Neuropeptides Involved in Facial Nerve Regeneration
Abstract
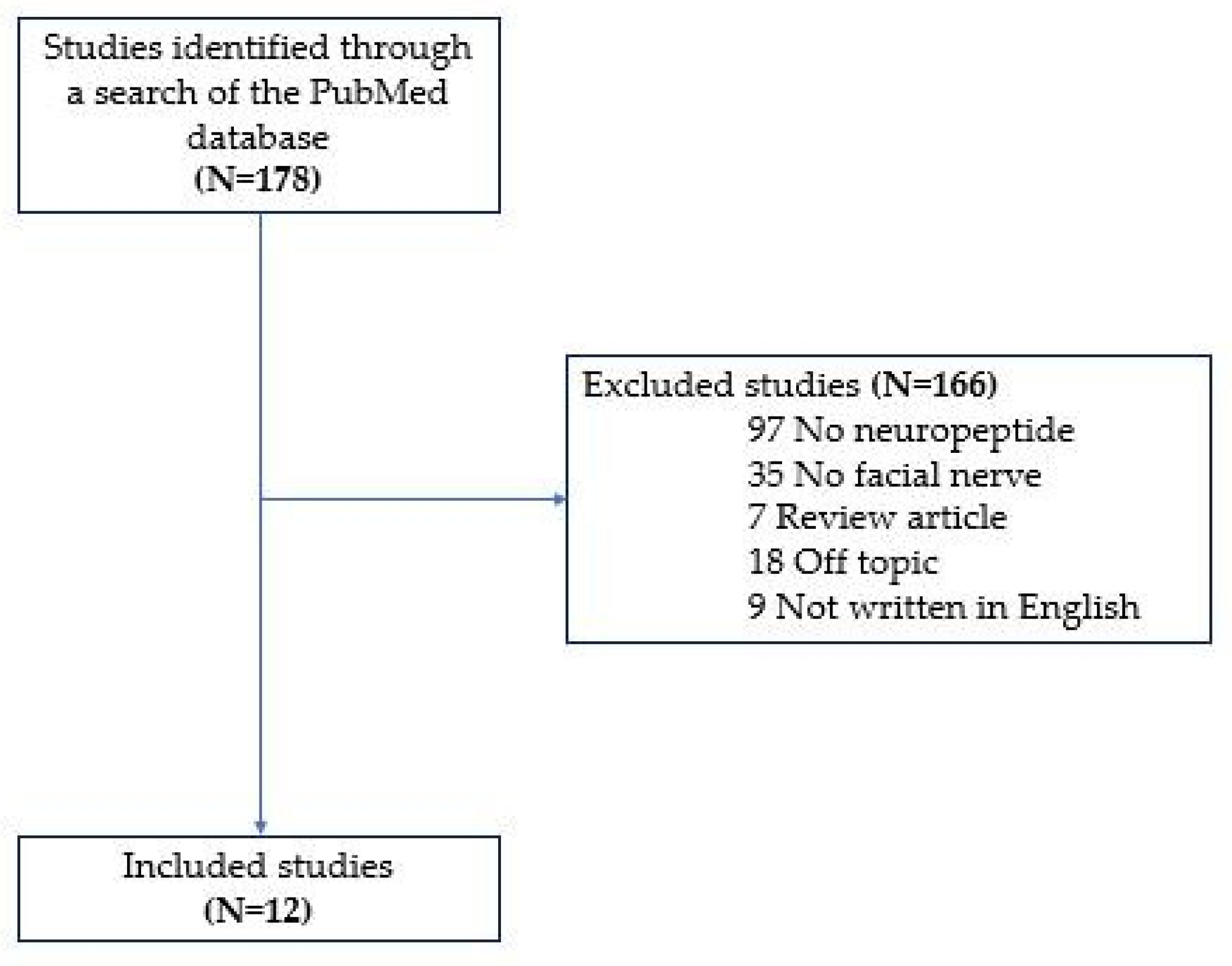
1. Introduction
2. Anatomy and Physiology of the Nervous System and Facial Nerves
2.1. Differences between Central and Peripheral Nervous Systems
2.2. Facial Nerve Function and Mechanisms of Nerve Recovery after Injury
3. Neuropeptides
3.1. General Concept
3.2. Roles of Neuropeptides in Facial Nerve Regeneration
3.2.1. Calcitonin Gene-Related Peptide (CGRP)
3.2.2. Galanin
3.2.3. Pituitary Adenylyl Cyclase-Activating Peptide (PACAP)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mattox, D.E. Clinical disorders of the facial nerve. In Cummings Otolaryngology-Head and Neck Surgery, 6th ed.; Mosby: St. Louis, MO, USA, 2015; pp. 2617–2628. [Google Scholar]
- Vrabec, J.T.; Lin, J.W. Acute paralysis of the facial nerve. In Baileys Head & Neck Surgery Otolaryngology, 5th ed.; Lippincott Williams & Wilikins: Philadelphia, PA, USA, 2014; pp. 2503–2518. [Google Scholar]
- Lee, H.Y.; Byun, J.Y.; Park, M.S.; Yeo, S.G. Steroid-antiviral treatment improves the recovery rate in patients with severe Bell’s palsy. Am. J. Med. 2013, 126, 336–341. [Google Scholar] [CrossRef]
- Kang, H.M.; Jung, S.Y.; Byun, J.Y.; Park, M.S.; Yeo, S.G. Steroid plus antiviral treatment for Bell’s palsy. J. Intern. Med. 2015, 277, 532–539. [Google Scholar] [CrossRef]
- Hoffman, P.N. A conditioning lesion induces changes in gene expression and axonal transport that enhance regeneration by increasing the intrinsic growth state of axons. Exp. Neurol. 2010, 223, 11–18. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Principles of neuronal regeneration. Acta Neurochir. Suppl. 1996, 66, 103–106. [Google Scholar]
- Moran, L.B.; Graeber, M.B. The facial nerve axotomy model. Brain Res. Rev. 2004, 44, 154–178. [Google Scholar] [CrossRef]
- Sauer, C.S.; Phetsanthad, A.; Riusech, O.L.; Li, L. Developing mass spectrometry for the quantitative analysis of neuropeptides. Expert Rev. Proteom. 2021, 18, 607–621. [Google Scholar] [CrossRef]
- Hartmann, M.C.; Pleil, K.E. Circuit and neuropeptide mechanisms of the paraventricular thalamus across stages of alcohol and drug use. Neuropharmacology 2021, 198, 108748. [Google Scholar] [CrossRef] [PubMed]
- Borbely, E.; Scheich, B.; Helyes, Z. Neuropeptides in learning and memory. Neuropeptides 2013, 47, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, D.; Kramarska, E.; Mackiewicz, P.; Orczyk-Pawiłowicz, M.; Lundy, F.T. Mammalian Neuropeptides as Modulators of Microbial Infections: Their Dual Role in Defense versus Virulence and Pathogenesis. Int. J. Mol. Sci. 2021, 22, 3658. [Google Scholar] [CrossRef]
- Cho, Y.C. Recent trends in nerve regeneration research. Mol. Cell. Biol. Newsl. 2014, 7, 1–4. [Google Scholar]
- Chen, M.S.; Huber, A.B.; van der Haar, M.E.; Frank, M.; Schnell, L.; Spillmann, A.A.; Christ, F.M.; Schwab, M.E. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 2000, 403, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Vielmetter, J.; Lottspeich, F.; Stuermer, C.A. The monoclonal antibody E587 recognizes growing (new and regenerating) retinal axons in the goldfish retinotectal pathway. J. Neurosci. 1991, 11, 3581–3593. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tan, A.M.; Colletti, M.; Rorai, A.T.; Skene, J.H.P.; Levine, J.M. Antibodies against the NG2 Proteoglycan Promote the Regeneration of Sensory Axons within the Dorsal Columns of the Spinal Cord. J. Neurosci. 2006, 26, 4729–4739. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Geoffroy, C.G.; Chan, A.F.; Tolentino, K.E.; Crawford, M.J.; Leal, M.A.; Kang, B.; Zheng, B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron 2010, 66, 663–670. [Google Scholar] [CrossRef]
- Rudge, J.S.; Silver, J. Inhibition of neurite outgrowth on astroglial scars in vitro. J. Neurosci. 1990, 10, 3594–3603. [Google Scholar] [CrossRef]
- Eicher, S.A.; Coker, N.J.; Alford, B.R.; Igarashi, M.; Smith, R.J. A comparative study of the fallopian canal at the meatal foramen and labyrinthine segment in young children and adults. Arch. Otolaryngol. Head Neck Surg. 1990, 116, 1030–1035. [Google Scholar] [CrossRef]
- Nakashima, S.; Sando, I.; Takahashi, H.; Fujita, S. Computer-aided 3-D reconstruction and measurement of the facial canal and facial nerve. I. Cross-sectional area and diameter: Preliminary report. Laryngoscope 1993, 103, 1150–1156. [Google Scholar] [CrossRef]
- Gordon, T.; Borschel, G.H. The use of the rat as a model for studying peripheral nerve regeneration and sprouting after complete and partial nerve injuries. Exp. Neurol. 2017, 287, 331–347. [Google Scholar] [CrossRef]
- Arthur-Farraj, P.; Coleman, M.P. Lessons from Injury: How Nerve Injury Studies Reveal Basic Biological Mechanisms and Therapeutic Opportunities for Peripheral Nerve Diseases. Neurotherapeutics 2021, 2021, 1–22. [Google Scholar]
- Vargas, M.E.; Yamagishi, Y.; Tessier-Lavigne, M.; Sagasti, A. Live imaging of calcium dynamics during axon degeneration reveals two functionally distinct phases of calcium influx. J. Neurosci. 2015, 35, 15026–15038. [Google Scholar] [CrossRef]
- Pellegatta, M.; Taveggia, C. The complex work of proteases and secretases in Wallerian degeneration: Beyond neuregulin-1. Front. Cell. Neurosci. 2019, 13, 93. [Google Scholar] [CrossRef]
- Gordon, T. Peripheral nerve regeneration and muscle reinnervation. Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef] [PubMed]
- Blanquie, O.; Bradke, F. Cytoskeleton dynamics in axon regeneration. Curr. Opin. Neurobiol. 2018, 51, 60–69. [Google Scholar] [CrossRef]
- Hoffman, P.N.; Lasek, R.J. Axonal transport of the cytoskeleton in regenerating motor neurons: Constancy and change. Brain Res. 1980, 202, 317–333. [Google Scholar] [CrossRef]
- Tetzlaff, W.; Bisby, M.A.; Kreutzberg, G.W. Changes in cytoskeletal proteins in the rat facial nucleus following axotomy. J. Neurosci. 1988, 8, 3181–3189. [Google Scholar] [CrossRef] [PubMed]
- Bosse, F.; Hasenpusch-Theil, K.; Küry, P.; Müller, H.W. Gene expression profiling reveals that peripheral nerve regeneration is a consequence of both novel injury-dependent and reactivated developmental processes. J. Neurochem. 2006, 96, 1441–1457. [Google Scholar] [CrossRef]
- Byun, J.Y. Facial nerve disorder—Anatomy and evaluation of facial nerve. In Otorhinolaryngology—Head and Neck Surgery, 2nd ed.; Koonja Publishing Inc.: Seoul, Korea, 2018; p. 931. [Google Scholar]
- Adkins, W.Y.; Osguthrope, J.D. Management of trauma of the facial nerve. Otolaryngol. Clin. N. Am. 1991, 24, 587–611. [Google Scholar] [CrossRef]
- Faleiros, M.C. Importance of the stapedial reflex in the diagnosis of several pathologies. Rev. Laryngol. Otol. Rhinol. 2000, 121, 345–348. [Google Scholar]
- Pardal-Fernandez, J.M.; Garcia-Alvarez, G.; Jerez-Garcia, P.; Marco-Giner, J.; Almodovar-Alvarez, C. Peripheral facial paralysis. The value of clinical neurophysiology. Rev. Neurol. 2003, 36, 991–996. [Google Scholar]
- Nemeroff, C.B. (Ed.) The Neurobiology of Neuropeptides. In Neuropeptides and Psychiatric Disorders; American Psychiatric Press, Inc.: Washington, DC, USA, 1991; pp. 3–11. [Google Scholar]
- Nemeroff, C.B.; Bissette, G. Neuropeptides in psychiatic disorders. In American Handbook of Psychiatry, 4th ed.; Berger, P.A., Brodie, H.K.H., Eds.; Basic Books: New York, NY, USA, 1986; Volume 8, pp. 64–110. [Google Scholar]
- Kim, Y.H.; Shim, J.C. Neuropeptides in Clinical Psychiatric Research: Endorphins and Cholecystokinins. Korean J. Biol. Psychiatry 1998, 5, 34–45. [Google Scholar]
- Zhang, Y.; Zhang, H.; Jiang, B.; Tong, X.; Yan, S.; Lu, J. Current views on neuropeptides in atopic dermatitis. Exp. Dermatol. 2021, 30, 1588–1597, Online ahead of print. [Google Scholar] [CrossRef]
- Oda, N.; Miyahara, N.; Taniguchi, A.; Morichika, D.; Senoo, S.; Fujii, U.; Itano, J.; Gion, Y.; Kiura, K.; Kanehiro, A.; et al. Requirement for neuropeptide Y in the development of type 2 responses and allergen-induced airway hyperresponsiveness and inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L407–L417. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, L.; Xie, J.; Shi, L. The Emerging Role of Neuropeptides in Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 646726. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, F.; Vanselow, J.T.; Schlosser, A.; Wegener, C.; Rössler, W. Neuropeptides in the desert ant Cataglyphis fortis: Mass spectrometric analysis, localization, and age-related changes. J. Comp. Neurol. 2017, 525, 901–918. [Google Scholar] [CrossRef] [PubMed]
- Botelho, M.; Cavadas, C. Neuropeptide Y: An Anti-Aging Player? Trends Neurosci. 2015, 38, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.L.; Li, G.Z.; Chen, S.Z.; Wang, J.J.; Olson, J.E.; Xia, H.J.; Lazartigues, E.; Zhu, Y.L.; Chen, Y.F.; Zheng, J.L.; et al. Angiotensin converting enzyme 2/Ang-(1-7)/mas axis protects brain from ischemic injury with a tendency of age-dependence. CNS Neurosci. Ther. 2014, 20, 452–459. [Google Scholar] [CrossRef]
- Lasagni Vitar, R.M.; Rama, P.; Ferrari, G. The two-faced effects of nerves and neuropeptides in corneal diseases. Prog. Retin. Eye Res. 2021, 7, 100974, Online ahead of print. [Google Scholar] [CrossRef]
- Sumeraga, G.; Pilmane, M. Distribution of neuropeptides in nasal and nasopharyngeal mucosa in patients with the post nasal drip syndrome. Anthropology 2011, 20, 389–404. [Google Scholar] [CrossRef]
- Baraniuk, J.N.; Laliner, M.A. Neuropeptides and nasal secretion. J. Allergy Clin. Immunol. 1990, 86, 620–627. [Google Scholar] [CrossRef]
- Saika, T.; Senba, E.; Noguchi, K.; Sato, M.; Kubo, T.; Matsunaga, T.; Tohyama, M. Changes in expression of peptides in rat facial motoneurons after facial nerve crushing and resection. Brain Res. Mol. Brain Res. 1991, 11, 187–196. [Google Scholar] [CrossRef]
- Makwana, M.; Werner, A.; Acosta-Saltos, A.; Gonitel, R.; Pararajasingham, A.; Ruff, C.; Rumajogee, P.; Cuthill, D.; Galiano, M.; Bohatschek, M.; et al. Peripheral Facial Nerve Axotomy in Mice Causes Sprouting of Motor Axons into Perineuronal Central White Matter: Time Course and Molecular Characterization. J. Comp. Neurol. 2010, 518, 699–721. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kobayashi, S.; Shimizu-Okabe, C.; Okabe, A.; Moon, C.; Shin, T.; Takayama, C. Changes in the expression and localization of signaling molecules in mouse facial motor neurons during regeneration of facial nerves. J. Chem. Neuroanat. 2018, 88, 13–21. [Google Scholar] [CrossRef]
- Haas, C.A.; Dumoulin, F.L.; Lazar, P.; Raivich, G.; Reddington, M.; Streit, W.J.; Kreutzberg, G.W. The role of calcitonin gene-related peptide in the regenerating facial nucleus. In The Facial Nerve; Springer Science and Business Media LLC: Berlin, Germany, 1994; pp. 71–74. [Google Scholar]
- Dumoulin, F.L.; Raivich, G.; Streit, W.J.; Kreutzberg, G.W. Differential Regulation of Calcitonin Gene-related Peptide (CGRP) in Regenerating Rat Facial Nucleus and Dorsal Root Ganglion. Eur. J. Neurosci. 1991, 3, 338–342. [Google Scholar] [CrossRef]
- Mohri, D.; Satomi, F.; Kondo, E.; Fukuoka, T.; Sakagami, M.; Noguchi, K. Change in gene expression in facial nerve nuclei and the effect of superoxide dismutase in a rat model of ischemic facial paralysis. Brain Res. 2001, 893, 227–236. [Google Scholar] [CrossRef]
- Streit, W.J.; Dumoulin, F.L.; Raivich, G.; Kreutzberg, G.W. Calcitonin gene-related peptide increases in rat facial motoneurons after peripheral nerve transection. Neurosci. Lett. 1989, 101, 143–148. [Google Scholar] [CrossRef]
- Suarez, V.; Guntinas-Lichius, O.; Streppel, M.; Ingorokva, S.; Grosheva, M.; Neiss, W.F.; Angelov, D.N.; Klimaschewski, L. The axotomy-induced neuropeptides galanin and pituitary adenylate cyclase-activating peptide promote axonal sprouting of primary afferent and cranial motor neurones. Eur. J. Neurosci. 2006, 24, 1555–1564. [Google Scholar] [CrossRef]
- Burazin, T.C.; Gundlach, A.L. Inducible galanin and GalR2 receptor system in motor neuron injury and regeneration. J. Neurochem. 1998, 71, 879–882. [Google Scholar] [CrossRef]
- Kimura, H.; Kawatani, M.; Ito, E.; Ishikawa, K. Effects of pituitary adenylate cyclase-activating polypeptide on facial nerve recovery in the Guinea pig. Laryngoscope 2003, 113, 1000–1006. [Google Scholar] [CrossRef]
- Armstrong, B.D.; Abad, C.; Chhith, S.; Rodriguez, W.; Cheung-Lau, G.; Trinh, V.; Waschek, J.A. Restoration of axotomy-induced PACAP gene induction in SCID mice with CD4+ T-lymphocytes. Neuroreport 2004, 15, 2647–2650. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.D.; Abad, C.; Chhith, S.; Cheung-Lau, G.; Hajji, O.E.; Nobuta, H.; Waschek, J.A. Impaired nerve regeneration and enhanced neuroinflammatory response in mice lacking pituitary adenylyl cyclase activating peptide. Neuroscience 2008, 151, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.A.; Streit, W.J.; Kreutzberg, G.W. Rat facial motoneurons express increased levels of calcitonin gene-related peptide mRNA in response to axotomy. J. Neurosci. Res. 1990, 27, 270–275. [Google Scholar] [CrossRef] [PubMed]
| Congenital Mononeural agenesis Congenital facial paralysis Congenital unilateral lower lip palsy Facial paralysis with other deficits Möbius syndrome Hemifacial macrosomia Oculoauriculovertebral dysplasia Poland syndrome Secondary to teratogens Thalidomide Rubella Polyneuritis Bell palsy Herpes zoster Guillain-Barré syndrome Autoimmune disease Lyme disease HIV infection Kawasaki disease Tuberculosis Trauma Temporal bone fracture Barotrauma Birth trauma Facial laceration Penetrating injury | Facial Burn Radiation injury Otitis media Acute bacterial Chronic bacterial Cholesteatoma Metabolic and systemic diseases Sarcoidosis Melkersson-Rosenthal syndrome Neurologic disorders HIV infection Cerebrovascular disorders Tumors Schwannoma Glomus tumor Primary parotid tumor Leukemia Histiocytosis Meningioma Rhabdomyosarcoma Metastatic tumor Recurrent Iatrogenic Mastoid surgery Parotid surgery |
| Peptide Source | Neuropeptides |
|---|---|
| Nervous system, nerve fibers | CGRP, gastrin-releasing peptide, VIP, NPY, Subs P, PACAP, galanin, FRAP, Brain natriuretic peptide |
| Brain peptides | Leucine enkephalin, methionine enkephalin, Subs P, Gastrin, VIP, Brain GF, brain-derived neurohormone, neurotensin, insulin, glucagon |
| Cerebral cortex, hippocampus, amygdala | CCK |
| Hypothalamic peptides | TRH, LHRH, GHIH, CRH, somatostatin, CCK |
| Pituitary peptides | ACTH, -endorphin, -MSH, PRL, LH, TSH, GH, vasopressin, oxytocin, lipotropin |
| Sympathetic nerves | Noradrenaline, serotonin, NPY |
| Parasympathetic nerves | Acetylcholine, VIP, NPY, galanin, met-enkephalin |
| Corneal epithelial cells, endothelial cells, stromal cells, and corneal limbal stem cells, keratocyte | NGF, NT3, NT4, EGF, BDNF, GDNF, Endothelin, NPY, Subs P |
| Lacrimal gland, macrophages, fibroblasts | EGF |
| Nasal mucosa glands | PGP, NPY, VIP, Subs P, CGRP, CgA |
| Nasal mucosal blood vessels | PGP, NPY, VIP, Subs P, CGRP, CgA |
| Nasopharyngeal mucosa glands | PGP, NPY, VIP, Subs P, CGRP, CgA |
| Nasopharyngeal mucosa blood vessels | PGP, NPY, VIP, Subs P, CGRP, CgA |
| Dermal layer of normal skin | Brain natriuretic peptide |
| Mast cells, keratinocytes, lymphocyte, monocyte, chromaffin cells, and eosinophils | Endothelin, NPY, Subs P |
| Opioid peptides | Dynorphin, -endorphin, enkephalins |
| Circulating peptides | Angiotensin, calcitonin, glucagon |
| Author, Year [Ref.] | Molecule | Experimental Design | Evaluation/Technique | Results | Conclusions |
|---|---|---|---|---|---|
| Streit et al., 1989 [51] | CGRP | Rat Facial nerve transection | Structural, molecular changes in facial nucleus Immunohistochemistry, radioimmunoassay | Expression in perikarya dendrites and axons of facial motoneurons: CGRP increase at +15H, maximal levels on Day 6, return to normal after 5–6 weeks. Before glial changes | CGRP: increased facial motoneurons after axotomy |
| Haas et al., 1990 [57] | CGRP | Rat Facial nerve transection | CGRP expression, localization in facial nuclei Northern blot analysis, in situ hybridization histochemistry (ISHH) | Peak CGRP expression at 16H, return to basal levels at Day 9. CGRP mRNA is expressed in 50% of motoneurons | Early and strong induction of CGRP expression in 50% of motoneurons in the injured facial nucleus |
| Dumoulin et al., 1991 [49] | CGRP | Rat Facial nerve transected | CGRP expression in regenerating the facial motor nucleus Radioimmunoassay Northern blot analysis | Biphasic five-fold response (Days 3 and 21) in regenerating motoneurons, elevation persists after 45 days No second peak if another resection and ligation of the nerve is performed | CGRP signaling factor: First increase: regulation of astrocyte reaction Second increase: muscle reinnervation |
| Saika et al., 1992 [45] | Alpha CGRP, beta CGRP, galanin, CCK | Rats Facial nerve crushed or transected | Effect of axonal regeneration on peptide production ÌSHH Comparison control rats, axotomized rats (nerve crush or transection) ISHH | Crushed nerve: Alpha CGRP: single peak and return to normal at 6 weeks Beta CGRP: transient early increase and return to normal at 2 weeks CCK: no response Galanin: delayed response, shorter than after nerve cut Transected nerve: alpha CGRP 2 peaks increase, persist at 8 weeks; beta CGRP: transient early increase than decrease; CCK, galanin: delayed response and persistent elevation | Alpha CGRP, CCK, and galanin increase parallel axon regeneration: trophic effect in motoneuron regeneration Beta CGRP decrease: role in neurotransmission Expression level correlated with nerve recovery. More rapid return to normal after crush compared to the transected nerve |
| Mohri et al. 2001 [50] | CGRP, c-Jun, GAP-43 | Rat Ischemic facial nerve injury | Change of gene expression in facial nuclei after facial nerve ischemia Effect of SOD (superoxide dismutase) on CGRP ISHH | CGRP expression is less elevated and detected earlier after ischemia compared to axotomy. Peaks at Days 3 and 14 (Day 21 in axotomy) SOD, which is a free radical-scavenging enzyme, decreases CGRP expression | CGRP expression changes depending on the extent of nerve damage. Free radicals generated by ischemia partially responsible for ischemic nerve damage and change in gene expression in motoneurons |
| Burazin et al., 1998 [53] | Galanin GaIRl, GalR2 | Rats Facial nerve crushed or transected | temporal changes in galanin, receptors Ga1R1 and Ga1R2 expression in facial motor neurons ISHH | Galanin, GalR2 detected in the facial nucleus on the side of nerve injury but absent on the contralateral side GalR1 not detected GalR2 mRNA increased after 3 days, peaking after 7 days and returning to normal after 14 days (consistent with the time course of axonal regeneration) | Selective upregulation of Ga1R2 after facial nerve injury The receptor may represent an active “autoreceptor” involved in nerve degeneration or regeneration |
| Makwana, 2010 [46] | Galanin CCRP | Mice Facial nerve transection | Origin, time course, molecular characteristics of sprouting neurites Facial nuclei | Sprouting axons galanin + and CGRP + in and around the facial motor nucleus in white matter, from axotomized motoneuron Delayed appearance of sprouting galanin+ (Days 7 to 42) | Galanin and CGRP secreted by facial motor nucleus occur early, with peak expression following injury, later secretion of galanin, coincides with central sprouting and neuronal cell death, neurite-outgrowth enhancing properties of galanin |
| Kim et al. (2018) [47] | ChAT, CGRP, Galanin, Gephyrin, KCC2 | Mice (n = 42) Facial nerve transection | Molecule expression facial nucleus, time course of neural functional recovery Immunohistochemistry | Galanin immunolabeling was detected in both axons and cell bodies of FMNs after suturing. Galanin returned to normal level at 1 month (before facial function recovery) Markedly increased CGRP immunolabeling in the genu, nerve root, and FMNs. CGRP expression returned to normal levels when facial functions recovered at Day 60 | Changes in CGRP expression during nerve regenerations may be an objective marker of regeneration. However, galanin may be a marker for axon injury |
| Kimura et al., 2004 [54] | PACAP | Guinea pig Facial nerve transection PACAP injected in injured nerve | Effect of PACAP in GAP-43, GDNF, CMAP. Comparison of PACAP-treated versus non-treated groups | Accelerated reappearance of CMAP Increase and prolonged level of GDNF and myelin | PACAP facilitated the recovery of CMAP, the number of myelinated axons, or both. PACAP can promote nerve regeneration |
| Armstrong et al., 2004 [55] | PACAP CD4+ | Mice immunodeficient (SCID) Facial nerve transection | Effect of CD4 + cells on PACAP induction in motor neurons after facial nerve axotomy Facial nuclei | SCID mice: loss of PACAP gene induction after axotomy CD4 + enriched splenocytes partially restored an upregulation of PACAP | CD4 + lymphocytes play a critical role in the induction of PACAP expression after facial nerve injury. CD4 + cell-dependent induction of PACAP may play a role in nerve/immune cell interaction facilitating nerve regeneration |
| Suarez et al., 2006 [52] | Galanin, PACAP | Rat Facial nerve transection | Effects of galanin and PACAP on axonal elongation and sprouting | Axonal length and the number of branch points significantly increased in the presence of galanin or PACAP (2–5 μm) | Galanin and PACAP: neurotrophic molecules inducing peripheral axon sprouting. However, it has a limitation due to the stimulation of collateral axon mis-branching |
| Armstrong et al., 2007 [56] | PACAP | Mice PACAP deficient Facial nerve axotomy and crush | Effects of PACAP on: - facial motor neurons - microglial activation - specific cytokine responses facial nuclei in the brain stem | Deletion of PACAP resulted in: - no differences in motor neuron survival vs. wild-type mice - delayed axon regeneration - reduced numbers of regenerating axons - altered microglial response - amplified inflammatory response in the FMN and nerve site (8- to 12-fold elevation of proinflammatory cytokines TNF-alpha, IL-6, and IFN-gamma) | PACAP: induced nerve regeneration and stimulated microglial activation in the brainstem facial moto nucleus; provided temporal control of the inflammatory immune response |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, I.; Kim, Y.; Kang, D.; Jung, J.; Kim, S.; Rim, H.; Kim, S.; Yeo, S.-G. Neuropeptides Involved in Facial Nerve Regeneration. Biomedicines 2021, 9, 1575. https://doi.org/10.3390/biomedicines9111575
Kim I, Kim Y, Kang D, Jung J, Kim S, Rim H, Kim S, Yeo S-G. Neuropeptides Involved in Facial Nerve Regeneration. Biomedicines. 2021; 9(11):1575. https://doi.org/10.3390/biomedicines9111575
Chicago/Turabian StyleKim, Inhyeok, Yonjae Kim, Daewoong Kang, Junyang Jung, Sungsoo Kim, Hwasung Rim, Sanghoon Kim, and Seung-Geun Yeo. 2021. "Neuropeptides Involved in Facial Nerve Regeneration" Biomedicines 9, no. 11: 1575. https://doi.org/10.3390/biomedicines9111575
APA StyleKim, I., Kim, Y., Kang, D., Jung, J., Kim, S., Rim, H., Kim, S., & Yeo, S.-G. (2021). Neuropeptides Involved in Facial Nerve Regeneration. Biomedicines, 9(11), 1575. https://doi.org/10.3390/biomedicines9111575








