutant Libraries and Screening against C. elegans
2.4. Cloning of P. tunicata Gene (HP1) into pBAD24 Vector
2.5. Nematode-Killing Assay
2.6. Egg Hatching and Brood Size Assay
2.7. Bacterial Colonisation Assay
2.8. Enzymatic Assay
2.9. Necrosis Assay
2.10. Microscopy Imaging
2.11. Protein Extraction, SDS PAGE and Protein Modelling
2.12. Statistical Analysis
3. Results
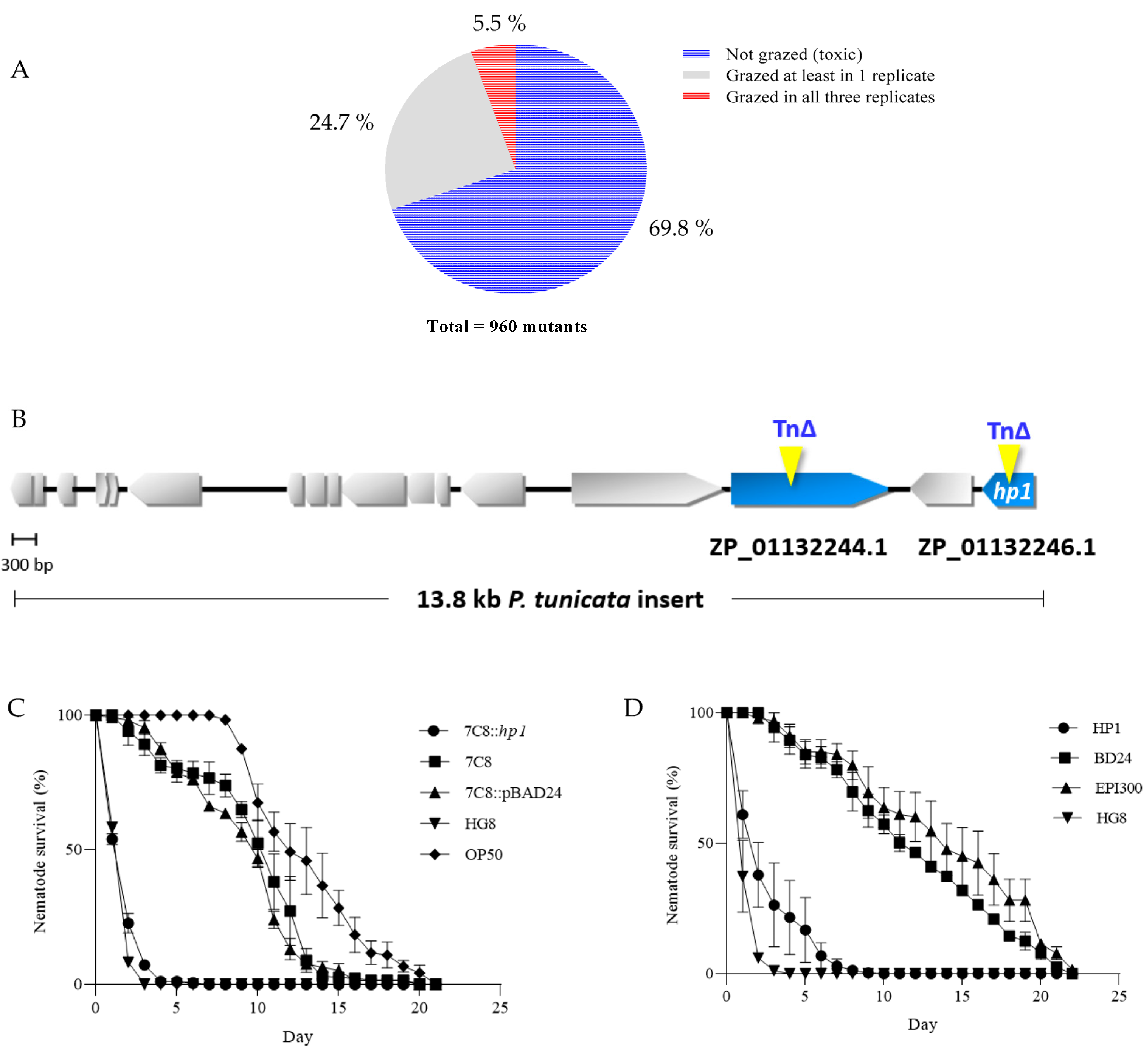
3.1. Generation of HG8 Transposon Mutants and Restoration of 7C8 Mutant Attenuated Activity upon Complementation with HP1
3.2. Expression of Individual HP1 in E. coli Reduced C. elegans Survival
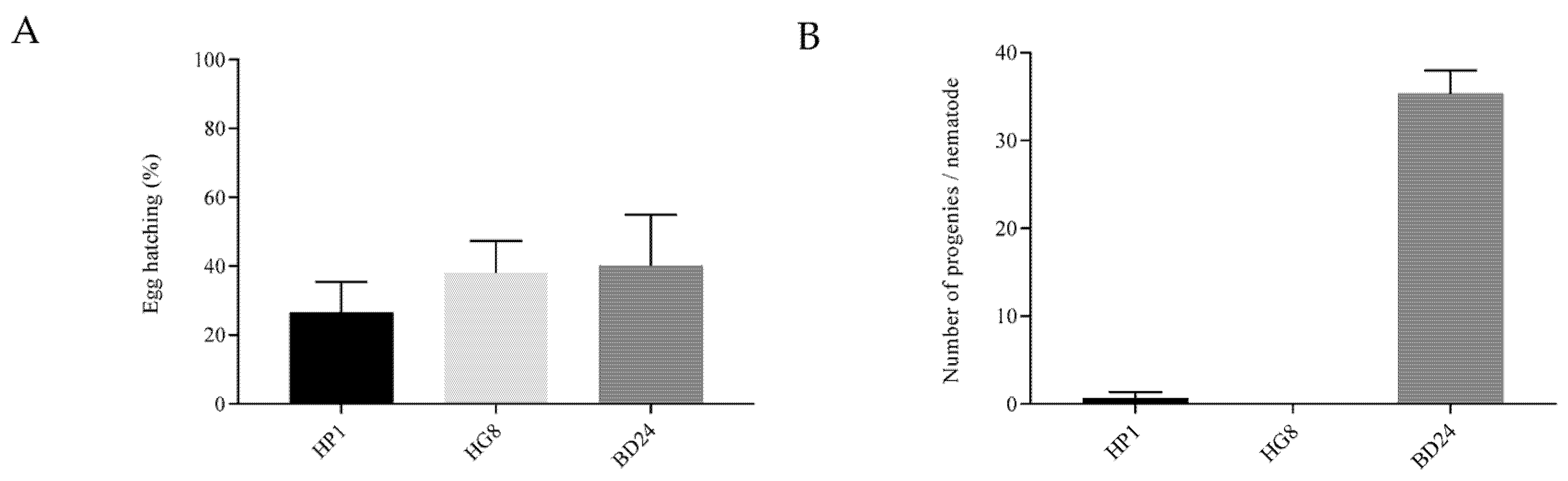
3.3. Exposure to HP1 and HG8 Does Not Affect C. elegans Eggs’ Hatching Efficiency but Decreases the Brood Size
3.4. HP1::GFP and HG8::GFP Bacterial Strains Can Colonise and Persist in C. elegans Gastrointestinal System
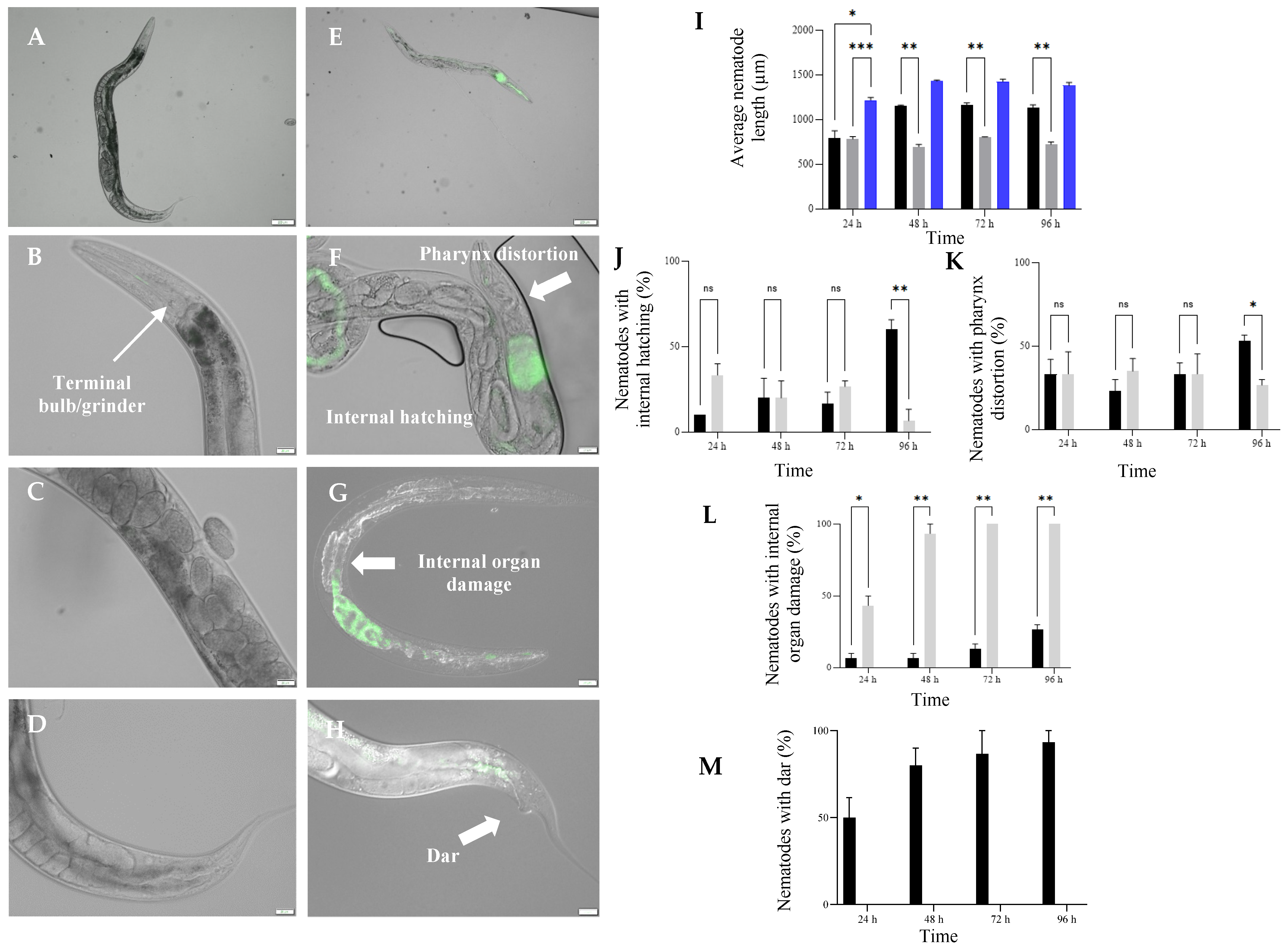
3.5. Exposure to HP1::GFP and HG8::GFP Resulted in Morphological Changes in C. elegans
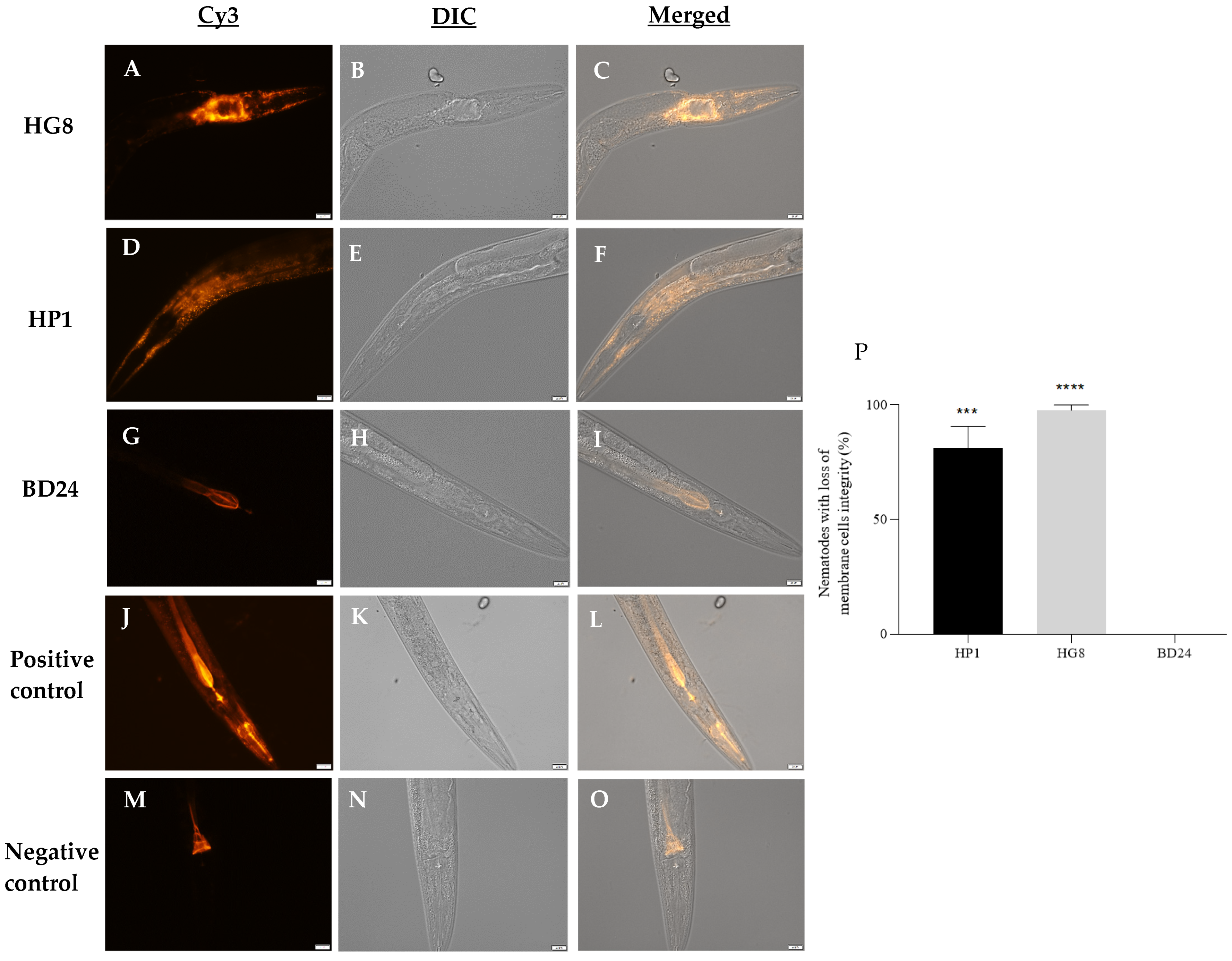
3.6. Exposure to HP1::GFP and HG8::GFP Results in Loss of C. elegans Cell Membrane Integrity
3.7. HP1 and HG8 Protein Extracts Are Toxic against C. elegans
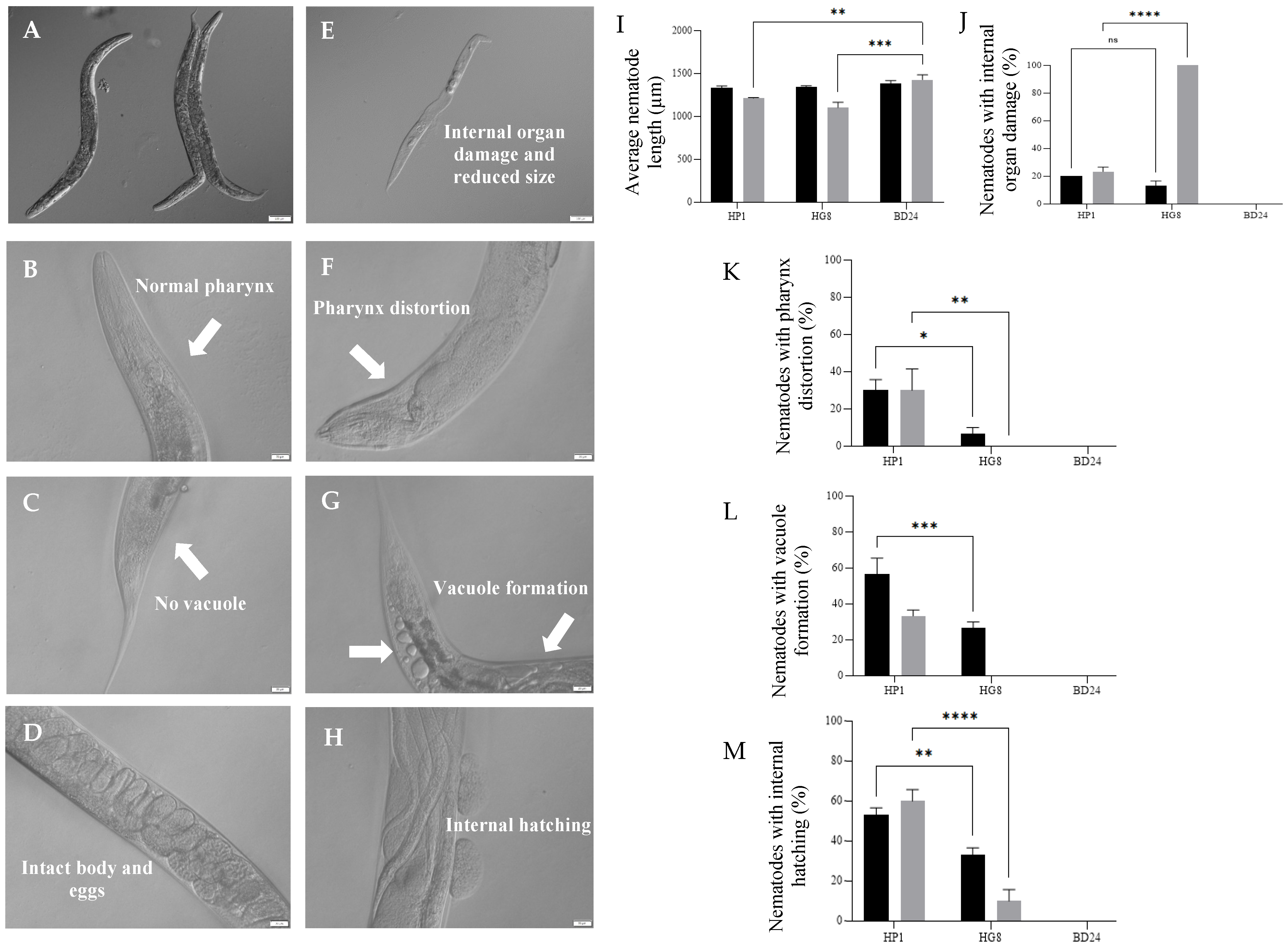
3.8. Nkp-1 Treatment Causes Physical Damage to C. elegans Cells
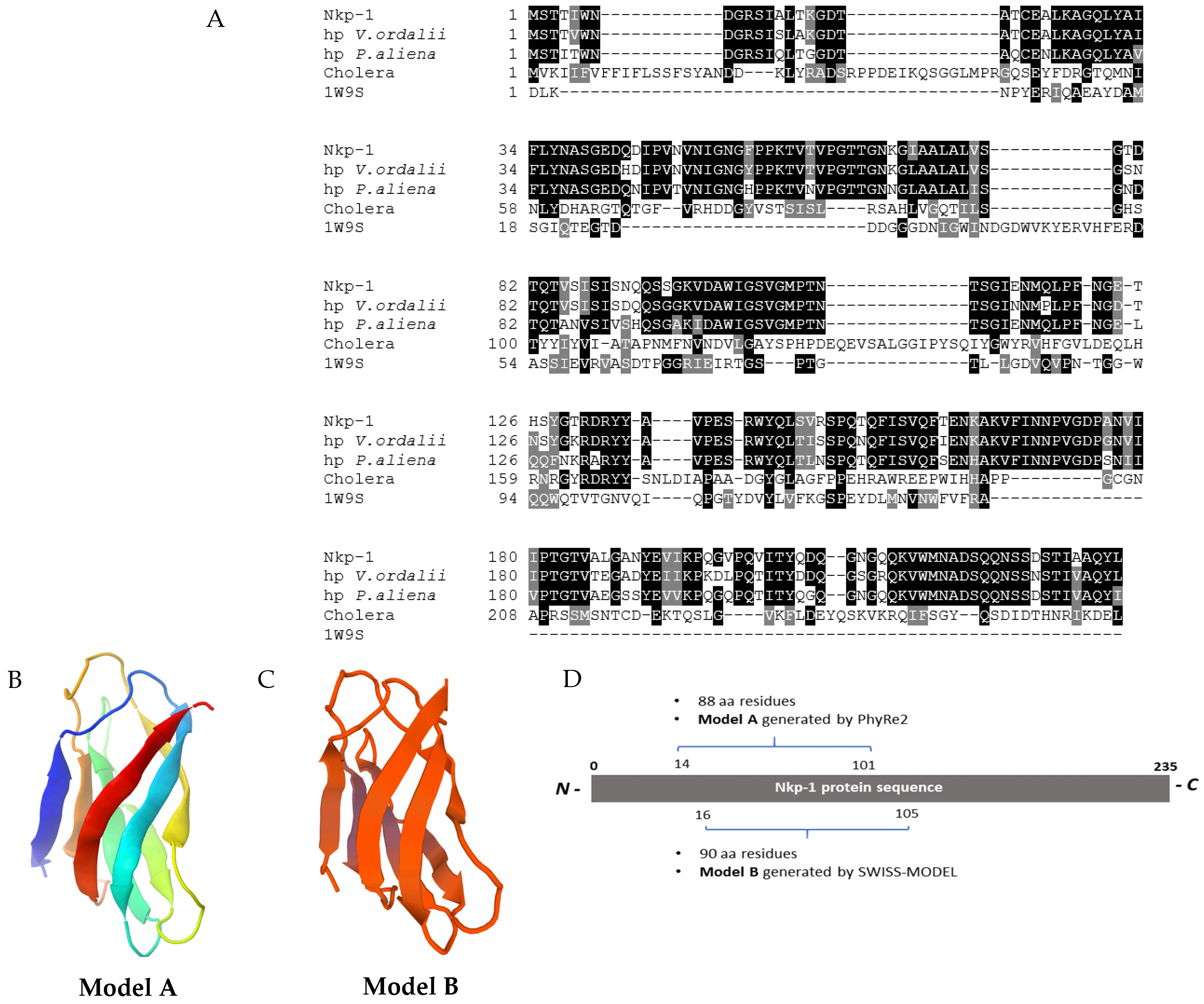
3.9. Nkp-1 Harbours a Carbohydrate-Binding Module
4. Discussion
4.1. HP1 Is Responsible for Nkp-1 Expression
4.2. Nkp-1 Expressing E. coli Clones Kill C. elegans via a Proposed Step-by-Step Mode of Action (MOA)
4.3. Dar Formation and Internal Hatching; C. elegans Response against Nkp-1 Expressing Bacteria
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziegelbauer, K.; Speich, B.; Mäusezahl, D.; Bos, R.; Keiser, J.; Utzinger, J. Effect of sanitation on soil-transmitted helminth infection: Systematic review and meta-analysis. PLoS Med. 2012, 9, e1001162. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Spigt, M.; Bezabih, A.M.; Pavon, I.L.; Dinant, G.-J.; Velasco, R.B. Efficacy of handwashing with soap and nail clipping on intestinal parasitic infections in school-aged children: A factorial cluster randomized controlled trial. PLoS Med. 2015, 12, e1001837. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Soil-Transmitted Helminth Infections. Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 28 September 2021).
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef]
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; den Nijs, L.; Hockland, S.; Maafi, Z.T. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J., Gheysen, G., Fenoll, C., Eds.; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Coyne, D.L.; Cortada, L.; Dalzell, J.J.; Claudius-Cole, A.O.; Haukeland, S.; Luambano, N.; Talwana, H. Plant-parasitic nematodes and food security in Sub-Saharan Africa. Annu. Rev. Phytopathol. 2018, 56, 381–403. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.L.; Liwanag, H.J.; Snyder, J.S.; Akogun, O.; Belizario, V., Jr.; Freeman, M.C.; Gyorkos, T.W.; Imtiaz, R.; Keiser, J.; Krolewiecki, A.; et al. Toward the 2020 goal of soil-transmitted helminthiasis control and elimination. PLoS Negl. Trop. Dis. 2018, 12, e0006606. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, C.; Pulaski, C.N.; Bourguinat, C.; Keller, K.; Prichard, R.K.; Geary, T.G. Clinical validation of molecular markers of macrocyclic lactone resistance in Dirofilaria immitis. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Holden-Dye, L.; Walker, R. Anthelmintic drugs and nematocides: Studies in Caenorhabditis elegans. In WormBook: The Online Review of C. elegans Biology; The C. elegans Research Community, Ed.; WormBook: Pasadena, CA, USA, 2014; pp. 1–12. [Google Scholar] [CrossRef]
- Fanelli, E.; Troccoli, A.; Tarasco, E.; De Luca, F. Molecular characterization and functional analysis of the Hb-Hsp90-1 gene in relation to temperature changes in Heterorhabditis bacteriophora. Front. Physiol. 2021, 12, 136. [Google Scholar] [CrossRef]
- Lourenço-Tessutti, I.T.; Souza Junior, J.D.A.; Martins-de-Sa, D.; Viana, A.A.B.; Carneiro, R.M.D.G.; Togawa, R.C.; de Almeida-Engler, J.; Batista, J.A.N.; Silva, M.C.M.; Fragoso, R.R.; et al. Knock-down of heat-shock protein 90 and isocitrate lyase gene expression reduced root-knot nematode reproduction. Phytopathology 2015, 105, 628–637. [Google Scholar] [CrossRef]
- Midkiff, D.; San-Miguel, A. Microfluidic technologies for high throughput screening through sorting and on-chip culture of C. elegans. Molecules 2019, 24, 4292. [Google Scholar] [CrossRef] [PubMed]
- Giunti, S.; Andersen, N.; Rayes, D.; De Rosa, M.J. Drug discovery: Insights from the invertebrate Caenorhabditis elegans. Pharmacol. Res. Perspect. 2021, 9, e00721. [Google Scholar] [CrossRef]
- Romano, G.; Costantini, M.; Sansone, C.; Lauritano, C.; Ruocco, N.; Ianora, A. Marine microorganisms as a promising and sustainable source of bioactive molecules. Mar. Environ. Res. 2017, 128, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Salikin, N.H.; Nappi, J.; Majzoub, M.E.; Egan, S. Combating parasitic nematode infections, newly discovered antinematode compounds from marine epiphytic bacteria. Microorganisms 2020, 8, 1963. [Google Scholar] [CrossRef]
- Bernbom, N.; Ng, Y.Y.; Kjelleberg, S.; Harder, T.; Gram, L. Marine bacteria from Danish coastal waters show antifouling activity against the marine fouling bacterium Pseudoalteromonas sp. strain S91 and zoospores of the green alga Ulva australis independent of bacteriocidal activity. Appl. Environ. Microbiol. 2011, 77, 8557–8567. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, S.S.; Strube, M.L.; Bech, P.K.; Gram, L.; Sonnenschein, E.C. Marine chitinolytic Pseudoalteromonas represents an untapped reservoir of bioactive potential. mSystems 2019, 4, e00060-19. [Google Scholar] [CrossRef]
- Ballestriero, F.; Thomas, T.; Burke, C.; Egan, S.; Kjelleberg, S. Identification of compounds with bioactivity against the nematode Caenorhabditis elegans by a screen based on the functional genomics of the marine bacterium Pseudoalteromonas tunicata D2. Appl. Environ. Microbiol. 2010, 76, 5710–5717. [Google Scholar] [CrossRef]
- Franks, A.; Egan, S.; Holmström, C.; James, S.; Lappin-Scott, H.; Kjelleberg, S. Inhibition of fungal colonization by Pseudoalteromonas tunicata provides a competitive advantage during surface colonization. Appl. Environ. Microbiol. 2006, 72, 6079–6087. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Daim, M.F. Characterising the Antinematodal Activity of the Marine Bacterium Pseudoalteromonas tunicata When Screened against Caenorhabditis elegans. Bachelor’ Thesis, The University of New South Wales, Sydney, Australia, 2012. [Google Scholar]
- Burke, C.; Thomas, T.; Egan, S.; Kjelleberg, S. The use of functional genomics for the identification of a gene cluster encoding for the biosynthesis of an antifungal tambjamine in the marine bacterium Pseudoalteromonas tunicata. Env. Microbiol. 2007, 9, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Guzman, L.M.; Belin, D.; Carson, M.J.; Beckwith, J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1995, 177, 4121–4130. [Google Scholar] [CrossRef] [PubMed]
- Stretton, S.; Techkarnjanaruk, S.; McLennan, A.M.; Goodman, A.E. Use of green fluorescent protein to tag and investigate gene expression in marine bacteria. Appl. Env. Microbiol. 1998, 64, 2554–2559. [Google Scholar] [CrossRef] [PubMed]
- Stiernagle, T. Maintenance of C. elegans. In WormBook: The Online Review of C. elegans Biology; The C. elegans Research Community, Ed.; WormBook: Pasadena, CA, USA, 2006; pp. 1–11. [Google Scholar] [CrossRef]
- Seidman, C.E.; Struhl, K.; Sheen, J.; Jessen, T. Introduction of plasmid DNA into cells. Curr. Protoc. Mol. Biol. 1997, 37, 1–8. [Google Scholar] [CrossRef]
- Wei, J.-Z.; Siehl, D.L.; Hou, Z.; Rosen, B.; Oral, J.; Taylor, C.G.; Wu, G. An enterotoxin-like binary protein from Pseudomonas protegens with potent nematicidal activity. Appl. Environ. Microbiol. 2017, 83, e00942-17. [Google Scholar] [CrossRef]
- Nappi, J. Discovery of Novel Bioactive Metabolites from Marine Epiphytic Bacteria and Assessment of Their Ecological Role. Ph.D. Thesis, The University of New South Wales, Sydney, Australia, 2019. [Google Scholar]
- Sant’anna, V.; Vommaro, R.C.; de Souza, W. Caenorhabditis elegans as a model for the screening of anthelminthic compounds: Ultrastructural study of the effects of albendazole. Exp. Parasitol. 2013, 135, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bischof, L.J.; Huffman, D.; Aroian, R. Assays for toxicity studies in C. elegans with Bt crystal proteins. Methods Mol. Biol. 2006, 351, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Garsin, D.A.; Sifri, C.D.; Mylonakis, E.; Qin, X.; Singh, K.V.; Murray, B.E.; Calderwood, S.B.; Ausubel, F.M. A Simple model host for identifying Gram-positive virulence factors. Proc. Natl. Acad. Sci. USA 2001, 98, 10892–10897. [Google Scholar] [CrossRef]
- Portal-Celhay, C.; Bradley, E.R.; Blaser, M.J. Control of intestinal bacterial proliferation in regulation of lifespan in Caenorhabditis elegans. BMC Microbiol. 2012, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Galdino, A.C.M.; Branquinha, M.H.; Santos, A.L.S.; Viganor, L. Pseudomonas aeruginosa and its arsenal of proteases: Weapons to battle the host. In Pathophysiological Aspects of Proteases; Chakraborti, S., Dhalla, N., Eds.; Springer: Singapore, 2017; pp. 381–397. [Google Scholar] [CrossRef]
- Hu, J.; Cai, W.; Wang, C.; Du, X.; Lin, J.; Cai, J. Purification and characterization of alkaline lipase production by Pseudomonas aeruginosa HFE733 and application for biodegradation in food wastewater treatment. Biotechnol. Biotechnol. Equip. 2018, 32, 583–590. [Google Scholar] [CrossRef]
- Nursyam, H.; Prihanto, A.A.; Warasari, N.I.; Saadah, M.; Masrifa, R.E.; Nabila, N.A.; Istiqfarin, N.; Siddiq, I.J. The isolation and identification of endophytic bacteria from mangrove (Sonneratia alba) that produces gelatinase. IOP Conf. Ser. Earth Environ. Sci. 2018, 137, 12056. [Google Scholar] [CrossRef]
- Sarker, K.N.; Das Mohapatra, P.K.; Dutta, S. Use of low cost natural resources for enhanced chitinase production and optimization using CCD and RSM: A new initiative for bio-control of plant pathogen. Indian Phytopathol. 2019, 72, 281–300. [Google Scholar] [CrossRef]
- Waschkowitz, T.; Rockstroh, S.; Daniel, R. Isolation and characterization of metalloproteases with a novel domain structure by construction and screening of metagenomic libraries. Appl. Environ. Microbiol. 2009, 75, 2506–2516. [Google Scholar] [CrossRef]
- Balan, S.S.; Nethaji, R.; Sankar, S.; Jayalakshmi, S. Production of gelatinase enzyme from Bacillus spp isolated from the sediment sample of Porto Novo coastal sites. Asian Pac. J. Trop. Biomed. 2012, 2, S1811–S1816. [Google Scholar] [CrossRef]
- Samad, M.Y.A.; Razak, C.N.A.; Salleh, A.B.; Yunus, W.Z.W.; Ampon, K.; Basri, M. A plate assay for primary screening of lipase activity. J. Microbiol. Methods 1989, 9, 51–56. [Google Scholar] [CrossRef]
- Lamine, B.M.; Lamine, B.M.; Bouziane, A. Optimisation of the chitinase production by Serratia marcescens DSM 30121T and biological control of locusts. J. Biotechnol. Biomater. 2012, 2, 3–7. [Google Scholar] [CrossRef]
- Kourtis, N.; Nikoletopoulou, V.; Tavernarakis, N. Small heat-shock proteins protect from heat-stroke-associated neurodegeneration. Nature 2012, 490, 213. [Google Scholar] [CrossRef]
- Zhang, F.; Peng, D.; Cheng, C.; Zhou, W.; Ju, S.; Wan, D.; Yu, Z.; Shi, J.; Deng, Y.; Wang, F. Bacillus thuringiensis crystal protein Cry6Aa triggers Caenorhabditis elegans necrosis pathway mediated by aspartic protease (ASP-1). PLoS Pathog. 2016, 12, e1005389. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhi, D.; Li, C.; Liu, D.; Zhang, J.; Tian, J.; Wang, X.; Ren, H.; Li, H. Infection and immune response in the nematode Caenorhabditis elegans elicited by the phytopathogen Xanthomonas. J. Microbiol. Biotechnol. 2014, 24, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Waterborg, J.H. The Lowry method for protein quantitation. In The Protein Protocols Handbook, 3rd ed.; Walker, J.M., Ed.; Springer’s Humana Press: Totowa, NJ, USA, 2009; pp. 7–10. [Google Scholar]
- Cleveland, D.W.; Fischer, S.G.; Kirschner, M.W.; Laemmli, U.K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J. Biol. Chem. 1977, 252, 1102–1106. [Google Scholar] [CrossRef]
- Laemmli, U.K.; Favre, M. Maturation of the head of bacteriophage T4: I. DNA packaging events. J. Mol. Biol. 1973, 80, 575–599. [Google Scholar] [CrossRef]
- Perkins, D.N.; Pappin, D.J.C.; Creasy, D.M.; Cottrell, J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophor. Int. J. 1999, 20, 3551–3567. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Mantel, N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 1966, 50, 163–170. [Google Scholar]
- Harrington, D. Linear rank tests in survival analysis. In Encyclopedia of Biostatistics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Moustafa, I.; Connaris, H.; Taylor, M.; Zaitsev, V.; Wilson, J.C.; Kiefel, M.J.; Von Itzstein, M.; Taylor, G. Sialic acid recognition by Vibrio cholerae neuraminidase. J. Biol. Chem. 2004, 279, 40819–40826. [Google Scholar] [CrossRef] [PubMed]
- Neal, C.J.; Jobling, M.G.; Holmes, R.K.; Hol, W.G.J. Structural biology: Structural basis for the activation of cholera toxin by human ARF6-GTP. Science 2005, 309, 1093–1096. [Google Scholar] [CrossRef]
- Van Bueren, A.L.; Morland, C.; Gilbert, H.J.; Boraston, A.B. Family 6 carbohydrate binding modules recognize the non reducing end of beta-1,3-linked glucans by presenting a unique ligand binding surface. J. Biol. Chem. 2005, 280, 530–537. [Google Scholar] [CrossRef]
- Fujimoto, Z.; Suzuki, N.; Kishine, N.; Ichinose, H.; Momma, M.; Kimura, A.; Funane, K. Carbohydrate-binding architecture of the multi-modular α-1, 6-glucosyltransferase from Paenibacillus sp. 598K, which produces α-1, 6-glucosyl-α-glucosaccharides from starch. Biochem. J. 2017, 474, 2763–2778. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, P.; Moretti, S.; Xenarios, I.; Orobitg, M.; Montanyola, A.; Chang, J.-M.; Taly, J.-F.; Notredame, C. T-Coffee: A web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res. 2011, 39, W13–W17. [Google Scholar] [CrossRef]
- Rahul, S.; Chandrashekhar, P.; Hemant, B.; Chandrakant, N.; Laxmikant, S.; Satish, P. Nematicidal activity of microbial pigment from Serratia marcescens. Nat. Prod. Res. 2014, 28, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Charuchaibovorn, S.; Sanprasert, V.; Sutthanont, N.; Hu, Y.; Abraham, A.; Ostroff, G.R.; Aroian, R.V.; Jaleta, T.G.; Lok, J.B.; Nuchprayoon, S. Bacillus thuringiensis Cry5B is active against Strongyloides stercoralis in vitro. Am. J. Trop. Med. Hyg. 2019, 101, 1177–1182. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes Database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 2008, 37, D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Sainz-Polo, M.A.; Valenzuela, S.V.; González, B.; Pastor, F.I.J.; Sanz-Aparicio, J. Structural analysis of glucuronoxylan-specific Xyn30D and its attached CBM35 domain gives insights into the role of modularity in specificity. J. Biol. Chem. 2014, 289, 31088–31101. [Google Scholar] [CrossRef]
- Zhang, X.; Rogowski, A.; Zhao, L.; Hahn, M.G.; Avci, U.; Knox, J.P.; Gilbert, H.J. Understanding how the complex molecular architecture of mannan-degrading hydrolases contributes to plant cell wall degradation. J. Biol. Chem. 2014, 289, 2002–2012. [Google Scholar] [CrossRef]
- Katsimpouras, C.; Dimarogona, M.; Petropoulos, P.; Christakopoulos, P.; Topakas, E. A thermostable GH26 endo-β-mannanase from Myceliophthora thermophila capable of enhancing lignocellulose degradation. Appl. Microbiol. Biotechnol. 2016, 100, 8385–8397. [Google Scholar] [CrossRef] [PubMed]
- Teh, A.-H.; Fazli, N.H.; Furusawa, G. Crystal structure of a neoagarobiose-producing GH16 family β-agarase from Persicobacter sp. CCB-QB2. Appl. Microbiol. Biotechnol. 2019, 104, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Suyotha, W.; Oguro, N.; Matsui, T.; Shiga, S.; Itoh, T.; Hibi, T.; Tanaka, Y.; Wakayama, M.; Makabe, K. Crystal structure of the catalytic unit of GH 87-type α-1,3-glucanase Agl-KA from Bacillus circulans. Sci. Rep. 2019, 9, 15295. [Google Scholar] [CrossRef]
- Feng, D.; Chen, Z.; Wang, Z.; Zhang, C.; He, K.; Guo, S. Domain III of Bacillus thuringiensis Cry1Ie toxin plays an important role in binding to peritrophic membrane of Asian corn borer. PLoS ONE 2015, 10, e0136430. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, Á.; Porteous-Álvarez, A.J.; Del Val, M.; Casquero, P.A.; Escriche, B. Toxicity of five Cry proteins against the insect pest Acanthoscelides obtectus (Coleoptera: Chrisomelidae: Bruchinae). J. Invertebr. Pathol. 2020, 169, 107295. [Google Scholar] [CrossRef] [PubMed]
- Michel, G.; Barbeyron, T.; Kloareg, B.; Czjzek, M. The family 6 carbohydrate-binding modules have coevolved with their appended catalytic modules toward similar substrate specificity. Glycobiology 2009, 19, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Berninsone, P.M. Carbohydrates and glycosylation. In WormBook: The Online Review of C. elegans Biology; The C. elegans Research Community, Ed.; WormBook: Pasadena, CA, USA, 2018. [Google Scholar] [CrossRef]
- Jing, X.; Yuan, Y.; Wu, Y.; Wu, D.; Gong, P.; Gao, M. Crystal structure of Bacillus thuringiensis Cry7Ca1 toxin active against Locusta migratoria manilensis. Protein Sci. 2019, 28, 609–619. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Wang, Z.; Zhong, B.; Shu, C.; Zhang, J. Replacement of loop2 and 3 of Cry1Ai in domain II affects specificity to the economically important insect Bombyx mori. J. Invertebr. Pathol. 2020, 169, 107296. [Google Scholar] [CrossRef] [PubMed]
- Deist, B.R.; Rausch, M.A.; Fernandez-Luna, M.T.; Adang, M.J.; Bonning, B.C. Bt toxin modification for enhanced efficacy. Toxins 2014, 6, 3005–3027. [Google Scholar] [CrossRef] [PubMed]
- Karlova, R.; Weemen-Hendriks, M.; Naimov, S.; Ceron, J.; Dukiandjiev, S.; de Maagd, R.A. Bacillus thuringiensis δ-endotoxin Cry1Ac domain III enhances activity against Heliothis virescens in some, but not all Cry1-Cry1Ac hybrids. J. Invertebr. Pathol. 2005, 88, 169–172. [Google Scholar] [CrossRef] [PubMed]
- López Pazos, S.A. Three-dimensional structure of Bacillus thuringiensis toxins: A review. Acta Biológica Colomb. 2007, 12, 19–32. [Google Scholar]
- Jankowska, E.; Parsons, L.M.; Song, X.; Smith, D.F.; Cummings, R.D.; Cipollo, J.F. A Comprehensive Caenorhabditis elegans N-Glycans shotgun array. Glycobiology 2018, 28, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Albertson, D.G.; Thompson, J.N. The pharynx of Caenorhabditis elegans. Philos. Trans. R. Soc. London. B Biol. Sci. 1976, 275, 299–325. [Google Scholar] [CrossRef] [PubMed]
- Avery, L.; Shtonda, B.B. Food transport in the C. elegans pharynx. J. Exp. Biol. 2003, 206, 2441–2457. [Google Scholar] [CrossRef] [PubMed]
- Griffitts, J.S.; Haslam, S.M.; Yang, T.; Garczynski, S.F.; Mulloy, B.; Morris, H.; Cremer, P.S.; Dell, A.; Adang, M.J.; Aroian, R.V. Glycolipids as receptors for Bacillus thuringiensis crystal toxin. Science 2005, 307, 922–925. [Google Scholar] [CrossRef]
- Wei, J.-Z.; Hale, K.; Carta, L.; Platzer, E.; Wong, C.; Fang, S.-C.; Aroian, R.V. Bacillus thuringiensis crystal proteins that target nematodes. Proc. Natl. Acad. Sci. USA 2003, 100, 2760–2765. [Google Scholar] [CrossRef] [PubMed]
- Timmons, A.K.; Meehan, T.L.; Gartmond, T.D.; McCall, K. Use of necrotic markers in the Drosophila ovary. In Necrosis: Methods in Molecular Biology; McCall, K., Klein, C., Eds.; Springer’s Humana Press: Totowa, NJ, USA, 2013; Volume 1004, pp. 215–228. [Google Scholar] [CrossRef]
- Dementiev, A.; Board, J.; Sitaram, A.; Hey, T.; Kelker, M.S.; Xu, X.; Hu, Y.; Vidal-Quist, C.; Chikwana, V.; Griffin, S.; et al. The pesticidal Cry6Aa toxin from Bacillus thuringiensis is structurally similar to HlyE-family alpha pore-forming toxins. BMC Biol. 2016, 14, 71. [Google Scholar] [CrossRef]
- Szczesny, P.; Iacovache, I.; Muszewska, A.; Ginalski, K.; van der Goot, F.G.; Grynberg, M. Extending the aerolysin family: From bacteria to vertebrates. PLoS ONE 2011, 6, e20349. [Google Scholar] [CrossRef] [PubMed]
- Cowell, S.; Aschauer, W.; Gruber, H.J.; Nelson, K.L.; Buckley, J.T. The erythrocyte receptor for the channel-forming toxin aerolysin is a novel glycosylphosphatidylinositol-anchored protein. Mol. Microbiol. 1997, 25, 343–350. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iacovache, I.; van der Goot, F.G.; Pernot, L. Pore formation: An ancient yet complex form of attack. Biochim. Biophys. Acta Biomembr. 2008, 1778, 1611–1623. [Google Scholar] [CrossRef]
- Peng, D.; Wan, D.; Cheng, C.; Ye, X.; Sun, M. Nematode-specific cadherin CDH-8 acts as a receptor for Cry5B toxin in Caenorhabditis elegans. Appl. Microbiol. Biotechnol. 2018, 102, 3663–3673. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.-H.; Xue, K.S.; Tang, L.; Williams, P.L.; Wang, J.-S. Aflatoxin B(1)-induced developmental and DNA damage in Caenorhabditis elegans. Toxins 2017, 9, 9. [Google Scholar] [CrossRef]
- Iatsenko, I.; Boichenko, I.; Sommer, R.J. Bacillus thuringiensis DB27 produces two novel protoxins, Cry21Fa1 and Cry21Ha1, which act synergistically against nematodes. Appl. Environ. Microbiol. 2014, 80, 3266–3275. [Google Scholar] [CrossRef] [PubMed]
- Marroquin, L.D.; Elyassnia, D.; Griffitts, J.S.; Feitelson, J.S.; Aroian, R.V. Bacillus thuringiensis (Bt) toxin susceptibility and isolation of resistance mutants in the nematode Caenorhabditis elegans. Genetics 2000, 155, 1693–1699. [Google Scholar] [CrossRef]
- Galimov, E.R.; Pryor, R.E.; Poole, S.E.; Benedetto, A.; Pincus, Z.; Gems, D. Coupling of rigor mortis and intestinal necrosis during C. elegans organismal death. Cell Rep. 2018, 22, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gilliat, A.F.; Ziehm, M.; Turmaine, M.; Wang, H.; Ezcurra, M.; Yang, C.; Phillips, G.; McBay, D.; Zhang, W.B. Two forms of death in ageing Caenorhabditis elegans. Nat. Commun. 2017, 8, 15458. [Google Scholar] [CrossRef]
- Haskins, K.A.; Russell, J.F.; Gaddis, N.; Dressman, H.K.; Aballay, A. Unfolded protein response genes regulated by CED-1 are required for Caenorhabditis elegans innate immunity. Dev. Cell 2008, 15, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Tavernarakis, N.; Driscoll, M. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca2+ release from the endoplasmic reticulum. Neuron 2001, 31, 957–971. [Google Scholar] [CrossRef]
- Nikoletopoulou, V.; Tavernarakis, N. Chapter Six—Necrotic cell death in Caenorhabditis elegans. In Methods in Enzymology; Ashkenazi, A., Wells, J.A., Yuan, J., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 545, pp. 127–155. [Google Scholar] [CrossRef]
- Durai, S.; Balamurugan, K. Vibrio: Caenorhabditis elegans as a laboratory model for Vibrio infections. In Laboratory Models for Foodborne Infections, 1st ed.; Liu, D., Ed.; CRC Press: Boca Raton, FL, USA, 2017; p. 413. [Google Scholar]
- Somasiri, P.; Behm, C.A.; Adamski, M.; Wen, J.; Verma, N.K. Transcriptional response of Caenorhabditis elegans when exposed to Shigella flexneri. Genomics 2020, 112, 774–781. [Google Scholar] [CrossRef]
- Brito, C.; Cabanes, D.; Sarmento Mesquita, F.; Sousa, S. Mechanisms protecting host cells against bacterial pore-forming toxins. Cell. Mol. Life Sci. 2019, 76, 1319–1339. [Google Scholar] [CrossRef] [PubMed]
- Cinar, H.N.; Kothary, M.; Datta, A.R.; Tall, B.D.; Sprando, R.; Bilecen, K.; Yildiz, F.; McCardell, B. Vibrio cholerae hemolysin is required for lethality, developmental delay, and intestinal vacuolation in Caenorhabditis elegans. PLoS ONE 2010, 5, e11558. [Google Scholar] [CrossRef] [PubMed]
- Irazoqui, J.E.; Urbach, J.M.; Ausubel, F.M. Evolution of host innate defence: Insights from Caenorhabditis elegans and primitive invertebrates. Nat. Rev. Immunol. 2010, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Pukkila-Worley, R.; Ausubel, F.M. Immune defense mechanisms in the Caenorhabditis elegans intestinal epithelium. Curr. Opin. Immunol. 2012, 24, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Los, F.C.O.; Randis, T.M.; Aroian, R.V.; Ratner, A.J. Role of pore-forming toxins in bacterial infectious diseases. Microbiol. Mol. Biol. Rev. 2013, 77, 173. [Google Scholar] [CrossRef]
- Sellegounder, D.; Pandian, S.; Balamurugan, K. Changes in Caenorhabditis elegans exposed to Vibrio Parahaemolyticus. J. Microbiol. Biotechnol. 2011, 21, 1026–1035. [Google Scholar] [CrossRef]
- JebaMercy, G.; Balamurugan, K. Effects of sequential infections of Caenorhabditis elegans with Staphylococcus aureus and Proteus mirabilis. Microbiol. Immunol. 2012, 56, 825–835. [Google Scholar] [CrossRef]
- Mosser, T.; Matic, I.; Leroy, M. Bacterium-induced internal egg hatching frequency is predictive of life span in Caenorhabditis elegans populations. Appl. Environ. Microbiol. 2011, 77, 8189–8192. [Google Scholar] [CrossRef]
- De Souza, C.; Simpson-Louredo, L.; Mota, H.F.; Faria, Y.V.; de Oliveira Cabral, F.; dos Santos Colodette, S.; Canellas, M.E.F.C.; Cucinelli, A.D.E.S.; de Luna, M.D.G.; Santos, C.D.S.; et al. Virulence potential of Corynebacterium striatum towards Caenorhabditis elegans. Antonie Leeuwenhoek 2019, 112, 1331–1340. [Google Scholar] [CrossRef]
- Hodgkin, J.; Kuwabara, P.E.; Corneliussen, B. A novel bacterial pathogen, Microbacterium nematophilum, induces morphological change in the nematode C. elegans. Curr. Biol. 2000, 10, 1615–1618. [Google Scholar] [CrossRef]
- Jain, C.; Yun, M.; Politz, S.M.; Rao, R.P. A pathogenesis assay using Saccharomyces cerevisiae and Caenorhabditis elegans reveals novel roles for yeast AP-1, Yap1, and host dual oxidase BLI-3 in fungal pathogenesis. Eukaryot Cell 2009, 8, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Battisti, J.M.; Watson, L.A.; Naung, M.T.; Drobish, A.M.; Voronina, E.; Minnick, M.F. Analysis of the Caenorhabditis elegans innate immune response to Coxiella burnetii. Innate Immun. 2017, 23, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, H.R.; Hodgkin, J. The ERK MAP Kinase cascade mediates tail swelling and a protective response to rectal infection in C. elegans. Curr. Biol. 2004, 14, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Chew, Y.L.; Schafer, W.; McMullan, R. Identification of a conserved, orphan G protein-coupled receptor required for efficient pathogen clearance in Caenorhabditis elegans. Infect. Immun. 2019, 87, e00034-19. [Google Scholar] [CrossRef] [PubMed]



| Strain or Vector | Relevant Characteristic or Genotype | Reference or Source |
|---|---|---|
| Strain | ||
| E. coli | ||
| EPI300-T1R | F-mcrA ∆(mrrhsdRMSmcrBC) Φ80dlacZ∆M15∆lacX74 recA1 endA1 araD139 ∆(ara, leu) 7697 galU galK λ-rpsL nupG trfA tonA dhfr | Epicentre |
| EPI300 | F-λ-mcrA Δ(mrr-hsdRMS-mcrBC) Φ80dlacZΔM15 Δ(lac)X74 recA1 endA1 araD139 Δ(ara, leu)7697 galU galK rpsL (StrR) nupG’ trfA dhfr | Epicentre |
| OP50 | Uracil auxotroph | [20] |
| DH5α | ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17 (rk– mk+) supE44 thi-1 gyrA relA1, carrying pBAD24 vector | CMSI, UNSW |
| HP1 | EPI300 transformed with pBAD24HP1 (NCBI Accession: ZP_01132246.1) | This study |
| BD24 | EPI300 transformed with empty pBAD24 vector | This study |
| HG8 | EPI300-T1R transformed with pCC1FOS™::ZP_ 01132230.1 to ZP_ 01132246.1 | [18] |
| HP1::GFP | HP1 transformed with p519ngfp plasmid | This study |
| BD24::GFP | BD24 transformed with p519ngfp plasmid | This study |
| HG8::GFP | HG8 transformed with p519ngfp plasmid | This study |
| 7C8 | HG8 transposon mutant library showing mutation at HP1 | [21] |
| 7C8::HP1 | HG8 transposon mutant library showing mutation at ZP_01132246.1, complemented with pBAD24HP1 | This study |
| 7C8::pBAD24 | HG8 transposon mutant library showing mutation at ZP_01132246.1, complemented with empty pBAD24 vector | This study |
| P. aeruginosa ATCC 9027 | Clinical sample | American Type Culture Collection (ATCC®) |
| Vector | ||
| pCC1FOS™::ZP_ 01132230.1 to ZP_ 01132246.1 a | Fosmid backbone for genomic library of Pseudoalteromonas tunicata D2 carrying a wild type D2 insert (13.8 kb) expressing putative anti-nematode activity, Cmr | [18,22] |
| pCC1FOS™::ZP_ 01132230.1 to ZP_01132246.1 with EZ-Tn5™ Δ ZP_01132246.1 a | HG8 fosmid mutated by EZ-Tn5™ transposon on P. tunicata wild type gene ZP_01132246, Cmr, Kanr | [21] |
| pBAD24 a | F-, Δ(argF-lac)169, φ80dlacZ58(M15), glnX44(AS), λ−, rfbC1, gyrA96(NalR), recA1, endA1, spoT1, thiE1, hsdR17, pBAD24 | [23] |
| pBAD24HP1 a | P. tunicata D2 wild type gene (ZP_01132246.1) cloned downstream the pBAD promoter, Ampr | This study |
| p519ngfp | High-copy-number plasmid with constitutive GFP expression; Kmr | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salikin, N.H.; Dubois, M.; Nappi, J.; Lebhar, H.; Marquis, C.; Egan, S. Novel Nematode-Killing Protein-1 (Nkp-1) from a Marine Epiphytic Bacterium Pseudoalteromonas tunicata. Biomedicines 2021, 9, 1586. https://doi.org/10.3390/biomedicines9111586
Salikin NH, Dubois M, Nappi J, Lebhar H, Marquis C, Egan S. Novel Nematode-Killing Protein-1 (Nkp-1) from a Marine Epiphytic Bacterium Pseudoalteromonas tunicata. Biomedicines. 2021; 9(11):1586. https://doi.org/10.3390/biomedicines9111586
Chicago/Turabian StyleSalikin, Nor Hawani, Malak Dubois, Jadranka Nappi, Helene Lebhar, Christopher Marquis, and Suhelen Egan. 2021. "Novel Nematode-Killing Protein-1 (Nkp-1) from a Marine Epiphytic Bacterium Pseudoalteromonas tunicata" Biomedicines 9, no. 11: 1586. https://doi.org/10.3390/biomedicines9111586
APA StyleSalikin, N. H., Dubois, M., Nappi, J., Lebhar, H., Marquis, C., & Egan, S. (2021). Novel Nematode-Killing Protein-1 (Nkp-1) from a Marine Epiphytic Bacterium Pseudoalteromonas tunicata. Biomedicines, 9(11), 1586. https://doi.org/10.3390/biomedicines9111586






