Abstract
Oral mucositis (OM) is a painful condition caused by chemotherapeutic or radiotherapeutic cancer treatments, occurring in patients with different tumour characteristics and locations. OM greatly impacts a patient’s quality of life and cancer recovery. Current OM management strategies are not providing sufficient prevention and treatment; new approaches to injury management are needed. Studies on the benefit of omega-3 free fatty acids (FFA) in human health have increased significantly in recent years. FFA properties have been studied extensively, including their potential therapeutic use in inflammatory conditions. However, omega-3 FFA’s use as a supplementary treatment for OM has not been clinically tested. Preliminary evidence suggests that utilising FFA to manage OM could be a useful strategy for lesion management, assisting with healthy oral mucosa recovery. This review will describe the incidence, risk factors, biology of OM and the current treatment strategies, leading to a discussion of the utility of omega-3 FFA as a novel therapeutic agent for OM.
1. Introduction
Oral mucositis (OM) is a severe form of acute inflammation and ulceration in the oral mucosa that can be induced by oncological therapy. OM induces erythema and swelling in the oral mucosa, followed by generalised ulceration and bleeding that can spread further from the oral cavity to the digestive tract and that is capable of causing debilitating effects for patients with OM. Effects include pain, inflammation, compromised oral hygiene, an increased risk for local and systemic infections as well as impaired nutrition [1,2,3,4]. The OM doubles the risk of systemic infections and quadruples the risk of death in cancer patients. Supportive care approaches to managing symptoms are commonly used in the OM condition. However, due the complicated pathobiology, those interventions are often not efficient and effective for all patients. Therefore, OM relief still represents an unmet need.
The aim of this review is to update knowledge about the concept, incidence and pathogenesis of OM, to examine the well-established therapeutic strategies in the prevention or treatment of OM and to discuss how omega-3 free fatty acids supplements could be used to treat OM patients.
2. Incidence and Risk Assessment
The incidence of OM in patients with various types of cancer ranges from approximately 30% to 40% to almost 100% [5]. However, the most severe and debilitating type of OM observed in cancer patients is caused by head and neck radiation therapy, where it manifests in almost all patients [6]. OM develops in approximately 60% to 85% of patients undergoing hematopoietic stem cell transplantation (HSCT) and 20% to 40% of patients receiving conventional chemotherapies [7]. The use of concomitant chemotherapy and targeted agents increases OM risk [5]. The true prevalence of OM as an adverse effect in oncological treatment has potentially been underreported. OM incidence and severity vary depending on the treatment modality. Often, its severity is clinically observed in patients receiving chemotherapy, radiotherapy, or a combination of the two. Radio-induced OM occurs in head and neck cancer patients who receive cumulative radiation doses ranging from <32 gray (Gy) to greater than 65 Gy. However, dose fractionation protocols and differences in RT techniques result in different incidence rates [8]. Add to these variables the locations and intensities of OM that are associated with the vast range of chemotherapeutic drugs. The highest incidence of OM occurs in patients that receive antimetabolites, platin-derived DNA abductors, taxanes, anthracyclines, irinotecan and alkylating agents [9]. More research is required to understand the true prevalence of OM pathology in oncological therapies.
OM adversely affects several clinical outcomes for patients. Cancer patients who develop this comorbidity typically experience a decreased tolerance for therapy, are at a higher risk of readmission and tend to have longer hospitalisation periods than patients without OM [10]. An intensive care unit study revealed that the most commonly diagnosed alterations in the oral cavity were an imbalance in the oral microbiota, oral candidiasis, salivary flow changes and mucositis [11]. Furthermore, OM negatively affects the nutritional status of cancer patients since dysphagia (difficulty in feeding) with solid and liquid food, dysarthria (poor coordination of the speech muscles) and odynophagia (pain or burning sensation when swallowing) affect food intake and other nutritional supplementation [12]. Additionally, symptoms related to developmental pathology, from lower-grade oral burning to severe pain and spontaneous bleeding that disrupts routine feeding, may lead to cachexia (the loss of skeletal muscle and fat tissues), requiring parenteral nutrition via a nasogastric tube [2].
Comorbidities alongside OM can lead to severe systemic disorders, immunosuppression and even sepsis [1,3,7]. Therefore, many studies have been seeking strategies to aid faster recoveries of inflammatory pathological conditions by reducing the rate of oral cavity contamination by harmful microorganisms [11]. OM is a prevalent, adverse effect of cancer treatment that has likely been underreported despite its negative ramifications in patients’ lives [12]. As a consequence, considerable research is being carried out to determine agents and strategies which promote the prevention of OM and the recovery from its disruptions to patients’ quality of life.
3. Biomolecular Mechanisms of OM
Radiation-induced and chemotherapy-induced OM have similar developmental mechanisms [1]. The cascade of biological events responsible for the genesis of OM begins with the induction of DNA damage caused by radiation or chemotherapeutic cancer therapy [6,13] (Figure 1).
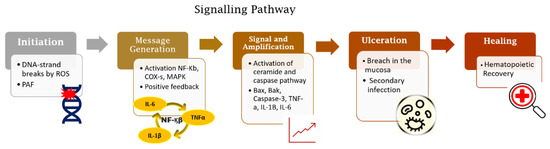
Figure 1.
Five-phase pathobiological model of oral mucositis. Based on this model, the process trigger is DNA damage induced by radio-chemotherapy followed by activation of inflammatory pathways together with apoptosis. These processes lead to the loss of integrity of the mucosal barrier and subsequent wound formation. The end of the signalling pathway occurs spontaneously after cessation of tissue damage.
The genesis of OM in neoplastic treatments occurs with or without mucosal cell DNA damage. Radiation-induced OM takes place when DNA degrades in oral basal-epithelial cells, while chemotherapy-induced OM occurs when basal cells become damaged by chemotherapeutic agents present in the systemic circulation [14]. In radiation-induced OM, reactive oxygen species (ROS) are produced in response to DNA damage. The production of ROS negatively impacts the epithelium by causing irreversible DNA damage, promoting cell apoptosis. It is important to note that, at this stage, patients do not present any clinical symptoms, but the biological disruptions will have already occurred in the submucosa, advancing the progression of OM [13,14,15]. With submucosal injury, initiators of inflammation are triggered (Figure 2). Taken together, these endogenous mechanisms trigger a cascade of biological events and inflammatory pathways that initiate tissue damage in the oral mucosa [5,13,16,17,18].
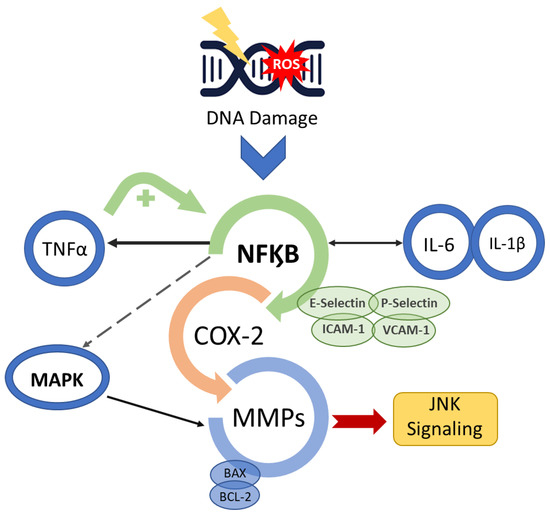
Figure 2.
Mucositis signal amplification. Anti-cancer treatment activates the transcription factor nuclear factor-κB (NF-κB) after damage to basal epithelial cells and cells in the underlying tissue. The break in double-stranded DNA, the generation of ROS and PAF release through platelet aggregation leads to cell death and/or injury. NF-κB up-regulation initiates a positive feedback loop and consequently amplifies the production of pro-inflammatory cytokines (TNF-α, interleukin (IL)-6 and IL-1β) as well as the transcription of genes encoding MAPK, COX-2 and tyrosine-kinase signalling molecules, prompting activation of apoptotic genes (BAX and BCL-2) and matrix metalloproteinases.
Additionally, enzymatic and fibroblast activation occurs, further accelerating cell apoptosis in the oral submucosa, advancing OM [19]. The activation of the pro-inflammatory NF-kB pathway also induces the expression of various adhesive molecules, including E-selectin, P-selectin, ICAM-1, vascular adhesion molecule 1 (VCAM-1) and angiogenesis molecules [18,20,21]. Consequently, c-Jun N-terminal kinase (JNK) signalling is triggered, initiating a fibronectin breakdown and leading to macrophage activation [14,15,22,23]. NF-kB also affects genes in the B-cell lymphoma 2 (BCL2) family, which may directly induce cell apoptosis [24].
There is a change in the interface integrity between the epithelium and the submucosa in the basement membrane, which is mediated by the transcription factor that activates protein 1 (AP1), which controls the genes that regulate matrix metalloproteinases (MMP) production [13,14,22,23,24]. The modification in the production of MMP causes destruction of the subepithelial matrix and, consequently, may enhance the effectiveness of other signals, such as those carried out by TNF-α [13].
Tissue-destructive processes result in visible inflammation and ulceration in the oral mucosa, often leading to bacterial colonisation and further aggravating tissue damage. Alterations in the oral microbial communities occur during cancer treatments, leading to microbiota imbalances and consequently to an increase in normally contained populations of microorganisms [25]. The presence of pro-inflammatory microbiota influences the severity of OM. For example, the increased amount of Porphyromonas gingivalis and the oral yeasts Candida glabrata and Candida kefyr alter the recovery capacity of epithelial cells of oral mucosa, leading to a delay in the wound healing capacity [26]. Ruptures in the oral mucosal tissue thereafter led to microbial colonisation and growth, creating a risk of infection for patients. The open wounds in the oral cavity caused by OM allow opportunistic bacteria, such as Actinomyces, Lactobacillus, Bifidobacterium and Eubacterium, to colonise the tissue and release toxins into the submucosa, causing damage and leading to infection. This also increases the risk of septicaemia in patients with neutropenia [27,28,29].
The inflammatory process culminates with wound healing associated with hematopoietic recovery, the re-establishment of balanced local microbial flora and an absence of factors that interfere with wound healing, such as infection resolution and diminished mechanical irritation [16,30,31]. The resulting repair of the extracellular matrix leads to mucosal renewal and healing via stimulated proliferation, migration, adhesion and differentiation of compromised tissues in the submucosa [15,32]. Nevertheless, residual angiogenesis in the tissue after an OM episode induces higher risk of future episodes [33].
The course of OM progression is not only associated with treatment factors but also with patients’ characteristics such as body mass profiles, renal and hepatic function, local oral factors and genetics [5,31,34]. Despite OM being an independent risk factor for the development of infections, several researchers have demonstrated that microbial flora is not a primary causative factor for the developmental pathology [23]. Once the individual risks for mucositis have been identified, efforts to interfere in the inflammatory responses from different treatment approaches will naturally follow. Therefore, the therapy-based strategy involves the concomitant use of agents that are able to act in different phases of the pathogenesis of mucositis [34].
4. Prevention and Management Strategies
OM treatment is a miscellany of therapies that quest the control of the diseases and the symptoms relieved. Therefore, the clinical practice guidelines from the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO) summarized the standard protocol to manage the OM in cancer patients [32,34]. The proposed intervention strategy is to begin with the care in oral health with a combination of toothbrushing and flossing [35]. The strategy is followed by the elimination of any type of irritant and the control of the proliferation of oral pathogenic microflora through mechanical removal, combined with the individual use of mouth rinses to maintain oral hygiene. Complete oral examinations and dental interventions are critical components performed in conjunction with oncological treatments [32,34,35].
Oral cryotherapy and photobiomodulation (PBM) therapy have been utilised preventively to reduce the impact of the treatment toxicity in the oral mucosa [36]. The PBM is recommended for the prevention and treatment of OM in patients receiving cancer treatments. Several studies have demonstrated the effectiveness of anti-inflammatory effects in supporting tissue repair [13,33,37,38,39,40,41,42,43,44,45]. Nevertheless, clinical evidence still shows that some patients present recurring episodes of OM during their cancer therapy despite being treated with LLLT [37].
Pharmacological agents (pentoxifylline, benzydamine hydrochloride, thalidomide and simvastatin) currently utilized to prevent and treat OM have variable efficacy rates and significant side effects, rendering this treatment strategy less than ideal [13,14,15]. The requirement to reduce the side effects of pharmacological agents and increase the possibility of a patient’s fast recovery elicited the need for research to demonstrate the benefits of utilising natural resources and herbal medicines to manage the OM wound and related inflammatory conditions [3,33]. For this reason, several natural products such as chamomile, essentials oils from manuka (Leptospermum scoparium) and kanuka (Kunzea ericoides), vitamins A, B12 and E, folate, glutamine, aloe vera and curcumin have been studied [46,47,48,49]. In addition, several studies have investigated the mechanisms of action of n-3 fatty acids (or omega-3 fatty acids) against several diseases, with observed successes that are likely due to the fatty acids’ anti-inflammatory effects [50].
Researchers have demonstrated that the use of a combination of agents and physical strategies can provide anti-inflammatory, analgesic and anti-microbial effects that can be used to manage cancer-therapy-induced OM in general. The combination strategy has been promising for patients’ symptom relief and wellness during the OM course [13,37].
5. Omega-3: Inflammation Reduction and Tissue Homeostasis Recovery
The PUFAs are a part of the group of fats (lipids) that are the main components of cellular membranes [51]. The shift in cell membrane compositions could be the mechanism of anti-inflammatory agents as well as immune cell activations [52,53]. The synthesis of eicosanoids (prostaglandins, prostacyclins, thromboxanes and leukotrienes) is generated by the presence of omega-3 (eicosapentaenoic acid (EPA; C20: 5ω-3), docosahexaenoic acid (DHA; C22: 6ω-3)) and omega-6 (arachidonic acid (AA)) incorporated into the cell membrane [53]. Further, metabolic enzymes act on EPA and DHA, producing metabolites that activate other anti-inflammatory pathways and weaken the inflammatory action of immune cells [54,55] (Figure 3).
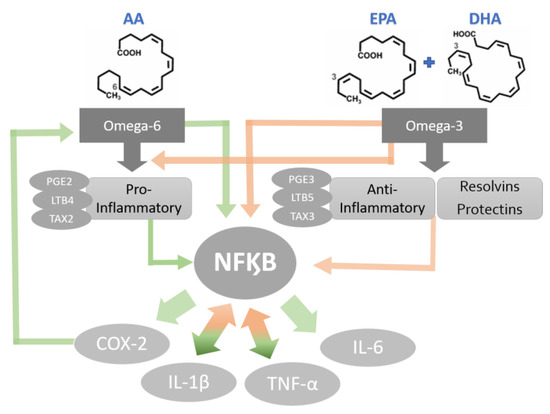
Figure 3.
Potential mechanisms of PUFA-activated cytokine production. Green bold arrows indicate activation or up-regulation and red bold arrows represent inhibition or down regulation.
Omega-3 PUFAs have been proven to inhibit inflammatory processes in several ways. The blockage of the AA cascade is an anti-inflammatory mechanism frequently studied as the pathway is linked with the daily omega-3 (EPA and DHA) supplementation [56,57,58,59]. The increased bioavailability of omega-3 leads to an additional level of immunoregulation by these FFAs [56,57]. Publications show that AA metabolites, such as prostaglandin and leukotrienes, are involved in oral health and diseases, including the modulation of salivary gland inflammation [60]. Although the role of AA metabolites in OM is not well established, there is evidence that topical prostaglandin-E2 (PGE2) application is effective in reducing chemoradiotherapy-induced OM [61]. Therefore, EPA and DHA used to reduce the oral mucosa inflammatory process should be further investigated to elucidate whether the FFAs promote inhibition of pro-inflammatory cascades during the OM development. The action of PUFAs on the immune system is related not only to the profile alteration of eicosanoids during inflammatory activation but also to the alteration of pro-inflammatory protein production, including cytokines and adhesion molecules [56].
A prominent anti-inflammatory mechanism of action of omega-3 is its epigenetic inhibition of NF-kB in response to inflammatory stimuli [59]. This epigenetic alteration is also responsible for the decrease in the expression levels of adhesion molecules, inflammatory cytokines and COX-2 metabolites [62,63]. In addition, FFA can block the translocation of NF-kB to the nucleus by inducing peroxisome proliferator-activated receptor gamma (PPARg) target genes [64]. This translocation inhibition leads to a decrease in the production of cytokines such as TNF-α, IL-1β and IL-6 [55,62,63,64]. According to Calder (2013), the omega-3 FFAs elicit an effect on inflammatory genes’ expression via the inhibition of the activation of the transcription factor NF-kB in response to exogenous inflammatory stimuli [63].
As previously mentioned, NF-kB is a primary driver in the process of mucositis pathobiology and the severity of the disease is associated with the pathway activators. Their activation leads to further production of the principal inflammatory target cytokines, which, in turn, amplifies the response [18]. Omega-3 blocking the NF-kB migration to the nucleus could decrease the production of cytokines responsible for the positive feedback and affect the development of the OM signal amplification phase. The blockade of this stage alters the biological environment to reduce the message generation that activates the damaged response pathways. Consequently, there would be a stop in the cytokines’ feedback loops and a slowdown of tissue injury. However, as the source of damage remains due the chemotherapeutic or irradiation insult, the mucosa still presents molecular alterations that would delay the total tissue recovery until the end of oncological treatment.
In addition to acting in several pathways, omega-3 FFA modulates immune cells’ activity. PUFAs act directly on several immune cells. FFA supplementation modulates neutrophil function, including migration and phagocytic capacity as well as the production of reactive oxygen species and cytokines. It stimulates macrophage cells to produce and secrete cytokines and chemokines, to increase capacity of phagocytosis and the cells polarization and to modulate T cell activation [55].
Mucosa wound recovery in cancer patients could be supported by the daily intake of these natural supplements, not only to increase the immune cell’s capacity to act directly in the mucosa but also in the systemic immunoregulation. During the OM development, there is an activation of inflammatory cascades that affect mast cells, neutrophils and natural killer cells, leading to the production of AA metabolites, toxic phagocytic products (oxygen metabolites, nitric oxide, collagenases, etc.), toxic lymphocyte products, neuropeptides and various components of the plasma proteolytic cascades [65]. In this condition, FFAs would be able to reduce inflammation through immune cell regulation and support wound reepithelialisation.
6. Evidence of Omega-3 as Therapeutic Strategy
Natural and herbal remedies have been precursors to numerous medicines that are commercialised and utilised today [66]. A novel strategy to speed up the recovery of OM is to improve patients’ nutrition by increasing their intake of omega-3. This nutritive change may be particularly effective in promoting tissue recovery, decreasing inflammation and improving the body’s natural immune response to OM [67]. The anti-inflammatory and healing properties of omega-3 fatty acids make them exciting potential pharmaceutical agents for several pathological conditions. Evidence suggests that local and systemic levels of inflammatory mediators, combined with EPA and DHA oral supplementation, may encourage inflammation resolution, PMN down-regulation and wound reepithelialisation [68].
In a critical review, the anti-inflammatory potential of the compounds has also been evaluated in the management of different cancer therapy side effects, such as anorexia-cachexia syndrome, pain, depression and paraneoplastic syndromes. The authors concluded, through preclinical evidence, that omega-3 PUFAs and their metabolites might modulate the main pathways underlying complications secondary to cancer [69]. The evidence that PUFAs are promising for handling the toxicity effects of the oncologic treatment is an initial point to evaluate their use in the OM prevention and treatment.
Although omega-3 may be a promising agent to treat and manage various pathologies, there are few studies that investigate how and if omega-3 fatty acids aid in the healing of wounds from different aetiologies (Table 1). Omega-3 activity in the recovery of epithelial cells has been demonstrated in clinical trial studies. The FFA capacity to reduce swelling and pain without debridement of the necrotic tissue in cutaneous wound healing was demonstrated. In addition, the studies have shown that FFA increases proinflammatory cytokine production, PMN down-regulation and wound reepithelialization [56,68]. Although the process of wound healing in the oral mucosa is starkly different to other tissues, the evidence from the studies gives a broad sense for the way that omega-3 could act in mucosal recovery.

Table 1.
Summary of the characteristics of studies investigating the effectiveness of omega-3 (ω-3) PUFAS on different ulcers.
Animal studies have demonstrated that supplementing the diet with 0.2% to 0.4% of the total animal weight with omega-3 was associated with accelerating ulcer healing in murine mucosal models [70,71]. Hashemipour et al. concluded that omega-3 was effective in wound re-epithelialization, increasing the average thickness of the epithelium and encouraging inflammation resolution [71]. The use of FFA in mucosal recovery also specifically increases the formation of granulation tissue, encourages fibroblast action and reduces the severity and size of oral wounds [70,71]. Despite both studies having been conducted using animal models, the results agree with the general understanding of the mechanism action of omega-3 in tissue inflammatory processes.
Two double-blind, placebo-controlled mucosa cell-based clinical trials assessed the effects of the systemic use of omega-3 on the treatment of recurrent aphthous stomatitis. Both studies note that omega-3 treatment achieved a significant reduction in ulcer numbers, duration in the tissue and level of pain. Results indicate that the daily consumption of omega-3 capsules of 1000 mg (200 mg of DHA and 300 mg of EPA essential fatty acids) could be effective in the management of recurrent ulcers in the oral cavity [72,73]. Even though there are differences in etiopathology between aphthous stomatitis and OM, the study’s conclusion brings forth important considerations regarding mucosa recovery under omega-3 influence.
In one clinical study, patients received 2000 mg of fish oil (360 mg of EPA and 240 mg of DHA) omega-3 fatty acids and presented significantly less pain and irritation of the oral cavity [74]. Despite the limitations of this particular study, the clinical observations support the premise that dietary intake of omega-3 can affect molecular and cellular activities supporting tissue recovery [59]. Several separate experiments have also demonstrated the positive effects of omega-3 fatty acids in preventing complications associated with diseases and treatments across a range of cyclosporine use, hypertension, diabetes, arthritis, other inflammatory conditions, autoimmune disorders and cancer [54,59].
The American Heart Association (AHA) has suggested that a safe EPA and DHA dosage for healthy people is 0.5 g to 1.8 g daily [75]. This is equivalent to approximately one to two servings of fish per week. However, this recommendation is based on the calculated effective dosage for preventing cardiovascular diseases and their associated mortality. Specific dosage recommendations for the treatment of OM must be established before this treatment strategy is clinically implemented. Furthermore, AHA guidelines advise monitoring patients who consume high doses of EPA and DHA (>3 g/d) because of the potential complication of excessive bleeding [75]. Additionally, the AHA cautions consumers to be aware of their fish sources, as some species of fish contain high concentrations of toxins, such as methylmercury and dioxins [75]. This risk can be mitigated by consuming younger and smaller fish and consuming fish from low-risk waters. Generally, the benefits of consuming omega-3 fatty acids appear to outweigh the risks. Therefore, omega-3, in safe doses, could be a feasible intervention to aid in the recovery of OM. The increased use of natural medicine instead of the synthetic pharmaceuticals in the management of some diseases is mainly due to less adverse effects [76]. Studies have demonstrated the possibility of handling the healing of different wounds via the dietary intake of PUFAs based on their anti-inflammatory effects [56,68,70,71,72,73,74]. Altogether, it is possible to manage OM via the dietary intake of PUFAs due to their anti-inflammatory effects. In short, the anti-inflammatory mechanisms of PUFAs include decreasing immune cell recruitment, switching pro-inflammatory pathways and eliminating apoptotic cells through phagocytosis, resulting in tissue healing [76].
This review outcome agrees with the latest research findings, that PUFAs present a promising approach in addressing the lack or delay of the recovery of OM during cancer treatment, possibly improving the overall quality of life and, consequently, patients’ survival [77].
7. Conclusions
Evidence suggests that the dietary supplementation of FFA could be effective for managing the complications associated with cancer treatment, including the physiological development of OM. This intervention is especially relevant for cancer patients not responding to current OM treatment strategies. Nevertheless, this possibility needs to be better studied before it can be clinically implemented. Studies on the pathophysiology of OM, including its pharmacogenomic influences and epidemiology, will be helpful to better understand the disease. Meta-analyses on the best pharmaceutical agent combinations to treat OM will also be beneficial in this pursuit. Importantly, more primary research must be conducted on the anti-inflammatory mechanisms of omega-3 fatty acids to discern if this supplementation strategy will truly be efficacious for treating inflammatory diseases such as OM; if so, then the optimised dosage regime of omega-3 fatty acids for OM must be discovered before clinical implementation is possible.
Author Contributions
R.C.L. contributed to conception and design and drafted the manuscript; F.d.A.A. and J.L. contributed to conception and critically revised the manuscript; E.F. contributed to the revision of the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by New Zealand National Science Challenge High Value Nutrition grant number HVN 3719491 1905 and Beyond Capital MedTech Management Limited.
Acknowledgments
Auckland University of Technology Doctoral Scholarship to Lessa, R.C.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AA | Arachidonic acid |
| AHA | American Heart Association |
| ALA | Alpha-linolenic acid |
| AP1 | Activator protein 1. |
| BAX | BCL-2-like protein 4 |
| BCL2 | B-cell lymphoma 2 gene |
| COX-2 | Cyclooxygenase-2 |
| DHA | Docosahexaenoic acid |
| DNA | Deoxyribonucleic acid |
| EPA | Eicosapentaenoic acid |
| FFA | Free fatty acids |
| GPx | Glutathione peroxidase |
| HO-1 | Heme oxygenase 1 |
| HSCT | Hematopoietic stem cell transplantation |
| ICAM-1 | Endothelial- and leukocyte-associated transmembrane |
| ICU | Intensive care unit |
| IL-1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| JNK | c- Jun N-terminal kinase |
| LLLT | Low-level laser therapy |
| MAPK | Mitogenated protein kinase |
| MASCC/ISOO | The Mucositis Study Group of the Multinational Association for Supportive Care in Cancer and the International Society of Oral Oncology |
| MMP | Matrix metalloproteinase |
| MTX | Methotrexate |
| NF-kB | Nuclear factor-kape Beta |
| OM | Oral mucositis |
| ω-3 | Omega-3PAF: Platelet-activating factor |
| PBM | Photobiomodulation |
| PMN | Polymorphonuclear leukocytes |
| PPARg | Peroxisome Proliferator Activated Receptor Gamma |
| PUFA | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
| TNF-α | Tumour necrosis factor alpha |
| TPN | Total parenteral nutrition |
| U.S. FDA | United States Food and Drug Administration |
| VCAM-1 | Vascular adhesion molecule 1 |
References
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of oral mucositis in patients who have cancer. Dent. Clin. N. Am. 2008, 52, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Nonzee, N.J.; Dandade, N.A.; Patel, U.; Markossian, T.; Agulnik, M.; Argiris, A.; Patel, J.D.; Kern, R.C.; Munshi, H.G.; Calhoun, E.A.; et al. Evaluating the supportive care costs of severe radiochemotherapy-induced mucositis and pharyngitis: Results from a Northwestern University Costs of Cancer Program pilot study with head and neck and nonsmall cell lung cancer patients who received care at a county hospital, a Veterans Administration hospital, or a comprehensive cancer care center. Cancer 2008, 113, 1446–14452. [Google Scholar] [CrossRef] [PubMed]
- Alvariño-Martín, C.; Sarrión-Pérez, M.G. Prevention and treatment of oral mucositis in patients receiving chemotherapy. J. Clin. Exp. Dent. 2014, 6, e74–e80. [Google Scholar] [CrossRef] [PubMed]
- De Barros, P.A.V.; Rabelo Andrade, M.E.; de Vasconcelos Generoso, S.; Mendes Miranda, S.E.; Dos Reis, D.C.; Lacerda Leocádio, P.C.; de Sales E Souza, É.L.; Dos Santos Martins, F.; da Gama, M.A.S.; Cassali, G.D.; et al. Conjugated linoleic acid prevents damage caused by intestinal mucositis induced by 5-fluorouracil in an experimental model. Biomed. Pharmacother. 2018, 103, 1567–1576. [Google Scholar] [CrossRef]
- Villa, A.; Sonis, S.T. Mucositis: Pathobiology and management. Curr. Opin. Oncol. 2015, 27, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B. Mucositis Study Section of the Multinational Association for Supportive Care in Cancer; International Society for Oral Oncology. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Q.; Zheng, Z.; Liu, Z.; Meng, L.; Dong, L.; Jiang, X. Status of treatment and prophylaxis for radiation-induced oral mucositis in patients with head and neck cancer. Front. Oncol. 2021, 11, 642575. [Google Scholar] [CrossRef] [PubMed]
- Pulito, C.; Cristaudo, A.; Porta, C.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral mucositis: The hidden side of cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 210. [Google Scholar] [CrossRef]
- Naidu, M.U.; Ramana, G.V.; Rani, P.U.; Mohan, I.K.; Suman, A.; Roy, P. Chemotherapy-induced and/or radiation therapy-induced oral mucositis--complicating the treatment of cancer. Neoplasia 2004, 6, 423–431. [Google Scholar] [CrossRef]
- Silva, A.P.; Caruso, P.; Jaguar, G.C.; Carvalho, P.A.; Alves, F.A. Oral evaluation and procedures performed by dentists in patients admitted to the intensive care unit of a cancer center. Supportive Care Cancer 2014, 22, 2645–2650. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.I.; Campos, C.N.; Aarestrup, F.M.; Aarestrup, B.J. Oral mucositis in cancer treatment: Natural history, prevention and treatment. Mol. Clin. Oncol. 2014, 2, 337–340. [Google Scholar] [CrossRef]
- Treister, N.; Sonis, S. Mucositis: Biology and management. Curr. Opin. Otolaryngol. Head Neck Surg. 2007, 15, 123–129. [Google Scholar] [CrossRef]
- Sonis, S.T. A biological approach to mucositis. J. Supp. Oncol. 2004, 2, 21–32. [Google Scholar]
- Shankar, A.; Roy, S.; Bhandari, M.; Rath, G.K.; Biswas, A.S.; Kanodia, R.; Adhikari, N.; Sachan, R. Current Trends in Management of Oral Mucositis in Cancer Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Blijlevens, N.; Sonis, S. Palifermin (recombinant keratinocyte growth factor-1): A pleiotropic growth factor with multiple biological activities in preventing chemotherapy- and radiotherapy-induced mucositis. Ann. Oncol. 2007, 18, 817–826. [Google Scholar] [CrossRef]
- Razmara, F.; Khayamzadeh, M. An Investigation into the Prevalence and Treatment of Oral Mucositis After Cancer Treatment. Int. J. Cancer Manag. 2019, 12, e88405. [Google Scholar] [CrossRef]
- Logan, R.M.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M.K. Nuclear factor-κB (NF-κB) and cyclooxygenase-2 (COX-2) expression in the oral mucosa following cancer chemotherapy. Oral Oncol. 2007, 43, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Lionel, D.; Christophe, L.; Marc, A.; Jean-Luc, C. Oral mucositis induced by anticancer treatments: Physiopathology and treatments. Ther. Clin. Risk. Manag. 2006, 2, 159–168. [Google Scholar] [CrossRef]
- Mafra, C.A.D.C.C.; Vasconcelos, R.C.; de Medeiros, C.A.C.X.; Leitão, R.F.C.; Brito, G.A.C.; Costa, D.V.D.S.; Guerra, G.C.B.; de Araújo, R.F., Jr.; Medeiros, A.C.; de Araújo, A.A. Gliclazide Prevents 5-FU-Induced Oral Mucositis by Reducing Oxidative Stress, Inflammation, and P-Selectin Adhesion Molecules. Front. Physiol. 2019, 10, 327. [Google Scholar] [CrossRef]
- Sonis, S.T. The biologic role for nuclear factor-kappaB in disease and its potential involvement in mucosal injury associated with anti-neoplastic therapy. Crit. Rev. Oral Biol. Med. 2002, 13, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Al-Dasooqi, N.; Gibson, R.J.; Bowen, J.M.; Logan, R.M.; Stringer, A.M.; Keefe, D.M. Matrix metalloproteinases are possible mediators for the development of alimentary tract mucositis in the dark agouti rat. Exp. Biol. Med. 2010, 235, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Al-Dasooqi, N.; Sonis, S.T.; Bowen, J.M.; Bateman, E.; Blijlevens, N.; Gibson, R.J.; Logan, R.M.; Nair, R.G.; Stringer, A.M.; Yazbeck, R.; et al. Mucositis Study Group of Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO). Emerging evidence on the pathobiology of mucositis. Supportive Care Cancer 2013, 21, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. Pathobiology of oral mucositis: Novel insights and opportunities. J. Support Oncol. 2007, 5, 3–11. [Google Scholar] [PubMed]
- Vesty, A.; Gear, K.; Biswas, K.; Mackenzie, B.W.; Taylor, M.W.; Douglas, R.G. Oral microbial influences on oral mucositis during radiotherapy treatment of head and neck cancer. Supportive Care Cancer 2020, 28, 2683–2691. [Google Scholar] [CrossRef]
- Haverman, T.M.; Laheij, A.M.G.A.; Nie, M.; Deng, D.M.; Raber-Durlacher, J.E.; de Soet, J.J.; Rozema, F.R. Exploring the role of oral microorganisms in the pathogenesis of mucositis by assessing their impact on metabolic activity and reproductive capacity of epithelial cells in vitro. Supportive Care Cancer 2020, 28, 4729–4735. [Google Scholar] [CrossRef]
- Laheij, A.M.; de Soet, J.J. Can the oral microflora affect oral ulcerative mucositis? Curr. Opin. Supportive Palliat. Care 2014, 8, 180–187. [Google Scholar] [CrossRef]
- Van Saene, H.K.; Martin, M.V. Do microorganisms play a role in irradiation mucositis? Eur. J. Clin. Microbiol. Infect. Dis. 1990, 9, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Napeñas, J.J.; Brennan, M.T.; Bahrani-Mougeot, F.K.; Fox, P.C.; Lockhart, P.B. Relationship between mucositis and changes in oral microflora during cancer chemotherapy. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2007, 103, 48–59. [Google Scholar] [CrossRef]
- Hensley, M.L.; Hagerty, K.L.; Kewalramani, T.; Green, D.M.; Meropol, N.J.; Wasserman, T.H.; Cohen, G.I.; Emami, B.; Gradishar, W.J.; Mitchell, R.B.; et al. Clinical practice guideline update: Use of chemotherapy and radiation therapy protectants. J. Clin. Oncol. 2009, 27, 127–145. [Google Scholar] [CrossRef]
- Al-Ansari, S.; Zecha, J.A.; Barasch, A.; de Lange, J.; Rozema, F.R.; Raber-Durlacher, J.E. Oral mucositis induced by anticancer therapies. Curr. Oral. Health Rep. 2015, 2, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Jarvis, V.; Zadik, Y.; Barasch, A.; Ariyawardana, A.; Hovan, A.; Yarom, N.; Lalla, R.V.; Bowen, J.; Elad, S. Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO). Systematic review of miscellaneous agents for the management of oral mucositis in cancer patients. Supportive Care Cancer 2013, 21, 3223–3232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cinausero, M.; Aprile, G.; Ermacora, P.; Basile, D.; Vitale, M.G.; Fanotto, V.; Parisi, G.; Calvetti, L.; Sonis, S.T. New Frontiers in the Pathobiology and Treatment of Cancer Regimen-Related Mucosal Injury. Front. Pharmacol. 2017, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Peterson, D.E.; et al. Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014, 120, 1453–1461. [Google Scholar] [CrossRef]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef]
- Chaveli-López, B.; Bagán-Sebastián, J.V. Treatment of oral mucositis due to chemotherapy. J. Clin. Exp. Dent. 2016, 8, e201–e209. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, P.A.G.; Lessa, R.C.; Carraro, D.M.; Assis Pellizzon, A.C.; Jaguar, G.C.; Alves, F.A. Three photobiomodulation protocols in the prevention/treatment of radiotherapy-induced oral mucositis. Photodiagnosis Photodyn. Ther. 2020, 31, 101906. [Google Scholar] [CrossRef]
- Bensadoun, R.J.; Franquin, J.C.; Ciais, G.; Darcourt, V.; Schubert, M.M.; Viot, M.; Dejou, J.; Tardieu, C.; Benezery, K.; Nguyen, T.D.; et al. Low-energy He/Ne laser in the prevention of radiation-induced mucositis. A multicenter phase III randomized study in patients with head and neck cancer. Supportive Care Cancer 1999, 7, 244–252. [Google Scholar] [CrossRef]
- Arora, H.; Pai, K.M.; Maiya, A.; Vidyasagar, M.S.; Rajeev, A. Efficacy of He-Ne Laser in the prevention and treatment of radiotherapy-induced oral mucositis in oral cancer patients. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2008, 105, 180–186.e1. [Google Scholar] [CrossRef]
- Zanin, T.; Zanin, F.; Carvalhosa, A.A.; Castro, P.H.; Pacheco, M.T.; Zanin, I.C.; Brugnera, A., Jr. Use of 660-nm diode laser in the prevention and treatment of human oral mucositis induced by radiotherapy and chemotherapy. Photomed. Laser Surg. 2010, 28, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Simões, A.; de Campos, L.; de Souza, D.N.; de Matos, J.A.; Freitas, P.M.; Nicolau, J. Laser phototherapy as topical prophylaxis against radiation-induced xerostomia. Photomed. Laser Surg. 2010, 28, 357–363. [Google Scholar] [CrossRef]
- Gouvêa de Lima, A.; Villar, R.C.; de Castro, G., Jr.; Antequera, R.; Gil, E.; Rosalmeida, M.C.; Federico, M.H.; Snitcovsky, I.M. Oral mucositis prevention by low-level laser therapy in head-and-neck cancer patients undergoing concurrent chemoradiotherapy: A phase III randomised study. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 270–275. [Google Scholar] [CrossRef]
- Carvalho, P.A.; Jaguar, G.C.; Pellizzon, A.C.; Prado, J.D.; Lopes, R.N.; Alves, F.A. Evaluation of low-level laser therapy in the prevention and treatment of radiation-induced mucositis: A double-blind randomized study in head and neck cancer patients. Oral Oncol. 2011, 47, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Lopes Martins, A.F.; Nogueira, T.E.; Morais, M.O.; de Sousa-Neto, S.S.; Oton-Leite, A.F.; Valadares, M.C.; Aires Freitas, N.M.; Leles, C.R.; Mendonça, E.F. Cost-effectiveness randomized clinical trial on the effect of photobiomodulation therapy for prevention of radiotherapy-induced severe oral mucositis in a Brazilian cancer hospital setting. Supportive Care Cancer 2021, 29, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Pires Marques, E.C.; Piccolo Lopes, F.; Nascimento, I.C.; Morelli, J.; Pereira, M.V.; Machado Meiken, V.M.; Pinheiro, S.L. Photobiomodulation and photodynamic therapy for the treatment of oral mucositis in patients with cancer. Photodiagnosis Photodyn. Ther. 2020, 29, 101621. [Google Scholar] [CrossRef] [PubMed]
- Yarom, N.; Hovan, A.; Bossi, P.; Ariyawardana, A.; Jensen, S.B.; Gobbo, M.; Saca-Hazboun, H.; Kandwal, A.; Majorana, A.; Ottaviani, G.; et al. Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO). Systematic review of natural and miscellaneous agents, for the management of oral mucositis in cancer patients and clinical practice guidelines—Part 2: Honey, herbal compounds, saliva stimulants, probiotics, and miscellaneous agents. Supportive Care Cancer 2020, 28, 2457–2472. [Google Scholar] [CrossRef]
- Migliorati, C.A.; Oberle-Edwards, L.; Schubert, M. The role of alternative and natural agents, cryotherapy, and/or laser for management of alimentary mucositis. Supportive Care Cancer 2006, 14, 533–540. [Google Scholar] [CrossRef]
- Baharvand, M.; Jafari, S.; Mortazavi, H. Herbs in Oral Mucositis. J. Clin. Diagn Res. 2017, 11, ZE05–ZE11. [Google Scholar] [CrossRef] [PubMed]
- Aghamohamamdi, A.; Hosseinimehr, S.J. Natural Products for Management of Oral Mucositis Induced by Radiotherapy and Chemotherapy. Integr. Cancer Ther. 2016, 15, 60–68. [Google Scholar] [CrossRef]
- Kaur, M.; Sable, D.M.; Chowdhery, A.; Chavan, M. A Review of Omega 3 and it “s Role in Oral Diseases. Int. J. Curr. Adv. Res. 2016, 4, 921–925. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef] [PubMed]
- Surette, M.E. The science behind dietary omega-3 fatty acids. CMAJ 2008, 178, 177–180. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef]
- McDaniel, J.C.; Belury, M.; Ahijevych, K.; Blakely, W. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008, 16, 337–345. [Google Scholar] [CrossRef]
- Smith, P.C.; Martínez, C. Wound Healing in the Oral Mucosa. In Oral Mucosa in Health and Disease; Bergmeier, L., Ed.; Springer: Cham, Switzerland, 2018; pp. 77–90. [Google Scholar] [CrossRef]
- Kang, J.X.; Weylandt, K.H. Modulation of Inflammatory Cytokines by Omega-3 Fatty Acids. In Lipids in Health and Disease; Quinn, P.J., Wang, X., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 133–143. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Sommakia, S.; Baker, O.J. Regulation of inflammation by lipid mediators in oral diseases. Oral Dis. 2017, 23, 576–597. [Google Scholar] [CrossRef] [PubMed]
- Al-Taie, A.; Al-Shohani, A.D.; Albasry, Z.; Altaee, A. Current topical trends and novel therapeutic approaches and delivery systems for oral mucositis management. J. Pharm Bioall Sci. 2020, 12, 94–101. [Google Scholar] [CrossRef]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll- like receptor 4. J. Biol. Chem. 2001, 18, 16683–16689. [Google Scholar] [CrossRef]
- Calder, P.C. Immunonutrition. BMJ 2003, 327, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, W.; Vermeulen, L.; Delerive, P.; de Bosscher, K.; Staels, B.; Haegeman, G. A paradigm for gene regulation: Inflammation, NF-kappaB and PPAR. Adv. Exp. Med. Biol. 2003, 544, 181–196. [Google Scholar] [CrossRef]
- Alvarado, Y.; Bellm, L.A.; Giles, F.J. Oral Mucositis: Time for More Studies. Hematology 2020, 7, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Nagi, R.; Patil, D.J.; Rakesh, N.; Jain, S.; Sahu, S. Natural agents in the management of oral mucositis in cancer patients-systematic review. J. Oral Biol Craniofac Res. 2018, 8, 245–254. [Google Scholar] [CrossRef]
- Chow, O.; Barbul, A. Immunonutrition: Role in Wound Healing and Tissue Regeneration. Adv. Wound Care 2014, 3, 46–53. [Google Scholar] [CrossRef]
- McDaniel, J.C.; Massey, K.; Nicolaou, A. Fish oil supplementation alters levels of lipid mediators of inflammation in microenvironment of acute human wounds. Wound Repair Regen. 2011, 19, 189–200. [Google Scholar] [CrossRef]
- Freitas, R.D.S.; Campos, M.M. Protective Effects of Omega-3 Fatty Acids in Cancer- Related Complications. Nutrients 2019, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, N.N.; Aboubakr, S.H. Impact of Omega-3 on Healing of Buccal Traumatic Ulcer of Albino Rats (Histological and Immunohistological Study). Egypt. Dent. J. 2017, 63, 3193–3201. [Google Scholar] [CrossRef][Green Version]
- Hashemipour, M.A.; Ghasemi, A.R.; Dogaheh, M.A.; Torabi, M. Effects of Locally and Systemically Applied n-3 Fatty Acid on Oral Ulcer Recovery Process in Rats. Wounds 2012, 24, 258–266. [Google Scholar]
- El Khouli, A.M.; El-Gendy, E.A. Efficacy of omega-3 in treatment of recurrent aphthous stomatitis and improvement of quality of life: A randomizedrandomised, double-blind, placebo-controlled study. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2014, 117, 191–196. [Google Scholar] [CrossRef]
- Nosratzehi, T.; Akar, A. Efficacy of Omega-3 in Treatment of Recurrent Aphthous Stomatitis: A Randomised, Double-blind, Placebo-controlled Study. Chin. J. Dent. Res. 2016, 19, 159–164. [Google Scholar] [CrossRef]
- Hashemipour, M.A.; Barzegari, S.; Kakoie, S.; Aghahi, R.H. Effects of Omega-3 Fatty Acids Against Chemotherapy-induced Mucositis: A Double-blind Randomized Clinical Trial. Wounds 2017, 29, 360–366. [Google Scholar] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. AHA Nutrition Committee. American Heart Association. Omega-3 fatty acids and cardiovascular disease: New recommendations from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 151–152. [Google Scholar] [CrossRef]
- Serhan, C.N. Novel lipid mediators and resolution mechanisms in acute inflammation: To resolve or not? Am. J. Pathol. 2010, 177, 1576–1591. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.M.; Calder, P.C.; Ed Rainger, G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol. Ther. 2014, 141, 272–282. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).