Sirtuin 3 (SIRT3) Pathways in Age-Related Cardiovascular and Neurodegenerative Diseases
Abstract
1. Introduction
2. SIRT3 in Aging Biology
2.1. SIRT3, Oxidative Stress and Acetylation Status
2.2. SIRT3 and Visceral Fibrosis
3. SIRT3 in Cardiovascular Diseases
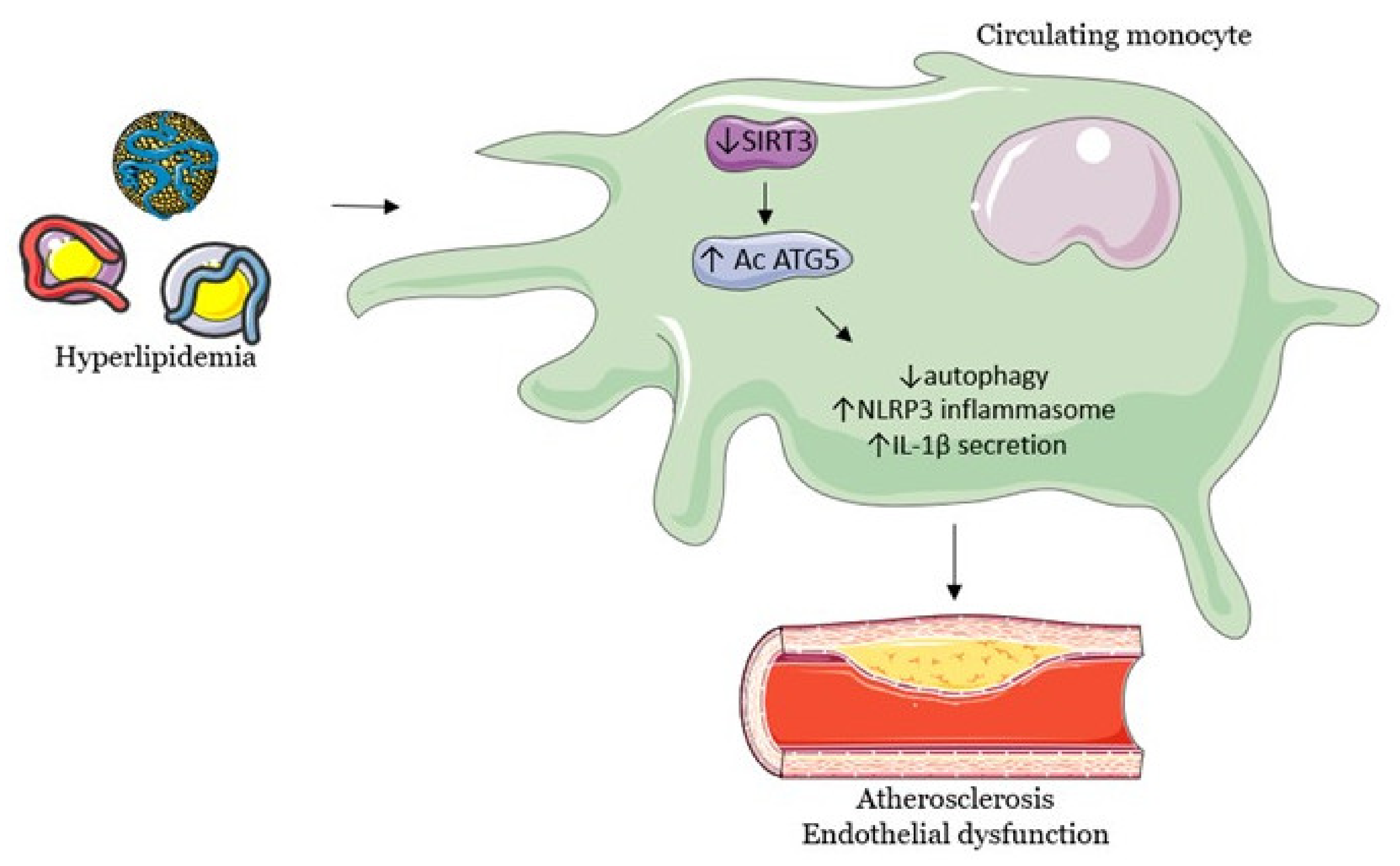
3.1. SIRT3 Modulates Endothelial Dysfunction, Hypertension and Atherosclerosis
3.2. Cardiac Hypertrophy
4. SIRT3 in Age-Related Neurodegenerative Diseases
4.1. Age-Related Hearing Loss
4.2. Alzheimer’s Disease
4.3. Parkinson’s Disease
5. SIRT3 Regulates Interventions That Enhance Health and Lifespan
5.1. Fasting
5.2. Physical Exercise
5.3. Exogenous Ketones
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AMPK | AMP-activated protein kinase |
| ANGII | Angiotensin II |
| APOE | Apolipoprotein E |
| ATP | Adenosine triphosphate |
| CVD | Cardiovascular diseases |
| FOXO3A | Forkhead box O3 |
| GABA | Gamma-Aminobutyric acid |
| GSK3β | Glycogen synthase kinase 3 beta |
| HIF1alpha | Hypoxia-inducible factor 1-alpha |
| HT | Hypertension |
| KO | Knock-out |
| MCP-1 | Monocyte chemoattractant protein 1 |
| MnSOD | Manganese superoxide dismutase |
| mRNA | Messenger RNA |
| mtROS | Mitochondrial ROS |
| mtDNA | Mitochondrial DNA |
| NAD+ | Nicotinamide adenine dinucleotide |
| NFkB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| NMNAT3 | Nicotinamide nucleotide adenylyl transferase 3 |
| NO | Nitric oxide |
| NSC | Neural stem cells |
| OPA1 | Optic atrophy 1 |
| PD | Parkinson’s disease |
| PGC-1α | Peroxisome proliferator-activated receptor-gamma coactivator |
| PPARα | Peroxisome proliferator-activated receptor |
| ROS | Reactive oxygen species |
| SIRTs | Sirtuins |
| SIRT3 | Sirtuin 3 |
| SNc | Substantia nigra pars compacta |
| SOD | Superoxide dismutase |
| TAC | Transverse aortic constriction |
| TGF-β | Transforming growth factor β |
| VCAM-1 | Vascular cell adhesion molecule 1 |
References
- Niccoli, T.; Partridge, L. Ageing as a Risk Factor for Disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Kassahun, H.; Croteau, D.L.; Scheibye-Knudsen, M.; Marosi, K.; Lu, H.; Shamanna, R.A.; Kalyanasundaram, S.; Bollineni, R.C.; Wilson, M.A.; et al. NAD+ Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell Metab. 2016, 24, 566–581. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.; et al. Mitophagy Inhibits Amyloid-β and Tau Pathology and Reverses Cognitive Deficits in Models of Alzheimer’s Disease. Nat. Neurosci. 2019, 22, 401–412. [Google Scholar] [CrossRef]
- Palmeira, C.M.; Teodoro, J.S.; Amorim, J.A.; Steegborn, C.; Sinclair, D.A.; Rolo, A.P. Mitohormesis and Metabolic Health: The Interplay between ROS, CAMP and Sirtuins. Free Radic. Biol. Med. 2019, 141, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Donmez, G.; Guarente, L. Aging and Disease: Connections to Sirtuins. Aging Cell 2010, 9, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Bonkowski, M.S.; Sinclair, D.A. Slowing Ageing by Design: The Rise of NAD+ and Sirtuin-Activating Compounds. Nat. Rev. Mol. Cell Biol. 2016, 17, 679–690. [Google Scholar] [CrossRef]
- Haigis, M.C.; Sinclair, D.A. Mammalian Sirtuins: Biological Insights and Disease Relevance. Annu. Rev. Pathol. 2010, 5, 253–295. [Google Scholar] [CrossRef]
- Banks, A.S.; Kon, N.; Knight, C.; Matsumoto, M.; Gutiérrez-Juárez, R.; Rossetti, L.; Gu, W.; Accili, D. SirT1 Gain of Function Increases Energy Efficiency and Prevents Diabetes in Mice. Cell Metab. 2008, 8, 333–341. [Google Scholar] [CrossRef]
- Hubbard, B.P.; Sinclair, D.A. Small Molecule SIRT1 Activators for the Treatment of Aging and Age-Related Diseases. Trends Pharmacol. Sci. 2014, 35, 146–154. [Google Scholar] [CrossRef]
- White, A.T.; Schenk, S. NAD(+)/NADH and Skeletal Muscle Mitochondrial Adaptations to Exercise. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E308–E321. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.E.; Sinclair, D.A. Sirtuins and NAD+ in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ. Res. 2018, 123, 868–885. [Google Scholar] [CrossRef] [PubMed]
- van de Ven, R.A.H.; Santos, D.; Haigis, M.C. Mitochondrial Sirtuins and Molecular Mechanisms of Aging. Trends Mol. Med. 2017, 23, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Nagasawa, K.; Münch, C.; Xu, Y.; Satterstrom, K.; Jeong, S.; Hayes, S.D.; Jedrychowski, M.P.; Vyas, F.S.; Zaganjor, E.; et al. Mitochondrial Sirtuin Network Reveals Dynamic SIRT3-Dependent Deacetylation in Response to Membrane Depolarization. Cell 2016, 167, 985–1000.e21. [Google Scholar] [CrossRef]
- Carrico, C.; Meyer, J.G.; He, W.; Gibson, B.W.; Verdin, E. The Mitochondrial Acylome Emerges: Proteomics, Regulation by Sirtuins, Metabolic and Disease Implications. Cell Metab. 2018, 27, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Rardin, M.J.; Newman, J.C.; Held, J.M.; Cusack, M.P.; Sorensen, D.J.; Li, B.; Schilling, B.; Mooney, S.D.; Kahn, C.R.; Verdin, E.; et al. Label-Free Quantitative Proteomics of the Lysine Acetylome in Mitochondria Identifies Substrates of SIRT3 in Metabolic Pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 6601–6606. [Google Scholar] [CrossRef]
- Cheng, A.; Yang, Y.; Zhou, Y.; Maharana, C.; Lu, D.; Peng, W.; Liu, Y.; Wan, R.; Marosi, K.; Misiak, M.; et al. Mitochondrial SIRT3 Mediates Adaptive Responses of Neurons to Exercise, and Metabolic and Excitatory Challenges. Cell Metab. 2016, 23, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Hazelton, J.L.; Petrasheuskaya, M.; Fiskum, G.; Kristián, T. Cyclophilin D Is Expressed Predominantly in Mitochondria of Gamma-Aminobutyric Acidergic Interneurons. J. Neurosci. Res. 2009, 87, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wu, Q.; Shi, J.; Zhou, S. Regulation of SIRT3 on Mitochondrial Functions and Oxidative Stress in Parkinson’s Disease. Biomed. Pharmacother. 2020, 132, 110928. [Google Scholar] [CrossRef]
- Ahn, B.-H.; Kim, H.-S.; Song, S.; Lee, I.H.; Liu, J.; Vassilopoulos, A.; Deng, C.-X.; Finkel, T. A Role for the Mitochondrial Deacetylase Sirt3 in Regulating Energy Homeostasis. Proc. Natl. Acad. Sci. USA 2008, 105, 14447–14452. [Google Scholar] [CrossRef]
- Kincaid, B.; Bossy-Wetzel, E. Forever Young: SIRT3 a Shield against Mitochondrial Meltdown, Aging, and Neurodegeneration. Front. Aging Neurosci. 2013, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Hallows, W.C.; Yu, W.; Smith, B.C.; Devries, M.K.; Devires, M.K.; Ellinger, J.J.; Someya, S.; Shortreed, M.R.; Prolla, T.; Markley, J.L.; et al. Sirt3 Promotes the Urea Cycle and Fatty Acid Oxidation during Dietary Restriction. Mol. Cell 2011, 41, 139–149. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Newman, J.C.; Wang, M.Z.; Ho, L.; Verdin, E. Mitochondrial Sirtuins: Regulators of Protein Acylation and Metabolism. Trends Endocrinol. Metab. TEM 2012, 23, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Lombard, D.B.; Alt, F.W.; Cheng, H.-L.; Bunkenborg, J.; Streeper, R.S.; Mostoslavsky, R.; Kim, J.; Yancopoulos, G.; Valenzuela, D.; Murphy, A.; et al. Mammalian Sir2 Homolog SIRT3 Regulates Global Mitochondrial Lysine Acetylation. Mol. Cell. Biol. 2007, 27, 8807–8814. [Google Scholar] [CrossRef]
- Schwer, B.; North, B.J.; Frye, R.A.; Ott, M.; Verdin, E. The Human Silent Information Regulator (Sir)2 Homologue HSIRT3 Is a Mitochondrial Nicotinamide Adenine Dinucleotide-Dependent Deacetylase. J. Cell Biol. 2002, 158, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Onyango, P.; Celic, I.; McCaffery, J.M.; Boeke, J.D.; Feinberg, A.P. SIRT3, a Human SIR2 Homologue, Is an NAD-Dependent Deacetylase Localized to Mitochondria. Proc. Natl. Acad. Sci. USA 2002, 99, 13653–13658. [Google Scholar] [CrossRef]
- Verdin, E.; Ott, M. 50 Years of Protein Acetylation: From Gene Regulation to Epigenetics, Metabolism and Beyond. Nat. Rev. Mol. Cell Biol. 2015, 16, 258–264. [Google Scholar] [CrossRef]
- Glozak, M.A.; Sengupta, N.; Zhang, X.; Seto, E. Acetylation and Deacetylation of Non-Histone Proteins. Gene 2005, 363, 15–23. [Google Scholar] [CrossRef]
- Choudhary, C.; Weinert, B.T.; Nishida, Y.; Verdin, E.; Mann, M. The Growing Landscape of Lysine Acetylation Links Metabolism and Cell Signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Xiao, Z.; Kwon, S.; Sun, X.; Ryerson, D.; Tkac, D.; Ma, P.; Wu, S.-Y.; Chiang, C.-M.; Zhou, E.; et al. A Dysregulated Acetyl/SUMO Switch of FXR Promotes Hepatic Inflammation in Obesity. EMBO J. 2015, 34, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Flick, F.; Lüscher, B. Regulation of Sirtuin Function by Posttranslational Modifications. Front. Pharmacol. 2012, 3, 29. [Google Scholar] [CrossRef]
- Hirschey, M.D.; Shimazu, T.; Goetzman, E.; Jing, E.; Schwer, B.; Lombard, D.B.; Grueter, C.A.; Harris, C.; Biddinger, S.; Ilkayeva, O.R.; et al. SIRT3 Regulates Mitochondrial Fatty-Acid Oxidation by Reversible Enzyme Deacetylation. Nature 2010, 464, 121–125. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Hua, L.; Dittenhafer-Reed, K.E.; Schwer, B.; Lombard, D.B.; Li, Y.; Bunkenborg, J.; Alt, F.W.; Denu, J.M.; et al. SIRT3 Deacetylates Mitochondrial 3-Hydroxy-3-Methylglutaryl CoA Synthase 2 and Regulates Ketone Body Production. Cell Metab. 2010, 12, 654–661. [Google Scholar] [CrossRef]
- Hebert, A.S.; Dittenhafer-Reed, K.E.; Yu, W.; Bailey, D.J.; Selen, E.S.; Boersma, M.D.; Carson, J.J.; Tonelli, M.; Balloon, A.J.; Higbee, A.J.; et al. Calorie Restriction and SIRT3 Trigger Global Reprogramming of the Mitochondrial Protein Acetylome. Mol. Cell 2013, 49, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-Y.; Lin, Y.-Y.; Zhu, H.; Chuang, L.-M.; Boeke, J.D. Protein Acetylation and Aging. Aging 2011, 3, 911–912. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Nguyen, C.U.; Chong, T.; Michel, C.R.; Fritz, K.S.; Reisdorph, N.; Knaub, L.; Reusch, J.E.B.; Pugazhenthi, S. SIRT3 Deficiency-Induced Mitochondrial Dysfunction and Inflammasome Formation in the Brain. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Yin, J.; Nielsen, M.; Li, S.; Shi, J. Ketones Improves Apolipoprotein E4-Related Memory Deficiency via Sirtuin 3. Aging 2019, 11, 4579–4586. [Google Scholar] [CrossRef]
- Kim, H.-S.; Patel, K.; Muldoon-Jacobs, K.; Bisht, K.S.; Aykin-Burns, N.; Pennington, J.D.; van der Meer, R.; Nguyen, P.; Savage, J.; Owens, K.M.; et al. SIRT3 Is a Mitochondria-Localized Tumor Suppressor Required for Maintenance of Mitochondrial Integrity and Metabolism during Stress. Cancer Cell 2010, 17, 41–52. [Google Scholar] [CrossRef]
- Ito, K.; Hirao, A.; Arai, F.; Takubo, K.; Matsuoka, S.; Miyamoto, K.; Ohmura, M.; Naka, K.; Hosokawa, K.; Ikeda, Y.; et al. Reactive Oxygen Species Act through P38 MAPK to Limit the Lifespan of Hematopoietic Stem Cells. Nat. Med. 2006, 12, 446–451. [Google Scholar] [CrossRef]
- Albani, D.; Ateri, E.; Mazzuco, S.; Ghilardi, A.; Rodilossi, S.; Biella, G.; Ongaro, F.; Antuono, P.; Boldrini, P.; Di Giorgi, E.; et al. Modulation of Human Longevity by SIRT3 Single Nucleotide Polymorphisms in the Prospective Study “Treviso Longeva (TRELONG)”. Age Dordr. Neth. 2014, 36, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Rose, G.; Dato, S.; Altomare, K.; Bellizzi, D.; Garasto, S.; Greco, V.; Passarino, G.; Feraco, E.; Mari, V.; Barbi, C.; et al. Variability of the SIRT3 Gene, Human Silent Information Regulator Sir2 Homologue, and Survivorship in the Elderly. Exp. Gerontol. 2003, 38, 1065–1070. [Google Scholar] [CrossRef]
- Hirschey, M.D.; Shimazu, T.; Jing, E.; Grueter, C.A.; Collins, A.M.; Aouizerat, B.; Stančáková, A.; Goetzman, E.; Lam, M.M.; Schwer, B.; et al. SIRT3 Deficiency and Mitochondrial Protein Hyperacetylation Accelerate the Development of the Metabolic Syndrome. Mol. Cell 2011, 44, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, D.; Rose, G.; Cavalcante, P.; Covello, G.; Dato, S.; De Rango, F.; Greco, V.; Maggiolini, M.; Feraco, E.; Mari, V.; et al. A Novel VNTR Enhancer within the SIRT3 Gene, a Human Homologue of SIR2, Is Associated with Survival at Oldest Ages. Genomics 2005, 85, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Halaschek-Wiener, J.; Amirabbasi-Beik, M.; Monfared, N.; Pieczyk, M.; Sailer, C.; Kollar, A.; Thomas, R.; Agalaridis, G.; Yamada, S.; Oliveira, L.; et al. Genetic Variation in Healthy Oldest-Old. PLoS ONE 2009, 4, e6641. [Google Scholar] [CrossRef]
- Pradhan, R.; Kumar, R.; Shekhar, S.; Rai, N.; Ambashtha, A.; Banerjee, J.; Pathak, M.; Dwivedi, S.N.; Dey, S.; Dey, A.B. Longevity and Healthy Ageing Genes FOXO3A and SIRT3: Serum Protein Marker and New Road Map to Burst Oxidative Stress by Withania Somnifera. Exp. Gerontol. 2017, 95, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Massudi, H.; Grant, R.; Braidy, N.; Guest, J.; Farnsworth, B.; Guillemin, G.J. Age-Associated Changes in Oxidative Stress and NAD+ Metabolism in Human Tissue. PLoS ONE 2012, 7, e42357. [Google Scholar] [CrossRef]
- Murtaza, G.; Khan, A.K.; Rashid, R.; Muneer, S.; Hasan, S.M.F.; Chen, J. FOXO Transcriptional Factors and Long-Term Living. Oxid. Med. Cell. Longev. 2017, 2017, 3494289. [Google Scholar] [CrossRef]
- Akasaki, Y.; Alvarez-Garcia, O.; Saito, M.; Caramés, B.; Iwamoto, Y.; Lotz, M.K. FoxO Transcription Factors Support Oxidative Stress Resistance in Human Chondrocytes. Arthritis Rheumatol. 2014, 66, 3349–3358. [Google Scholar] [CrossRef]
- Jacobs, K.M.; Pennington, J.D.; Bisht, K.S.; Aykin-Burns, N.; Kim, H.-S.; Mishra, M.; Sun, L.; Nguyen, P.; Ahn, B.-H.; Leclerc, J.; et al. SIRT3 Interacts with the Daf-16 Homolog FOXO3a in the Mitochondria, as Well as Increases FOXO3a Dependent Gene Expression. Int. J. Biol. Sci. 2008, 4, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Mehal, W.Z.; Iredale, J.; Friedman, S.L. Scraping Fibrosis: Expressway to the Core of Fibrosis. Nat. Med. 2011, 17, 552–553. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, N.R.; Bindu, S.; Pillai, V.B.; Samant, S.; Pan, Y.; Huang, J.-Y.; Gupta, M.; Nagalingam, R.S.; Wolfgeher, D.; Verdin, E.; et al. SIRT3 Blocks Aging-Associated Tissue Fibrosis in Mice by Deacetylating and Activating Glycogen Synthase Kinase 3β. Mol. Cell. Biol. 2015, 36, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Kops, G.J.P.L.; Dansen, T.B.; Polderman, P.E.; Saarloos, I.; Wirtz, K.W.A.; Coffer, P.J.; Huang, T.-T.; Bos, J.L.; Medema, R.H.; Burgering, B.M.T. Forkhead Transcription Factor FOXO3a Protects Quiescent Cells from Oxidative Stress. Nature 2002, 419, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Aquilano, K.; Vigilanza, P.; Baldelli, S.; Pagliei, B.; Rotilio, G.; Ciriolo, M.R. Peroxisome Proliferator-Activated Receptor Gamma Co-Activator 1alpha (PGC-1alpha) and Sirtuin 1 (SIRT1) Reside in Mitochondria: Possible Direct Function in Mitochondrial Biogenesis. J. Biol. Chem. 2010, 285, 21590–21599. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, J.; Xing, W.; Zhang, X.; Xu, J.; Zhang, H.; Chen, L.; Ning, X.; Ji, G.; Li, J.; et al. SIRT3 Deficiency Induces Endothelial Insulin Resistance and Blunts Endothelial-Dependent Vasorelaxation in Mice and Human with Obesity. Sci. Rep. 2016, 6, 23366. [Google Scholar] [CrossRef]
- Giralt, A.; Hondares, E.; Villena, J.A.; Ribas, F.; Díaz-Delfín, J.; Giralt, M.; Iglesias, R.; Villarroya, F. Peroxisome Proliferator-Activated Receptor-Gamma Coactivator-1alpha Controls Transcription of the Sirt3 Gene, an Essential Component of the Thermogenic Brown Adipocyte Phenotype. J. Biol. Chem. 2011, 286, 16958–16966. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wang, R.; Xue, Y.; Liu, X.; Zhang, H.; Chen, Y.; Fang, F.; Chang, Y. Sirtuin 3, a New Target of PGC-1alpha, Plays an Important Role in the Suppression of ROS and Mitochondrial Biogenesis. PLoS ONE 2010, 5, e11707. [Google Scholar] [CrossRef]
- Benigni, A.; Cassis, P.; Conti, S.; Perico, L.; Corna, D.; Cerullo, D.; Zentilin, L.; Zoja, C.; Perna, A.; Lionetti, V.; et al. Sirt3 Deficiency Shortens Life Span and Impairs Cardiac Mitochondrial Function Rescued by Opa1 Gene Transfer. Antioxid. Redox Signal. 2019, 31, 1255–1271. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Seok, S.; Yau, P.; Li, X.; Kemper, B.; Kemper, J.K. Obesity and Aging Diminish Sirtuin 1 (SIRT1)-Mediated Deacetylation of SIRT3, Leading to Hyperacetylation and Decreased Activity and Stability of SIRT3. J. Biol. Chem. 2017, 292, 17312–17323. [Google Scholar] [CrossRef]
- Lanza, I.R.; Short, D.K.; Short, K.R.; Raghavakaimal, S.; Basu, R.; Joyner, M.J.; McConnell, J.P.; Nair, K.S. Endurance Exercise as a Countermeasure for Aging. Diabetes 2008, 57, 2933–2942. [Google Scholar] [CrossRef]
- Kendrick, A.A.; Choudhury, M.; Rahman, S.M.; McCurdy, C.E.; Friederich, M.; Van Hove, J.L.K.; Watson, P.A.; Birdsey, N.; Bao, J.; Gius, D.; et al. Fatty Liver Is Associated with Reduced SIRT3 Activity and Mitochondrial Protein Hyperacetylation. Biochem. J. 2011, 433, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as Regulators of Metabolism and Healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.-I.; Kiess, W. Therapeutic Potential of SIRT1 and NAMPT-Mediated NAD Biosynthesis in Type 2 Diabetes. Front. Biosci. Landmark Ed. 2009, 14, 2983–2995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cui, S.; Bai, X.; Zhuo, L.; Sun, X.; Hong, Q.; Fu, B.; Wang, J.; Chen, X.; Cai, G. SIRT3 Overexpression Antagonizes High Glucose Accelerated Cellular Senescence in Human Diploid Fibroblasts via the SIRT3-FOXO1 Signaling Pathway. Age Dordr. Neth. 2013, 35, 2237–2253. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of Fibrosis: Therapeutic Translation for Fibrotic Disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Krenning, G.; Zeisberg, E.M.; Kalluri, R. The Origin of Fibroblasts and Mechanism of Cardiac Fibrosis. J. Cell. Physiol. 2010, 225, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Doyle, K.P.; Cekanaviciute, E.; Mamer, L.E.; Buckwalter, M.S. TGFβ Signaling in the Brain Increases with Aging and Signals to Astrocytes and Innate Immune Cells in the Weeks after Stroke. J. Neuroinflamm. 2010, 7, 62. [Google Scholar] [CrossRef]
- Meyer, T.E.; Kovács, S.J.; Ehsani, A.A.; Klein, S.; Holloszy, J.O.; Fontana, L. Long-Term Caloric Restriction Ameliorates the Decline in Diastolic Function in Humans. J. Am. Coll. Cardiol. 2006, 47, 398–402. [Google Scholar] [CrossRef]
- Varanita, T.; Soriano, M.E.; Romanello, V.; Zaglia, T.; Quintana-Cabrera, R.; Semenzato, M.; Menabò, R.; Costa, V.; Civiletto, G.; Pesce, P.; et al. The OPA1-Dependent Mitochondrial Cristae Remodeling Pathway Controls Atrophic, Apoptotic, and Ischemic Tissue Damage. Cell Metab. 2015, 21, 834–844. [Google Scholar] [CrossRef]
- Jin, J.; Wang, G.-L.; Timchenko, L.; Timchenko, N.A. GSK3beta and Aging Liver. Aging 2009, 1, 582–585. [Google Scholar] [CrossRef]
- Rehan, M.; Kurundkar, D.; Kurundkar, A.R.; Logsdon, N.J.; Smith, S.R.; Chanda, D.; Bernard, K.; Sanders, Y.Y.; Deshane, J.S.; Dsouza, K.G.; et al. Restoration of SIRT3 Gene Expression by Airway Delivery Resolves Age-Associated Persistent Lung Fibrosis in Mice. Nat. Aging 2021, 1, 205–217. [Google Scholar] [CrossRef] [PubMed]
- North Brian, J.; Sinclair David, A. The Intersection Between Aging and Cardiovascular Disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef]
- Giblin, W.; Skinner, M.E.; Lombard, D.B. Sirtuins: Guardians of Mammalian Healthspan. Trends Genet. TIG 2014, 30, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Rodrigues, A.R.; Tomada, N.; Fonseca, J.; Magalhães, A.; Gouveia, A.M.; Neves, D. Effects of Aging and Cardiovascular Disease Risk Factors on the Expression of Sirtuins in the Human Corpus Cavernosum. J. Sex. Med. 2015, 12, 2141–2152. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, K.N.; Chavez, P.; Gasowski, J.; Grodzicki, T.; Messerli, F.H. Hypertension in the Elderly: Some Practical Considerations. Clevel. Clin. J. Med. 2012, 79, 694–704. [Google Scholar] [CrossRef]
- Eirin, A.; Lerman, A.; Lerman, L.O. Mitochondria: A Pathogenic Paradigm in Hypertensive Renal Disease. Hypertension 2015, 65, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Civiletto, G.; Varanita, T.; Cerutti, R.; Gorletta, T.; Barbaro, S.; Marchet, S.; Lamperti, C.; Viscomi, C.; Scorrano, L.; Zeviani, M. Opa1 Overexpression Ameliorates the Phenotype of Two Mitochondrial Disease Mouse Models. Cell Metab. 2015, 21, 845–854. [Google Scholar] [CrossRef]
- Pillai, V.B.; Bindu, S.; Sharp, W.; Fang, Y.H.; Kim, G.; Gupta, M.; Samant, S.; Gupta, M.P. Sirt3 Protects Mitochondrial DNA Damage and Blocks the Development of Doxorubicin-Induced Cardiomyopathy in Mice. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H962–H972. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, N.R.; Samant, S.A.; Pillai, V.B.; Rajamohan, S.B.; Gupta, M.P. SIRT3 Is a Stress-Responsive Deacetylase in Cardiomyocytes That Protects Cells from Stress-Mediated Cell Death by Deacetylation of Ku70. Mol. Cell. Biol. 2008, 28, 6384–6401. [Google Scholar] [CrossRef] [PubMed]
- Frezza, C.; Cipolat, S.; Martins de Brito, O.; Micaroni, M.; Beznoussenko, G.V.; Rudka, T.; Bartoli, D.; Polishuck, R.S.; Danial, N.N.; De Strooper, B.; et al. OPA1 Controls Apoptotic Cristae Remodeling Independently from Mitochondrial Fusion. Cell 2006, 126, 177–189. [Google Scholar] [CrossRef]
- Scorrano, L.; Ashiya, M.; Buttle, K.; Weiler, S.; Oakes, S.A.; Mannella, C.A.; Korsmeyer, S.J. A Distinct Pathway Remodels Mitochondrial Cristae and Mobilizes Cytochrome c during Apoptosis. Dev. Cell 2002, 2, 55–67. [Google Scholar] [CrossRef]
- Arnoult, D.; Grodet, A.; Lee, Y.-J.; Estaquier, J.; Blackstone, C. Release of OPA1 during Apoptosis Participates in the Rapid and Complete Release of Cytochrome c and Subsequent Mitochondrial Fragmentation. J. Biol. Chem. 2005, 280, 35742–35750. [Google Scholar] [CrossRef] [PubMed]
- Dzeja, P.P.; Bortolon, R.; Perez-Terzic, C.; Holmuhamedov, E.L.; Terzic, A. Energetic Communication between Mitochondria and Nucleus Directed by Catalyzed Phosphotransfer. Proc. Natl. Acad. Sci. USA 2002, 99, 10156–10161. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Troppmair, J.; Sucher, R.; Hermann, M.; Saks, V.; Margreiter, R. Mitochondrial Subpopulations and Heterogeneity Revealed by Confocal Imaging: Possible Physiological Role? Biochim. Biophys. Acta 2006, 1757, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.I.E.; Giovannucci, D.R.; Blinder, G.; Shuttleworth, T.J.; Yule, D.I. Modulation of [Ca2+]i Signaling Dynamics and Metabolism by Perinuclear Mitochondria in Mouse Parotid Acinar Cells. J. Biol. Chem. 2004, 279, 12909–12917. [Google Scholar] [CrossRef]
- Torrealba, N.; Aranguiz, P.; Alonso, C.; Rothermel, B.A.; Lavandero, S. Mitochondria in Structural and Functional Cardiac Remodeling. Adv. Exp. Med. Biol. 2017, 982, 277–306. [Google Scholar] [CrossRef]
- Martín-Fernández, B.; Gredilla, R. Mitochondria and Oxidative Stress in Heart Aging. Age Dordr. Neth. 2016, 38, 225–238. [Google Scholar] [CrossRef]
- Abdullah, C.S.; Alam, S.; Aishwarya, R.; Miriyala, S.; Panchatcharam, M.; Bhuiyan, M.A.N.; Peretik, J.M.; Orr, A.W.; James, J.; Osinska, H.; et al. Cardiac Dysfunction in the Sigma 1 Receptor Knockout Mouse Associated With Impaired Mitochondrial Dynamics and Bioenergetics. J. Am. Heart Assoc. 2018, 7, e009775. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Sun, L.; Chen, W.; Zhou, Y.; Liu, K.; Chen, J.; Zhang, Z.; Zhang, C.; Tian, H. Sirtuin 3 Therapy Attenuates Aging Expression, Oxidative Stress Parameters, and Neointimal Hyperplasia Formation in Vein Grafts. Ann. Vasc. Surg. 2020, 64, 303–317. [Google Scholar] [CrossRef]
- López-Lluch, G.; Irusta, P.M.; Navas, P.; de Cabo, R. Mitochondrial Biogenesis and Healthy Aging. Exp. Gerontol. 2008, 43, 813–819. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative Stress and Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef]
- Montezano, A.C.; Dulak-Lis, M.; Tsiropoulou, S.; Harvey, A.; Briones, A.M.; Touyz, R.M. Oxidative Stress and Human Hypertension: Vascular Mechanisms, Biomarkers, and Novel Therapies. Can. J. Cardiol. 2015, 31, 631–641. [Google Scholar] [CrossRef]
- Tseng, A.H.-H.; Wu, L.-H.; Shieh, S.-S.; Wang, D.L. SIRT3 Interactions with FOXO3 Acetylation, Phosphorylation and Ubiquitinylation Mediate Endothelial Cell Responses to Hypoxia. Biochem. J. 2014, 464, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Wang, P.; Shen, Y.; Yuan, K.; Li, M.; Liang, W.; Que, H. Sirt3 Overexpression Alleviates Hyperglycemia-Induced Vascular Inflammation through Regulating Redox Balance, Cell Survival, and AMPK-Mediated Mitochondrial Homeostasis. J. Recept. Signal Transduct. Res. 2019, 39, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Huang, G.; Wei, T.; Gao, J.; Huang, C.; Sun, M.; Zhu, L.; Shen, W. Sirtuin 3-Induced Macrophage Autophagy in Regulating NLRP3 Inflammasome Activation. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2018, 1864, 764–777. [Google Scholar] [CrossRef]
- Goel, R.; Bhat, S.A.; Hanif, K.; Nath, C.; Shukla, R. Angiotensin II Receptor Blockers Attenuate Lipopolysaccharide-Induced Memory Impairment by Modulation of NF-ΚB-Mediated BDNF/CREB Expression and Apoptosis in Spontaneously Hypertensive Rats. Mol. Neurobiol. 2018, 55, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.L.; Benjamin, E.J.; Broderick, J.P.; Dyken, M.; Easton, J.D.; Feinberg, W.M.; Goldstein, L.B.; Gorelick, P.B.; Howard, G.; Kittner, S.J.; et al. American Heart Association Prevention Conference. IV. Prevention and Rehabilitation of Stroke. Risk Factors. Stroke 1997, 28, 1507–1517. [Google Scholar] [CrossRef]
- Goel, R.; Bhat, S.A.; Rajasekar, N.; Hanif, K.; Nath, C.; Shukla, R. Hypertension Exacerbates Predisposition to Neurodegeneration and Memory Impairment in the Presence of a Neuroinflammatory Stimulus: Protection by Angiotensin Converting Enzyme Inhibition. Pharmacol. Biochem. Behav. 2015, 133, 132–145. [Google Scholar] [CrossRef]
- Walker, K.A.; Power, M.C.; Gottesman, R.F. Defining the Relationship Between Hypertension, Cognitive Decline, and Dementia: A Review. Curr. Hypertens. Rep. 2017, 19, 24. [Google Scholar] [CrossRef]
- Iadecola, C.; Yaffe, K.; Biller, J.; Bratzke, L.C.; Faraci, F.M.; Gorelick, P.B.; Gulati, M.; Kamel, H.; Knopman, D.S.; Launer, L.J.; et al. Impact of Hypertension on Cognitive Function: A Scientific Statement From the American Heart Association. Hypertension 2016, 68, e67–e94. [Google Scholar] [CrossRef] [PubMed]
- Moonga, I.; Niccolini, F.; Wilson, H.; Pagano, G.; Politis, M. Alzheimer’s Disease Neuroimaging Initiative Hypertension Is Associated with Worse Cognitive Function and Hippocampal Hypometabolism in Alzheimer’s Disease. Eur. J. Neurol. 2017, 24, 1173–1182. [Google Scholar] [CrossRef]
- Dikalov, S.; Itani, H.; Richmond, B.; Vergeade, A.; Rahman, S.M.J.; Boutaud, O.; Blackwell, T.; Massion, P.P.; Harrison, D.G.; Dikalova, A. Tobacco Smoking Induces Cardiovascular Mitochondrial Oxidative Stress, Promotes Endothelial Dysfunction, and Enhances Hypertension. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H639–H646. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Dikalova, A.E. Crosstalk Between Mitochondrial Hyperacetylation and Oxidative Stress in Vascular Dysfunction and Hypertension. Antioxid. Redox Signal. 2019, 31, 710–721. [Google Scholar] [CrossRef]
- Tao, R.; Vassilopoulos, A.; Parisiadou, L.; Yan, Y.; Gius, D. Regulation of MnSOD Enzymatic Activity by Sirt3 Connects the Mitochondrial Acetylome Signaling Networks to Aging and Carcinogenesis. Antioxid. Redox Signal. 2014, 20, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Nazarewicz, R.R.; Bikineyeva, A.; Hilenski, L.; Lassègue, B.; Griendling, K.K.; Harrison, D.G.; Dikalova, A.E. Nox2-Induced Production of Mitochondrial Superoxide in Angiotensin II-Mediated Endothelial Oxidative Stress and Hypertension. Antioxid. Redox Signal. 2014, 20, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Itani, H.A.; Dikalova, A.E.; McMaster, W.G.; Nazarewicz, R.R.; Bikineyeva, A.T.; Harrison, D.G.; Dikalov, S.I. Mitochondrial Cyclophilin D in Vascular Oxidative Stress and Hypertension. Hypertension 2016, 67, 1218–1227. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Itani, H.A.; Nazarewicz, R.R.; McMaster, W.G.; Flynn, C.R.; Uzhachenko, R.; Fessel, J.P.; Gamboa, J.L.; Harrison, D.G.; Dikalov, S.I. Sirt3 Impairment and SOD2 Hyperacetylation in Vascular Oxidative Stress and Hypertension. Circ. Res. 2017, 121, 564–574. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Pandey, A.K.; Xiao, L.; Arslanbaeva, L.; Sidorova, T.; Lopez, M.G.; Billings Iv, F.T.; Verdin, E.; Auwerx, J.; Harrison, D.G.; et al. Mitochondrial Deacetylase Sirt3 Reduces Vascular Dysfunction and Hypertension While Sirt3 Depletion in Essential Hypertension Is Linked to Vascular Inflammation and Oxidative Stress. Circ. Res. 2020, 126, 439–452. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zeng, H.; Chen, S.T.; Roman, R.J.; Aschner, J.L.; Didion, S.; Chen, J.-X. Endothelial Specific SIRT3 Deletion Impairs Glycolysis and Angiogenesis and Causes Diastolic Dysfunction. J. Mol. Cell. Cardiol. 2017, 112, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ding, M.-L.; Wu, F.; He, W.; Li, J.; Zhang, X.-Y.; Xie, W.-L.; Duan, S.-Z.; Xia, W.-H.; Tao, J. Impaired Endothelial Repair Capacity of Early Endothelial Progenitor Cells in Hypertensive Patients With Primary Hyperaldosteronemia: Role of 5,6,7,8-Tetrahydrobiopterin Oxidation and Endothelial Nitric Oxide Synthase Uncoupling. Hypertension 2016, 67, 430–439. [Google Scholar] [CrossRef]
- He, J.; Liu, X.; Su, C.; Wu, F.; Sun, J.; Zhang, J.; Yang, X.; Zhang, C.; Zhou, Z.; Zhang, X.; et al. Inhibition of Mitochondrial Oxidative Damage Improves Reendothelialization Capacity of Endothelial Progenitor Cells via SIRT3 (Sirtuin 3)-Enhanced SOD2 (Superoxide Dismutase 2) Deacetylation in Hypertension. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1682–1698. [Google Scholar] [CrossRef]
- Wei, T.; Huang, G.; Gao, J.; Huang, C.; Sun, M.; Wu, J.; Bu, J.; Shen, W. Sirtuin 3 Deficiency Accelerates Hypertensive Cardiac Remodeling by Impairing Angiogenesis. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2017, 6, e006114. [Google Scholar] [CrossRef]
- Lin, J.-r.; Zheng, Y.-j.; Zhang, Z.-b.; Shen, W.-l.; Li, X.-d.; Wei, T.; Ruan, C.-c.; Chen, X.-h.; Zhu, D.-l.; Gao, P.-j. Suppression of Endothelial-to-Mesenchymal Transition by SIRT (Sirtuin) 3 Alleviated the Development of Hypertensive Renal Injury. Hypertension 2018, 72, 350–360. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Keep, R.F.; Kunkel, S.L.; Andjelkovic, A.V. Potential Role of MCP-1 in Endothelial Cell Tight Junction “Opening”: Signaling via Rho and Rho Kinase. J. Cell Sci. 2003, 116, 4615–4628. [Google Scholar] [CrossRef]
- Kabe, Y.; Ando, K.; Hirao, S.; Yoshida, M.; Handa, H. Redox Regulation of NF-KappaB Activation: Distinct Redox Regulation between the Cytoplasm and the Nucleus. Antioxid. Redox Signal. 2005, 7, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Traba, J.; Geiger, S.S.; Kwarteng-Siaw, M.; Han, K.; Ra, O.H.; Siegel, R.M.; Gius, D.; Sack, M.N. Prolonged Fasting Suppresses Mitochondrial NLRP3 Inflammasome Assembly and Activation via SIRT3-Mediated Activation of Superoxide Dismutase 2. J. Biol. Chem. 2017, 292, 12153–12164. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.H.H.; Shieh, S.-S.; Wang, D.L. SIRT3 Deacetylates FOXO3 to Protect Mitochondria against Oxidative Damage. Free Radic. Biol. Med. 2013, 63, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-C.; Tabima, D.M.; Dube, J.J.; Hughan, K.S.; Vanderpool, R.R.; Goncharov, D.A.; St Croix, C.M.; Garcia-Ocaña, A.; Goncharova, E.A.; Tofovic, S.P.; et al. SIRT3-AMPK Activation by Nitrite and Metformin Improves Hyperglycemia and Normalizes Pulmonary Hypertension Associated with Heart Failure with Preserved Ejection Fraction (PH-HFpEF). Circulation 2016, 133, 717–731. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.; Yan, X.; Zhang, C.; Liu, H.; Shan, X.; Li, J.; Yang, Y.; Huang, C.; Zhang, P.; et al. SIRT3-KLF15 Signaling Ameliorates Kidney Injury Induced by Hypertension. Oncotarget 2017, 8, 39592–39604. [Google Scholar] [CrossRef]
- Castrejón-Téllez, V.; Villegas-Romero, M.; Pérez-Torres, I.; Zarco, G.; Rubio-Ruiz, M.E.; Carreón-Torres, E.; Díaz-Díaz, E.; Grimaldo, O.E.; Guarner-Lans, V. Effect of Sucrose Ingestion at the End of a Critical Window That Increases Hypertension Susceptibility on Peripheral Mechanisms Regulating Blood Pressure in Rats. Role of Sirtuins 1 and 3. Nutrients 2019, 11, 309. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Yang, H.; Zhang, P.; Wu, F.; Li, Y.; Zhou, Y.; Zhang, X.; Ma, H.; Zhang, W.; et al. α-Linolenic Acid but Not Linolenic Acid Protects against Hypertension: Critical Role of SIRT3 and Autophagic Flux. Cell Death Dis. 2020, 11, 83–113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, M.; Zeng, X.; Yang, J.; Deng, H.; Yi, L.; Mi, M. Resveratrol Regulates Mitochondrial Reactive Oxygen Species Homeostasis through Sirt3 Signaling Pathway in Human Vascular Endothelial Cells. Cell Death Dis. 2014, 5, e1576. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.B.; Samant, S.; Sundaresan, N.R.; Raghuraman, H.; Kim, G.; Bonner, M.Y.; Arbiser, J.L.; Walker, D.I.; Jones, D.P.; Gius, D.; et al. Honokiol Blocks and Reverses Cardiac Hypertrophy in Mice by Activating Mitochondrial SIRT3. Nat. Commun. 2015, 6, 6656. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xue, R.-Q.; Lu, Y.; Yong, S.-Y.; Wu, Q.; Cui, Y.-L.; Zuo, X.-T.; Yu, X.-J.; Zhao, M.; Zang, W.-J. Choline Ameliorates Cardiac Hypertrophy by Regulating Metabolic Remodelling and UPRmt through SIRT3-AMPK Pathway. Cardiovasc. Res. 2019, 115, 530–545. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Yang, Z.; Liu, F.; Jin, Y.-G.; Zhang, N.; Ni, J.; Yuan, Y.; Liao, H.-H.; Wu, Q.-Q.; Xu, M.; et al. Sesamin Protects Against Cardiac Remodeling Via Sirt3/ROS Pathway. Cell. Physiol. Biochem. 2017, 44, 2212–2227. [Google Scholar] [CrossRef]
- Meng, G.; Liu, J.; Liu, S.; Song, Q.; Liu, L.; Xie, L.; Han, Y.; Ji, Y. Hydrogen Sulfide Pretreatment Improves Mitochondrial Function in Myocardial Hypertrophy via a SIRT3-dependent Manner. Br. J. Pharmacol. 2018, 175, 1126–1145. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, J.; Hu, O.; Huang, J.; Ran, L.; Chen, M.; Zhang, Y.; Zhou, X.; Zhu, J.; Zhang, Q.; et al. Dihydromyricetin Ameliorates Nonalcoholic Fatty Liver Disease by Improving Mitochondrial Respiratory Capacity and Redox Homeostasis Through Modulation of SIRT3 Signaling. Antioxid. Redox Signal. 2018, 30, 163–183. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, H.-Q.; Sun, L.-L.; Xu, M.-T.; Yu, J.; Liu, L.-L.; Zhang, J.-Y.; Wang, Y.-Q.; Wang, H.-X.; Bao, X.-F.; et al. Dihydromyricetin Attenuates Myocardial Hypertrophy Induced by Transverse Aortic Constriction via Oxidative Stress Inhibition and SIRT3 Pathway Enhancement. Int. J. Mol. Sci. 2018, 19, 2592. [Google Scholar] [CrossRef]
- Chen, T.; Li, J.; Liu, J.; Li, N.; Wang, S.; Liu, H.; Zeng, M.; Zhang, Y.; Bu, P. Activation of SIRT3 by Resveratrol Ameliorates Cardiac Fibrosis and Improves Cardiac Function via the TGF-β/Smad3 Pathway. Am. J. Physiol.-Heart Circ. Physiol. 2014, 308, H424–H434. [Google Scholar] [CrossRef]
- Pillai, V.B.; Sundaresan, N.R.; Kim, G.; Gupta, M.; Rajamohan, S.B.; Pillai, J.B.; Samant, S.; Ravindra, P.V.; Isbatan, A.; Gupta, M.P. Exogenous NAD Blocks Cardiac Hypertrophic Response via Activation of the SIRT3-LKB1-AMP-Activated Kinase Pathway. J. Biol. Chem. 2010, 285, 3133–3144. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, K.; Wang, Y.; Guo, R.; Liu, H.; Jia, C.; Sun, X.; Wu, C.; Wang, W.; Du, J.; et al. A Machine Learning-Driven Study Indicates Emodin Improves Cardiac Hypertrophy by Modulation of Mitochondrial SIRT3 Signaling. Pharmacol. Res. 2020, 155, 104739. [Google Scholar] [CrossRef]
- Jahanifar, F.; Astani, A.; Shekarforoosh, S.; Jamhiri, M.; Safari, F.; Zarei, F.; Safari, F. 1.25 Dihydroxyvitamin D3 Attenuates Hypertrophy Markers in Cardiomyoblast H9c2 Cells: Evaluation of Sirtuin3 MRNA and Protein Level. Int. J. Vitam. Nutr. Res. Int. Z. Vitam.-Ernahrungsforschung J. Int. Vitaminol. Nutr. 2019, 89, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Ma, Y.; You, J.; Li, Z.; Ding, Y.; He, P.; Lu, X.; Jiang, J.; Chen, S.; Liu, P. NMNAT3 Is Involved in the Protective Effect of SIRT3 in Ang II-Induced Cardiac Hypertrophy. Exp. Cell Res. 2016, 347, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Román-Azcona, M.S.; Pizarro-Delgado, J.; Planavila, A.; Villarroya, F.; Valenzuela-Alcaraz, B.; Crispi, F.; Sepúlveda-Martínez, Á.; Miguel-Escalada, I.; Ferrer, J.; et al. SIRT3-Mediated Inhibition of FOS through Histone H3 Deacetylation Prevents Cardiac Fibrosis and Inflammation. Signal Transduct. Target. Ther. 2020, 5, 14. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Gupta, M.; Kim, G.; Rajamohan, S.B.; Isbatan, A.; Gupta, M.P. Sirt3 Blocks the Cardiac Hypertrophic Response by Augmenting Foxo3a-Dependent Antioxidant Defense Mechanisms in Mice. J. Clin. Investig. 2009, 119, 2758–2771. [Google Scholar] [CrossRef] [PubMed]
- Koentges, C.; Pfeil, K.; Schnick, T.; Wiese, S.; Dahlbock, R.; Cimolai, M.C.; Meyer-Steenbuck, M.; Cenkerova, K.; Hoffmann, M.M.; Jaeger, C.; et al. SIRT3 Deficiency Impairs Mitochondrial and Contractile Function in the Heart. Basic Res. Cardiol. 2015, 110, 36. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Vaka, V.R.; He, X.; Booz, G.W.; Chen, J.-X. High-Fat Diet Induces Cardiac Remodelling and Dysfunction: Assessment of the Role Played by SIRT3 Loss. J. Cell. Mol. Med. 2015, 19, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, J.; Li, N.; Wang, S.; Liu, H.; Li, J.; Zhang, Y.; Bu, P. Mouse SIRT3 Attenuates Hypertrophy-Related Lipid Accumulation in the Heart through the Deacetylation of LCAD. PLoS ONE 2015, 10, e0118909. [Google Scholar] [CrossRef]
- He, X.; Zeng, H.; Chen, J.-X. Ablation of SIRT3 Causes Coronary Microvascular Dysfunction and Impairs Cardiac Recovery Post Myocardial Ischemia. Int. J. Cardiol. 2016, 215, 349–357. [Google Scholar] [CrossRef]
- Pillai, J.B.; Isbatan, A.; Imai, S.; Gupta, M.P. Poly(ADP-Ribose) Polymerase-1-Dependent Cardiac Myocyte Cell Death during Heart Failure Is Mediated by NAD+ Depletion and Reduced Sir2alpha Deacetylase Activity. J. Biol. Chem. 2005, 280, 43121–43130. [Google Scholar] [CrossRef]
- Münzel, T.; Gori, T.; Keaney, J.F.; Maack, C.; Daiber, A. Pathophysiological Role of Oxidative Stress in Systolic and Diastolic Heart Failure and Its Therapeutic Implications. Eur. Heart J. 2015, 36, 2555–2564. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Y.; Chen, W.; Xu, S.; Li, L.; Geng, Y.; Shen, A.; Gao, H.; Zhang, L.; Liu, S. SIRT3 Inhibits Cardiac Hypertrophy by Regulating PARP-1 Activity. Aging 2020, 12, 4178–4192. [Google Scholar] [CrossRef]
- Li, J.; Chen, T.; Xiao, M.; Li, N.; Wang, S.; Su, H.; Guo, X.; Liu, H.; Yan, F.; Yang, Y.; et al. Mouse Sirt3 Promotes Autophagy in AngII-Induced Myocardial Hypertrophy through the Deacetylation of FoxO1. Oncotarget 2016, 7, 86648–86659. [Google Scholar] [CrossRef]
- Hafner, A.V.; Dai, J.; Gomes, A.P.; Xiao, C.-Y.; Palmeira, C.M.; Rosenzweig, A.; Sinclair, D.A. Regulation of the MPTP by SIRT3-Mediated Deacetylation of CypD at Lysine 166 Suppresses Age-Related Cardiac Hypertrophy. Aging 2010, 2, 914–923. [Google Scholar] [CrossRef]
- Castillo, E.C.; Morales, J.A.; Chapoy-Villanueva, H.; Silva-Platas, C.; Treviño-Saldaña, N.; Guerrero-Beltrán, C.E.; Bernal-Ramírez, J.; Torres-Quintanilla, A.; García, N.; Youker, K.; et al. Mitochondrial Hyperacetylation in the Failing Hearts of Obese Patients Mediated Partly by a Reduction in SIRT3: The Involvement of the Mitochondrial Permeability Transition Pore. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2019, 53, 465–479. [Google Scholar] [CrossRef]
- McDonnell, E.; Peterson, B.S.; Bomze, H.M.; Hirschey, M.D. SIRT3 Regulates Progression and Development of Diseases of Aging. Trends Endocrinol. Metab. TEM 2015, 26, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Anamika, J.; Trigun, S.K. Sirtuin-3 Activation by Honokiol Restores Mitochondrial Dysfunction in the Hippocampus of the Hepatic Encephalopathy Rat Model of Ammonia Neurotoxicity. J. Biochem. Mol. Toxicol. 2021, 35, e22735. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ouyang, Y.; Yang, L.; Beal, M.F.; McQuibban, A.; Vogel, H.; Lu, B. Pink1 Regulates Mitochondrial Dynamics through Interaction with the Fission/Fusion Machinery. Proc. Natl. Acad. Sci. USA 2008, 105, 7070–7075. [Google Scholar] [CrossRef] [PubMed]
- Haun, F.; Nakamura, T.; Lipton, S.A. Dysfunctional Mitochondrial Dynamics in the Pathophysiology of Neurodegenerative Diseases. J. Cell Death 2013, 6, 27–35. [Google Scholar] [CrossRef]
- Lu, B. Mitochondrial Dynamics and Neurodegeneration. Curr. Neurol. Neurosci. Rep. 2009, 9, 212–219. [Google Scholar] [CrossRef]
- Reynolds, I.J.; Malaiyandi, L.M.; Coash, M.; Rintoul, G.L. Mitochondrial Trafficking in Neurons: A Key Variable in Neurodegeneration? J. Bioenerg. Biomembr. 2004, 36, 283–286. [Google Scholar] [CrossRef]
- Knott, A.B.; Perkins, G.; Schwarzenbacher, R.; Bossy-Wetzel, E. Mitochondrial Fragmentation in Neurodegeneration. Nat. Rev. Neurosci. 2008, 9, 505–518. [Google Scholar] [CrossRef]
- Srivastava, P.; Yadav, R.S. Efficacy of Natural Compounds in Neurodegenerative Disorders. Adv. Neurobiol. 2016, 12, 107–123. [Google Scholar] [CrossRef]
- Braidy, N.; Poljak, A.; Grant, R.; Jayasena, T.; Mansour, H.; Chan-Ling, T.; Smythe, G.; Sachdev, P.; Guillemin, G.J. Differential Expression of Sirtuins in the Aging Rat Brain. Front. Cell. Neurosci. 2015, 9, 167. [Google Scholar] [CrossRef]
- Kerr, J.S.; Adriaanse, B.A.; Greig, N.H.; Mattson, M.P.; Cader, M.Z.; Bohr, V.A.; Fang, E.F. Mitophagy and Alzheimer’s Disease: Cellular and Molecular Mechanisms. Trends Neurosci. 2017, 40, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Sauve, A.A.; Bai, P. Crosstalk between Poly(ADP-Ribose) Polymerase and Sirtuin Enzymes. Mol. Aspects Med. 2013, 34, 1168–1201. [Google Scholar] [CrossRef]
- Kwon, D.-N.; Park, W.-J.; Choi, Y.-J.; Gurunathan, S.; Kim, J.-H. Oxidative Stress and ROS Metabolism via Down-Regulation of Sirtuin 3 Expression in Cmah-Null Mice Affect Hearing Loss. Aging 2015, 7, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Someya, S.; Yu, W.; Hallows, W.C.; Xu, J.; Vann, J.M.; Leeuwenburgh, C.; Tanokura, M.; Denu, J.M.; Prolla, T.A. Sirt3 Mediates Reduction of Oxidative Damage and Prevention of Age-Related Hearing Loss under Caloric Restriction. Cell 2010, 143, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Adiele, R.C.; Adiele, C.A. Mitochondrial Regulatory Pathways in the Pathogenesis of Alzheimer’s Disease. J. Alzheimers Dis. JAD 2016, 53, 1257–1270. [Google Scholar] [CrossRef]
- Cadonic, C.; Sabbir, M.G.; Albensi, B.C. Mechanisms of Mitochondrial Dysfunction in Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 6078–6090. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, J.G.; Hipp, M.J.; Montine, T.J.; Kennedy, S.R. Mitochondrial DNA Mutations Increase in Early Stage Alzheimer Disease and Are Inconsistent with Oxidative Damage. Ann. Neurol. 2016, 80, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Lunnon, K.; Keohane, A.; Pidsley, R.; Newhouse, S.; Riddoch-Contreras, J.; Thubron, E.B.; Devall, M.; Soininen, H.; Kłoszewska, I.; Mecocci, P.; et al. Mitochondrial Genes Are Altered in Blood Early in Alzheimer’s Disease. Neurobiol. Aging 2017, 53, 36–47. [Google Scholar] [CrossRef]
- Emerit, J.; Edeas, M.; Bricaire, F. Neurodegenerative Diseases and Oxidative Stress. Biomed. Pharmacother. Biomed. Pharmacother. 2004, 58, 39–46. [Google Scholar] [CrossRef]
- Sutherland, G.T.; Chami, B.; Youssef, P.; Witting, P.K. Oxidative Stress in Alzheimer’s Disease: Primary Villain or Physiological by-Product? Redox Rep. Commun. Free Radic. Res. 2013, 18, 134–141. [Google Scholar] [CrossRef]
- Swomley, A.M.; Förster, S.; Keeney, J.T.; Triplett, J.; Zhang, Z.; Sultana, R.; Butterfield, D.A. Abeta, Oxidative Stress in Alzheimer Disease: Evidence Based on Proteomics Studies. Biochim. Biophys. Acta 2014, 1842, 1248–1257. [Google Scholar] [CrossRef]
- Kapogiannis, D.; Mattson, M.P. Disrupted Energy Metabolism and Neuronal Circuit Dysfunction in Cognitive Impairment and Alzheimer’s Disease. Lancet Neurol. 2011, 10, 187–198. [Google Scholar] [CrossRef]
- Calkins, M.J.; Reddy, P.H. Amyloid Beta Impairs Mitochondrial Anterograde Transport and Degenerates Synapses in Alzheimer’s Disease Neurons. Biochim. Biophys. Acta 2011, 1812, 507–513. [Google Scholar] [CrossRef]
- Wojsiat, J.; Zoltowska, K.M.; Laskowska-Kaszub, K.; Wojda, U. Oxidant/Antioxidant Imbalance in Alzheimer’s Disease: Therapeutic and Diagnostic Prospects. Oxid. Med. Cell. Longev. 2018, 2018, 6435861. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.I.; Milenkovic, I.; Regelsberger, G.; Kovacs, G.G. Distinct Patterns of Sirtuin Expression during Progression of Alzheimer’s Disease. Neuromol. Med. 2014, 16, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Yan, W.-Y.; Lei, Y.-H.; Wan, Z.; Hou, Y.-Y.; Sun, L.-K.; Zhou, J.-P. SIRT3 Regulation of Mitochondrial Quality Control in Neurodegenerative Diseases. Front. Aging Neurosci. 2019, 11, 313. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M.; Tong, M. Brain Metabolic Dysfunction at the Core of Alzheimer’s Disease. Biochem. Pharmacol. 2014, 88, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.F.; Boyle, J.P.; Green, K.N.; Pearson, H.A.; Peers, C. Hypoxic Remodelling of Ca2+ Mobilization in Type I Cortical Astrocytes: Involvement of ROS and pro-Amyloidogenic APP Processing. J. Neurochem. 2004, 88, 869–877. [Google Scholar] [CrossRef]
- Tamagno, E.; Guglielmotto, M.; Aragno, M.; Borghi, R.; Autelli, R.; Giliberto, L.; Muraca, G.; Danni, O.; Zhu, X.; Smith, M.A.; et al. Oxidative Stress Activates a Positive Feedback between the Gamma- and Beta-Secretase Cleavages of the Beta-Amyloid Precursor Protein. J. Neurochem. 2008, 104, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.J.; Guo, J.L.; Hurtado, D.E.; Kwong, L.K.; Mills, I.P.; Trojanowski, J.Q.; Lee, V.M.Y. The Acetylation of Tau Inhibits Its Function and Promotes Pathological Tau Aggregation. Nat. Commun. 2011, 2, 252. [Google Scholar] [CrossRef]
- Min, S.-W.; Chen, X.; Tracy, T.E.; Li, Y.; Zhou, Y.; Wang, C.; Shirakawa, K.; Minami, S.S.; Defensor, E.; Mok, S.A.; et al. Critical Role of Acetylation in Tau-Mediated Neurodegeneration and Cognitive Deficits. Nat. Med. 2015, 21, 1154–1162. [Google Scholar] [CrossRef]
- Min, S.-W.; Cho, S.-H.; Zhou, Y.; Schroeder, S.; Haroutunian, V.; Seeley, W.W.; Huang, E.J.; Shen, Y.; Masliah, E.; Mukherjee, C.; et al. Acetylation of Tau Inhibits Its Degradation and Contributes to Tauopathy. Neuron 2010, 67, 953–966. [Google Scholar] [CrossRef]
- Yin, J.; Han, P.; Song, M.; Nielsen, M.; Beach, T.G.; Serrano, G.E.; Liang, W.S.; Caselli, R.J.; Shi, J. Amyloid-β Increases Tau by Mediating Sirtuin 3 in Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 8592–8601. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zou, Y.; Zhang, M.; Zhao, N.; Tian, Q.; Gu, M.; Liu, W.; Shi, R.; Lü, Y.; Yu, W. Mitochondrial Sirt3 Expression Is Decreased in APP/PS1 Double Transgenic Mouse Model of Alzheimer’s Disease. Neurochem. Res. 2015, 40, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Tracy, T.E.; Sohn, P.D.; Minami, S.S.; Wang, C.; Min, S.-W.; Li, Y.; Zhou, Y.; Le, D.; Lo, I.; Ponnusamy, R.; et al. Acetylated Tau Obstructs KIBRA-Mediated Signaling in Synaptic Plasticity and Promotes Tauopathy-Related Memory Loss. Neuron 2016, 90, 245–260. [Google Scholar] [CrossRef]
- Han, P.; Tang, Z.; Yin, J.; Maalouf, M.; Beach, T.G.; Reiman, E.M.; Shi, J. Pituitary Adenylate Cyclase-Activating Polypeptide Protects against β-Amyloid Toxicity. Neurobiol. Aging 2014, 35, 2064–2071. [Google Scholar] [CrossRef]
- Lazarov, O.; Mattson, M.P.; Peterson, D.A.; Pimplikar, S.W.; Praag, H. van When Neurogenesis Encounters Aging and Disease. Trends Neurosci. 2010, 33, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yamamori, H.; Tatebayashi, Y.; Shafit-Zagardo, B.; Tanimukai, H.; Chen, S.; Iqbal, K.; Grundke-Iqbal, I. Failure of Neuronal Maturation in Alzheimer Disease Dentate Gyrus. J. Neuropathol. Exp. Neurol. 2008, 67, 78–84. [Google Scholar] [CrossRef]
- Waldau, B.; Shetty, A.K. Behavior of Neural Stem Cells in the Alzheimer Brain. Cell. Mol. Life Sci. 2008, 65, 2372–2384. [Google Scholar] [CrossRef]
- Ribeiro, M.F.; Genebra, T.; Rego, A.C.; Rodrigues, C.M.P.; Solá, S. Amyloid β Peptide Compromises Neural Stem Cell Fate by Irreversibly Disturbing Mitochondrial Oxidative State and Blocking Mitochondrial Biogenesis and Dynamics. Mol. Neurobiol. 2019, 56, 3922–3936. [Google Scholar] [CrossRef]
- Huijbers, W.; Schultz, A.P.; Papp, K.V.; LaPoint, M.R.; Hanseeuw, B.; Chhatwal, J.P.; Hedden, T.; Johnson, K.A.; Sperling, R.A. Tau Accumulation in Clinically Normal Older Adults Is Associated with Hippocampal Hyperactivity. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Putcha, D.; Brickhouse, M.; O’Keefe, K.; Sullivan, C.; Rentz, D.; Marshall, G.; Dickerson, B.; Sperling, R. Hippocampal Hyperactivation Associated with Cortical Thinning in Alzheimer’s Disease Signature Regions in Non-Demented Elderly Adults. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 17680–17688. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Losa, M.; Tracy, T.E.; Ma, K.; Verret, L.; Clemente-Perez, A.; Khan, A.S.; Cobos, I.; Ho, K.; Gan, L.; Mucke, L.; et al. Nav1.1-Overexpressing Interneuron Transplants Restore Brain Rhythms and Cognition in a Mouse Model of Alzheimer’s Disease. Neuron 2018, 98, 75–89.e5. [Google Scholar] [CrossRef]
- Mattson, M.P.; Cheng, B.; Davis, D.; Bryant, K.; Lieberburg, I.; Rydel, R.E. Beta-Amyloid Peptides Destabilize Calcium Homeostasis and Render Human Cortical Neurons Vulnerable to Excitotoxicity. J. Neurosci. Off. J. Soc. Neurosci. 1992, 12, 376–389. [Google Scholar] [CrossRef]
- Mattson, M.P.; Cheng, B.; Culwell, A.R.; Esch, F.S.; Lieberburg, I.; Rydel, R.E. Evidence for Excitoprotective and Intraneuronal Calcium-Regulating Roles for Secreted Forms of the Beta-Amyloid Precursor Protein. Neuron 1993, 10, 243–254. [Google Scholar] [CrossRef]
- Keller, J.N.; Pang, Z.; Geddes, J.W.; Begley, J.G.; Germeyer, A.; Waeg, G.; Mattson, M.P. Impairment of Glucose and Glutamate Transport and Induction of Mitochondrial Oxidative Stress and Dysfunction in Synaptosomes by Amyloid Beta-Peptide: Role of the Lipid Peroxidation Product 4-Hydroxynonenal. J. Neurochem. 1997, 69, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Connolly, N.M.C.; Prehn, J.H.M. The Metabolic Response to Excitotoxicity—Lessons from Single-Cell Imaging. J. Bioenerg. Biomembr. 2015, 47, 75–88. [Google Scholar] [CrossRef]
- Mattson, M.P. Excitotoxic and Excitoprotective Mechanisms: Abundant Targets for the Prevention and Treatment of Neurodegenerative Disorders. Neuromol. Med. 2003, 3, 65–94. [Google Scholar] [CrossRef]
- Woodbury, A.; Yu, S.P.; Wei, L.; García, P. Neuro-Modulating Effects of Honokiol: A Review. Front. Neurol. 2013, 4, 130. [Google Scholar] [CrossRef]
- Li, H.; Jia, J.; Wang, W.; Hou, T.; Tian, Y.; Wu, Q.; Xu, L.; Wei, Y.; Wang, X. Honokiol Alleviates Cognitive Deficits of Alzheimer’s Disease (PS1V97L) Transgenic Mice by Activating Mitochondrial SIRT3. J. Alzheimers Dis. JAD 2018, 64, 291–302. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Ni, C.; Song, G. Honokiol Attenuates Oligomeric Amyloid Β1-42-Induced Alzheimer’s Disease in Mice Through Attenuating Mitochondrial Apoptosis and Inhibiting the Nuclear Factor Kappa-B Signaling Pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 43, 69–81. [Google Scholar] [CrossRef]
- Hou, Y.; Lautrup, S.; Cordonnier, S.; Wang, Y.; Croteau, D.L.; Zavala, E.; Zhang, Y.; Moritoh, K.; O’Connell, J.F.; Baptiste, B.A.; et al. NAD+ Supplementation Normalizes Key Alzheimer’s Features and DNA Damage Responses in a New AD Mouse Model with Introduced DNA Repair Deficiency. Proc. Natl. Acad. Sci. USA 2018, 115, E1876–E1885. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Xia, L.; Shao, J.; Yin, S.; Cheng, C.Y.; Xia, W.; Gao, W.-Q. Adjudin Protects Rodent Cochlear Hair Cells against Gentamicin Ototoxicity via the SIRT3-ROS Pathway. Sci. Rep. 2015, 5, 8181. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.D.; Maqsood, S.; Huang, J.-Y.; Pan, Y.; Harkcom, W.; Li, W.; Sauve, A.; Verdin, E.; Jaffrey, S.R. Activation of SIRT3 by the NAD+ Precursor Nicotinamide Riboside Protects from Noise-Induced Hearing Loss. Cell Metab. 2014, 20, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yang, Y.; Hu, Y.; Sun, Y.; Du, Z.; Xie, Z.; Zhou, T.; Kong, W. Age-Related Decrease in the Mitochondrial Sirtuin Deacetylase Sirt3 Expression Associated with ROS Accumulation in the Auditory Cortex of the Mimetic Aging Rat Model. PLoS ONE 2014, 9, e88019. [Google Scholar] [CrossRef]
- Wang, J.; Gu, B.J.; Masters, C.L.; Wang, Y.-J. A Systemic View of Alzheimer Disease—Insights from Amyloid-β Metabolism beyond the Brain. Nat. Rev. Neurol. 2017, 13, 612–623. [Google Scholar] [CrossRef]
- Wojda, U. Alzheimer’s Disease Lymphocytes: Potential for Biomarkers? Biomark. Med. 2016, 10, 1–4. [Google Scholar] [CrossRef]
- Cosín-Tomàs, M.; Senserrich, J.; Arumí-Planas, M.; Alquézar, C.; Pallàs, M.; Martín-Requero, Á.; Suñol, C.; Kaliman, P.; Sanfeliu, C. Role of Resveratrol and Selenium on Oxidative Stress and Expression of Antioxidant and Anti-Aging Genes in Immortalized Lymphocytes from Alzheimer’s Disease Patients. Nutrients 2019, 11, 1764. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, D.M.; Herz, J.; Bu, G. Apolipoprotein E and Apolipoprotein E Receptors: Normal Biology and Roles in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006312. [Google Scholar] [CrossRef] [PubMed]
- Reiman, E.M.; Chen, K.; Alexander, G.E.; Caselli, R.J.; Bandy, D.; Osborne, D.; Saunders, A.M.; Hardy, J. Functional Brain Abnormalities in Young Adults at Genetic Risk for Late-Onset Alzheimer’s Dementia. Proc. Natl. Acad. Sci. USA 2004, 101, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Chételat, G.; Fouquet, M. Neuroimaging Biomarkers for Alzheimer’s Disease in Asymptomatic APOE4 Carriers. Rev. Neurol. 2013, 169, 729–736. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Valla, J.; Yaari, R.; Wolf, A.B.; Kusne, Y.; Beach, T.G.; Roher, A.E.; Corneveaux, J.J.; Huentelman, M.J.; Caselli, R.J.; Reiman, E.M. Reduced Posterior Cingulate Mitochondrial Activity in Expired Young Adult Carriers of the APOE Ε4 Allele, the Major Late-Onset Alzheimer’s Susceptibility Gene. J. Alzheimers Dis. JAD 2010, 22, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Turner, G.H.; Lin, H.; Coons, S.W.; Shi, J. Deficits in Spatial Learning and Memory Is Associated with Hippocampal Volume Loss in Aged Apolipoprotein E4 Mice. J. Alzheimers Dis. JAD 2011, 27, 89–98. [Google Scholar] [CrossRef]
- Yin, J.; Turner, G.H.; Coons, S.W.; Maalouf, M.; Reiman, E.M.; Shi, J. Association of Amyloid Burden, Brain Atrophy and Memory Deficits in Aged Apolipoprotein Ε4 Mice. Curr. Alzheimer Res. 2014, 11, 283–290. [Google Scholar] [CrossRef]
- Yin, J.; Li, S.; Nielsen, M.; Carcione, T.; Liang, W.S.; Shi, J. Sirtuin 3 Attenuates Amyloid-β Induced Neuronal Hypometabolism. Aging 2018, 10, 2874–2883. [Google Scholar] [CrossRef] [PubMed]
- Gusdon, A.M.; Callio, J.; Distefano, G.; O’Doherty, R.M.; Goodpaster, B.H.; Coen, P.M.; Chu, C.T. Exercise Increases Mitochondrial Complex I Activity and DRP1 Expression in the Brains of Aged Mice. Exp. Gerontol. 2017, 90, 1–13. [Google Scholar] [CrossRef]
- Santos-Alves, E.; Marques-Aleixo, I.; Rizo-Roca, D.; Torrella, J.R.; Oliveira, P.J.; Magalhães, J.; Ascensão, A. Exercise Modulates Liver Cellular and Mitochondrial Proteins Related to Quality Control Signaling. Life Sci. 2015, 135, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Haroutunian, V.; Katsel, P.; Cardozo, C.P.; Ho, L.; Buxbaum, J.D.; Pasinetti, G.M. PGC-1α Expression Decreases in the Alzheimer Disease Brain as a Function of Dementia. Arch. Neurol. 2009, 66, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Sajan, M.; Hansen, B.; Ivey, R.; Sajan, J.; Ari, C.; Song, S.; Braun, U.; Leitges, M.; Farese-Higgs, M.; Farese, R.V. Brain Insulin Signaling Is Increased in Insulin-Resistant States and Decreases in FOXOs and PGC-1α and Increases in Aβ1–40/42 and Phospho-Tau May Abet Alzheimer Development. Diabetes 2016, 65, 1892–1903. [Google Scholar] [CrossRef]
- Yin, J.; Nielsen, M.; Carcione, T.; Li, S.; Shi, J. Apolipoprotein E Regulates Mitochondrial Function through the PGC-1α-Sirtuin 3 Pathway. Aging 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, X.; Zhao, L. Human ApoE Isoforms Differentially Modulate Brain Glucose and Ketone Body Metabolism: Implications for Alzheimer’s Disease Risk Reduction and Early Intervention. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 6665–6681. [Google Scholar] [CrossRef] [PubMed]
- Chin, D.; Hagl, S.; Hoehn, A.; Huebbe, P.; Pallauf, K.; Grune, T.; Frank, J.; Eckert, G.P.; Rimbach, G. Adenosine Triphosphate Concentrations Are Higher in the Brain of APOE3- Compared to APOE4-Targeted Replacement Mice and Can Be Modulated by Curcumin. Genes Nutr. 2014, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-K.; Ji, Z.-S.; Dodson, S.E.; Miranda, R.D.; Rosenblum, C.I.; Reynolds, I.J.; Freedman, S.B.; Weisgraber, K.H.; Huang, Y.; Mahley, R.W. Apolipoprotein E4 Domain Interaction Mediates Detrimental Effects on Mitochondria and Is a Potential Therapeutic Target for Alzheimer Disease. J. Biol. Chem. 2011, 286, 5215–5221. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.L.; Kim, C.; Jimenez-Morales, D.; Newton, B.W.; Johnson, J.R.; Krogan, N.J.; Swaney, D.L.; Mahley, R.W. Neuronal Apolipoprotein E4 Expression Results in Proteome-Wide Alterations and Compromises Bioenergetic Capacity by Disrupting Mitochondrial Function. J. Alzheimers Dis. 2019, 68, 991–1011. [Google Scholar] [CrossRef]
- Elbaz, A.; Carcaillon, L.; Kab, S.; Moisan, F. Epidemiology of Parkinson’s Disease. Rev. Neurol. 2016, 172, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Peferoen, L.A.N.; Vogel, D.Y.S.; Breur, M.; van der Valk, P.; Baker, D.; van Noort, J.M. Inflammation in Neurodegenerative Diseases--an Update. Immunology 2014, 142, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Almalki, W.H.; Alzahrani, A.; Mahmoud El-Daly, M.E.-S.; Fadel Ahmed, A.-S.H.F. The Emerging Potential of SIRT-3 in Oxidative Stress-Inflammatory Axis Associated Increased Neuroinflammatory Component for Metabolically Impaired Neural Cell. Chem. Biol. Interact. 2021, 333, 109328. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Ma, Y.; Ma, Y.; Song, L.; Song, L.; Yu, L.; Yu, L.; Zhang, L.; Zhang, L.; et al. SIRT3 Deficiency Exacerbates P53/Parkin-mediated Mitophagy Inhibition and Promotes Mitochondrial Dysfunction: Implication for Aged Hearts. Int. J. Mol. Med. 2018, 41, 3517–3526. [Google Scholar] [CrossRef] [PubMed]
- Hauser, D.N.; Hastings, T.G. Mitochondrial Dysfunction and Oxidative Stress in Parkinson’s Disease and Monogenic Parkinsonism. Neurobiol. Dis. 2013, 51, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Abou-Sleiman, P.M.; Muqit, M.M.K.; Wood, N.W. Expanding Insights of Mitochondrial Dysfunction in Parkinson’s Disease. Nat. Rev. Neurosci. 2006, 7, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Bonifati, V.; Rizzu, P.; van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.J.; Squitieri, F.; Ibanez, P.; Joosse, M.; et al. Mutations in the DJ-1 Gene Associated with Autosomal Recessive Early-Onset Parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Valente, E.M.; Abou-Sleiman, P.M.; Caputo, V.; Muqit, M.M.K.; Harvey, K.; Gispert, S.; Ali, Z.; Del Turco, D.; Bentivoglio, A.R.; Healy, D.G.; et al. Hereditary Early-Onset Parkinson’s Disease Caused by Mutations in PINK1. Science 2004, 304, 1158–1160. [Google Scholar] [CrossRef]
- Shi, H.; Deng, H.-X.; Gius, D.; Schumacker, P.T.; Surmeier, D.J.; Ma, Y.-C. Sirt3 Protects Dopaminergic Neurons from Mitochondrial Oxidative Stress. Hum. Mol. Genet. 2017, 26, 1915–1926. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Deng, Y.-N.; Zhang, M.; Su, H.; Qu, Q.-M. SIRT3 Acts as a Neuroprotective Agent in Rotenone-Induced Parkinson Cell Model. Neurochem. Res. 2016, 41, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Zhang, T.; Liu, W.; Chen, Y. MiR-494-3p Modulates the Progression of in Vitro and in Vivo Parkinson’s Disease Models by Targeting SIRT3. Neurosci. Lett. 2018, 675, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.-X.; Li, X.; Dong, S.-Y.; Guo, Y.-J.; Liu, T.; Wu, Y.-C. SIRT3 Deacetylated and Increased Citrate Synthase Activity in PD Model. Biochem. Biophys. Res. Commun. 2017, 484, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Lin, C.-H.; Wu, R.-M.; Lin, J.-W.; Chang, C.-H.; Lai, M.-S. Antihypertensive Agents and Risk of Parkinson’s Disease: A Nationwide Cohort Study. PLoS ONE 2014, 9, e98961. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ruiz, C.; Villar-Cheda, B.; Dominguez-Meijide, A.; Garrido-Gil, P.; Guerra, M.J.; Labandeira-Garcia, J.L. Aging-Related Overactivity of the Angiotensin/AT1 Axis Decreases Sirtuin 3 Levels in the Substantia Nigra, Which Induces Vulnerability to Oxidative Stress and Neurodegeneration. J. Gerontol. Ser. A 2018, 75, 416–424. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of Intermittent Fasting on Health and Disease Processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent Metabolic Switching, Neuroplasticity and Brain Health. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Still, A.J.; Floyd, B.J.; Hebert, A.S.; Bingman, C.A.; Carson, J.J.; Gunderson, D.R.; Dolan, B.K.; Grimsrud, P.A.; Dittenhafer-Reed, K.E.; Stapleton, D.S.; et al. Quantification of Mitochondrial Acetylation Dynamics Highlights Prominent Sites of Metabolic Regulation. J. Biol. Chem. 2013, 288, 26209–26219. [Google Scholar] [CrossRef] [PubMed]
- Dittenhafer-Reed, K.E.; Richards, A.L.; Fan, J.; Smallegan, M.J.; Fotuhi Siahpirani, A.; Kemmerer, Z.A.; Prolla, T.A.; Roy, S.; Coon, J.J.; Denu, J.M. SIRT3 Mediates Multi-Tissue Coupling for Metabolic Fuel Switching. Cell Metab. 2015, 21, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Fann, D.Y.-W.; Santro, T.; Manzanero, S.; Widiapradja, A.; Cheng, Y.-L.; Lee, S.-Y.; Chunduri, P.; Jo, D.-G.; Stranahan, A.M.; Mattson, M.P.; et al. Intermittent Fasting Attenuates Inflammasome Activity in Ischemic Stroke. Exp. Neurol. 2014, 257, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Mattson, M.P. Dietary Restriction and 2-Deoxyglucose Administration Improve Behavioral Outcome and Reduce Degeneration of Dopaminergic Neurons in Models of Parkinson’s Disease. J. Neurosci. Res. 1999, 57, 195–206. [Google Scholar] [CrossRef]
- Mattson, M.P. Energy Intake and Exercise as Determinants of Brain Health and Vulnerability to Injury and Disease. Cell Metab. 2012, 16, 706–722. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, A.; Li, Y.-J.; Yang, Y.; Kishimoto, Y.; Zhang, S.; Wang, Y.; Wan, R.; Raefsky, S.M.; Lu, D.; et al. SIRT3 Mediates Hippocampal Synaptic Adaptations to Intermittent Fasting and Ameliorates Deficits in APP Mutant Mice. Nat. Commun. 2019, 10, 1886. [Google Scholar] [CrossRef]
- Forman, H.J. Redox Signaling: An Evolution from Free Radicals to Aging. Free Radic. Biol. Med. 2016, 97, 398–407. [Google Scholar] [CrossRef]
- Mattson, M.P.; Gleichmann, M.; Cheng, A. Mitochondria in Neuroplasticity and Neurological Disorders. Neuron 2008, 60, 748–766. [Google Scholar] [CrossRef] [PubMed]
- Kuchibhotla, K.V.; Goldman, S.T.; Lattarulo, C.R.; Wu, H.-Y.; Hyman, B.T.; Bacskai, B.J. Abeta Plaques Lead to Aberrant Regulation of Calcium Homeostasis in Vivo Resulting in Structural and Functional Disruption of Neuronal Networks. Neuron 2008, 59, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.J.; Mucke, L. Epilepsy and Cognitive Impairments in Alzheimer Disease. Arch. Neurol. 2009, 66, 435–440. [Google Scholar] [CrossRef]
- Minkeviciene, R.; Rheims, S.; Dobszay, M.B.; Zilberter, M.; Hartikainen, J.; Fülöp, L.; Penke, B.; Zilberter, Y.; Harkany, T.; Pitkänen, A.; et al. Amyloid Beta-Induced Neuronal Hyperexcitability Triggers Progressive Epilepsy. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 3453–3462. [Google Scholar] [CrossRef] [PubMed]
- Andrews-Zwilling, Y.; Bien-Ly, N.; Xu, Q.; Li, G.; Bernardo, A.; Yoon, S.Y.; Zwilling, D.; Yan, T.X.; Chen, L.; Huang, Y. Apolipoprotein E4 Causes Age- and Tau-Dependent Impairment of GABAergic Interneurons, Leading to Learning and Memory Deficits in Mice. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 13707–13717. [Google Scholar] [CrossRef]
- Villette, V.; Dutar, P. GABAergic Microcircuits in Alzheimer’s Disease Models. Curr. Alzheimer Res. 2017, 14, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.-M.; Adhihetty, P.J.; Buford, T.W.; Wohlgemuth, S.E.; Lees, H.A.; Nguyen, L.M.-D.; Aranda, J.M.; Sandesara, B.D.; Pahor, M.; Manini, T.M.; et al. The Impact of Aging on Mitochondrial Function and Biogenesis Pathways in Skeletal Muscle of Sedentary High- and Low-Functioning Elderly Individuals. Aging Cell 2012, 11, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.A.; Larkin, L.M.; Claflin, D.R.; Brooks, S.V. Age-Related Changes in the Structure and Function of Skeletal Muscles. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1091–1096. [Google Scholar] [CrossRef]
- Buso, A.; Comelli, M.; Picco, R.; Isola, M.; Magnesa, B.; Pišot, R.; Rittweger, J.; Salvadego, D.; Šimunič, B.; Grassi, B.; et al. Mitochondrial Adaptations in Elderly and Young Men Skeletal Muscle Following 2 Weeks of Bed Rest and Rehabilitation. Front. Physiol. 2019, 10, 474. [Google Scholar] [CrossRef]
- Koltai, E.; Bori, Z.; Osvath, P.; Ihasz, F.; Peter, S.; Toth, G.; Degens, H.; Rittweger, J.; Boldogh, I.; Radak, Z. Master Athletes Have Higher MiR-7, SIRT3 and SOD2 Expression in Skeletal Muscle than Age-Matched Sedentary Controls. Redox Biol. 2018, 19, 46–51. [Google Scholar] [CrossRef]
- Joseph, A.-M.; Adhihetty, P.J.; Leeuwenburgh, C. Beneficial Effects of Exercise on Age-Related Mitochondrial Dysfunction and Oxidative Stress in Skeletal Muscle. J. Physiol. 2016, 594, 5105–5123. [Google Scholar] [CrossRef]
- Corpas, R.; Solana, E.; De la Rosa, A.; Sarroca, S.; Griñán-Ferré, C.; Oriol, M.; Corbella, E.; Rodríguez-Farré, E.; Vina, J.; Pallàs, M.; et al. Peripheral Maintenance of the Axis SIRT1-SIRT3 at Youth Level May Contribute to Brain Resilience in Middle-Aged Amateur Rugby Players. Front. Aging Neurosci. 2019, 11, 352. [Google Scholar] [CrossRef]
- Hou, L.; Chen, W.; Liu, X.; Qiao, D.; Zhou, F.-M. Exercise-Induced Neuroprotection of the Nigrostriatal Dopamine System in Parkinson’s Disease. Front. Aging Neurosci. 2017, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Mahoney, J.R.; Allali, G.; Verghese, J. Physical Activity in Older Adults With Mild Parkinsonian Signs: A Cohort Study. J. Gerontol. A. Biol. Sci. Med. Sci. 2018, 73, 1682–1687. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.; Corrêa, C.L.; Lopez-Lopez, A.; Costa-Besada, M.A.; Diaz-Ruiz, C.; Labandeira-Garcia, J.L. Physical Exercise Improves Aging-Related Changes in Angiotensin, IGF-1, SIRT1, SIRT3, and VEGF in the Substantia Nigra. J. Gerontol. Ser. A 2018, 73, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.X.; Maalouf, M.; Han, P.; Zhao, M.; Gao, M.; Dharshaun, T.; Ryan, C.; Whitelegge, J.; Wu, J.; Eisenberg, D.; et al. Ketones Block Amyloid Entry and Improve Cognition in an Alzheimer’s Model. Neurobiol. Aging 2016, 39, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Muhammad, S.; Khan, M.A.; Chen, H.; Ridder, D.A.; Müller-Fielitz, H.; Pokorná, B.; Vollbrandt, T.; Stölting, I.; Nadrowitz, R.; et al. The β-Hydroxybutyrate Receptor HCA2 Activates a Neuroprotective Subset of Macrophages. Nat. Commun. 2014, 5, 3944. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, N.; Pinton, F.; Bahi-Buisson, N.; Dulac, O.; Chiron, C.; Nabbout, R. The Ketogenic Diet Improves Recently Worsened Focal Epilepsy. Dev. Med. Child Neurol. 2009, 51, 276–281. [Google Scholar] [CrossRef]
- Van der Auwera, I.; Wera, S.; Van Leuven, F.; Henderson, S.T. A Ketogenic Diet Reduces Amyloid Beta 40 and 42 in a Mouse Model of Alzheimer’s Disease. Nutr. Metab. 2005, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Vanitallie, T.B.; Nonas, C.; Di Rocco, A.; Boyar, K.; Hyams, K.; Heymsfield, S.B. Treatment of Parkinson Disease with Diet-Induced Hyperketonemia: A Feasibility Study. Neurology 2005, 64, 728–730. [Google Scholar] [CrossRef]
- Roberts, M.N.; Wallace, M.A.; Tomilov, A.A.; Zhou, Z.; Marcotte, G.R.; Tran, D.; Perez, G.; Gutierrez-Casado, E.; Koike, S.; Knotts, T.A.; et al. A Ketogenic Diet Extends Longevity and Healthspan in Adult Mice. Cell Metab. 2017, 26, 539–546.e5. [Google Scholar] [CrossRef] [PubMed]
- Bough, K.J.; Wetherington, J.; Hassel, B.; Pare, J.F.; Gawryluk, J.W.; Greene, J.G.; Shaw, R.; Smith, Y.; Geiger, J.D.; Dingledine, R.J. Mitochondrial Biogenesis in the Anticonvulsant Mechanism of the Ketogenic Diet. Ann. Neurol. 2006, 60, 223–235. [Google Scholar] [CrossRef]
- Hughes, S.D.; Kanabus, M.; Anderson, G.; Hargreaves, I.P.; Rutherford, T.; O’Donnell, M.; Cross, J.H.; Rahman, S.; Eaton, S.; Heales, S.J.R. The Ketogenic Diet Component Decanoic Acid Increases Mitochondrial Citrate Synthase and Complex I Activity in Neuronal Cells. J. Neurochem. 2014, 129, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Nylen, K.; Velazquez, J.L.P.; Sayed, V.; Gibson, K.M.; Burnham, W.M.; Snead, O.C. The Effects of a Ketogenic Diet on ATP Concentrations and the Number of Hippocampal Mitochondria in Aldh5a1(-/-) Mice. Biochim. Biophys. Acta 2009, 1790, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.M.; Rogawski, M.A. The Ketogenic Diet: Stoking the Powerhouse of the Cell. Epilepsy Curr. 2007, 7, 58–60. [Google Scholar] [CrossRef]
- Hasan-Olive, M.M.; Lauritzen, K.H.; Ali, M.; Rasmussen, L.J.; Storm-Mathisen, J.; Bergersen, L.H. A Ketogenic Diet Improves Mitochondrial Biogenesis and Bioenergetics via the PGC1α-SIRT3-UCP2 Axis. Neurochem. Res. 2019, 44, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaya, Y.; Bergman, C.; Lee, J.-H.; Wan, R.; King, M.T.; Mughal, M.R.; Okun, E.; Clarke, K.; Mattson, M.P.; Veech, R.L. A Ketone Ester Diet Exhibits Anxiolytic and Cognition-Sparing Properties, and Lessens Amyloid and Tau Pathologies in a Mouse Model of Alzheimer’s Disease. Neurobiol. Aging 2013, 34, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.K.; Sullivan, D.K.; Mahnken, J.D.; Burns, J.M.; Swerdlow, R.H. Feasibility and Efficacy Data from a Ketogenic Diet Intervention in Alzheimer’s Disease. Alzheimers Dement. 2018, 4, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Pitta, M.; Jiang, H.; Lee, J.-H.; Zhang, G.; Chen, X.; Kawamoto, E.M.; Mattson, M.P. Nicotinamide Forestalls Pathology and Cognitive Decline in Alzheimer Mice: Evidence for Improved Neuronal Bioenergetics and Autophagy Procession. Neurobiol. Aging 2013, 34, 1564–1580. [Google Scholar] [CrossRef] [PubMed]
- Klimova, N.; Long, A.; Kristian, T. Nicotinamide Mononucleotide Alters Mitochondrial Dynamics by SIRT3-Dependent Mechanism in Male Mice. J. Neurosci. Res. 2019, 97, 975–990. [Google Scholar] [CrossRef] [PubMed]

| Experimental Model | Intervention | The Effect of Intervention | Reference |
|---|---|---|---|
| Wild type mice | Angiotensin II induced hypertensive renal nephropathy | Decreased kidney SIRT3 expression; HT; Decreased renal function; | [120] |
| SIRT3 KO mice | Angiotensin II induced hypertensive renal nephropathy | Hypertensive nephropathy, renal fibrosis; | |
| Wild type mice | Angiotensin II induced hypertensive renal nephropathy + Honokiol (bioactive compound from Magnolia officinalis) | ↑ Renal function; ↑ SIRT3 expression; ↓ Kidney fibrosis; Activates SIRT3-KLF15 signaling pathway. | |
| Male newborn rats | Short-term sucrose ingestion in the early post-natal period | ↑ Blood pressure; ↓ Aortic SIRT3, SOD2 and endothelial NO synthase expression; ↑ Energy supply, ↓NADH to NAD+ oxidation; ↓SIRT3 activity. | [121] |
| Spontaneously hypertensive rats | α-Linolenic acid | ↓ HT; ↑ Endothelial function; Prevents HT-induced SIRT3 reduction and SOD2 hyperacetylation; ↓ Endothelial mtROS; Improvements were SIRT3-dependent. | [122] |
| Human umbilical vein endothelial cells | Resveratrol | ↓ Oxidative damage, mtROS, apoptosis; ↑ Cell viability; Activates AMPK-PGC-1α signaling, ↑ SIRT3 transcription, ↑ deacetylation+activation of mtROS clearing enzymes, ↑ complex 1 activity and ATP synthesis. | [123] |
| Experimental Model | Intervention | The Effect of Intervention | Reference |
|---|---|---|---|
| Adult male CD-1 mice with induced cardiac fibrosis | Honokiol | Protects against the appearance and progression of cardiac hypertrophy by activating mitochondrial SIRT3; Honokiol directly binds to SIRT3 and increases its enzymatic activity, affinity for NAD+ and gene expression. | [124] |
| Male Sprague Dawley rats; Transverse aortic constriction (TAC)-induced hypertrophy | Choline | Decreases cardiac hypertrophy and fibroses by activating SIRT3-AMPK-UPRmt signaling; Improves metabolic function; Increases serum beta-hydroxy butyrate and acetylcholine levels; Increases cardiac levels of enzymes required to metabolize ketone bodies and fatty acids that decrease cardiac hypertrophy. | [125] |
| TAC mice models | Sesamin | Decreases cardiac hypertrophy, fibrosis and inflammation; Improves cardiac function; Sesamin-induced reduction in hypertrophy is dependent on SIRT3, which decreases ROS; Increases SIRT3 and SOD2 expression and decreases FOXO3a phosphorylation. | [126] |
| In vitro neonatal rat cardiomyocytes hypertrophy model induced by ANGII | Hydrogen sulfide | ↑ SIRT3 promoter activity and expression; ↓ Hypertrophy, ↑ mt function, ↑ SOD2 and FOXO3a expression, ↓ oxidative stress; All hydrogen sulfide-induced changes were SIRT3-dependent. | [127] |
| Mice with TAC induced hypertrophy | ↓ Cardiac hypertrophy, ↓ ROS, ↓ Blood pressure; Restores myocardial mitochondrial structure, number and volume; ↑ OPA1, MFN1, MFN2 (mitochondrial fusion genes that increase respiratory chain efficiency) and pro PGC-1α, all the modifications being SIRT3 dependent. | [127] | |
| Mice with TAC induced hypertrophy | Dihydromyricetin | ↓ Hypertrophy, ↓ ROS, ↑ expression and activity of SIRT3, FOXO3a, SOD2; Activates AMPK-PGC1alpha-ERRalpha axis, which increases SIRT3 expression and leads to mtSOD2 deacetylation and decreased oxidative damage. | [128] [129] |
| Resveratrol | ↓ Cardiac hypertrophy and collagen deposition, ↑ Cardiac function, all in an SIRT3-dependent manner; In vitro, prevents fibroblast-myoblast differentiation by inhibiting TGFbeta-Smad3 signaling. | [130] | |
| NAD+ | ↓ Hypertrophy in a SIRT3-dependent manner by activating SIRT3-LKB1-AMPK signaling and culminates with decreased mTOR activity and decreased hypertrophy; Pathologic cardiac hypertrophy decreases Nampt and NAD+ levels (but not in exercise-induced hypertrophy). | [131] | |
| Emodin | ↑ PGC-1α-SIRT3 signaling. | [132] | |
| Angiotensin II induced hypertrophy in cardiomyoblast H9c2 cells | 1,25-OH vitamin D3 | ↓ Hypertrophy in a SIRT3 independent manner; SIRT3 expression was unaffected by the intervention. | [133] |
| Experimental Model | Intervention | The Effect of Intervention | Reference |
|---|---|---|---|
| In vitro: amyloid β oligomer-treated primary hippocampal neuronal cells In vivo: transgenic PS1V97L mouse model | Honokiol | Ameliorates mitochondrial dysfunction by activating SIRT3 and increasing its levels, which results in suppressed ROS, an increased ATP production, normalized mitochondrial membrane potential, delayed cognitive impairment; Decreases amyloid-β-induced hippocampal neuron apoptosis and improves cognitive performance. | [194] [195] [196] |
| DNA repair deficiency mouse (3xTgAD/Polβ+/−) | Nicotinamide riboside | Improves memory, learning and motor function; Decreases systemic inflammation, phosphorylated tau, DNA damage and apoptosis; Restores SIRT3 and SIRT6 levels; Restores synaptic plasticity in the hippocampus; Increases deacetylated SOD2 and increases neurogenesis; No effect on amyloid-β production. | [197] |
| In vitro and in vivo gentamicin-induced hair cell loss model | Adjudin | Protects against gentamicin-induced hair cell loss in rats’ cochleae by increasing SIRT3 mRNA and protein levels expression and decreasing ROS. | [198] |
| Noise-induced hearing loss mouse models | Nicotinamide riboside | Protects against degeneration of spiral ganglion neurites and noise-induced hearing loss in a SIRT3-dependent manner; Increases mitochondrial NAD+ and SIRT3 activity. | [199] |
| Four-week-old Sprague Dawley rats | d-Galactose-induced aging | Decreases SIRT3 expression, mtDNA lesions and SOD2 activity; Increases malondialdehyde and apoptosis levels in rats’ auditory cortices in natural and D-galactose-induced aging. | [200] |
| Peripheral lymphocytes from patients with AD | Resveratrol | AD patient lymphocytes show increased oxidative stress. Selenium administration did not modify the expression of SIRT3 or other longevity-related genes, but resveratrol upregulated SIRT3, SIRT1, SOD2 and NRF2 (a transcription factor that activates antioxidant response genes), that could be responsible for SIRT3 upregulation after resveratrol administration and provide protective effects in AD afflicted cells. | [201] [202] [203] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silaghi, C.N.; Farcaș, M.; Crăciun, A.M. Sirtuin 3 (SIRT3) Pathways in Age-Related Cardiovascular and Neurodegenerative Diseases. Biomedicines 2021, 9, 1574. https://doi.org/10.3390/biomedicines9111574
Silaghi CN, Farcaș M, Crăciun AM. Sirtuin 3 (SIRT3) Pathways in Age-Related Cardiovascular and Neurodegenerative Diseases. Biomedicines. 2021; 9(11):1574. https://doi.org/10.3390/biomedicines9111574
Chicago/Turabian StyleSilaghi, Ciprian N., Marius Farcaș, and Alexandra M. Crăciun. 2021. "Sirtuin 3 (SIRT3) Pathways in Age-Related Cardiovascular and Neurodegenerative Diseases" Biomedicines 9, no. 11: 1574. https://doi.org/10.3390/biomedicines9111574
APA StyleSilaghi, C. N., Farcaș, M., & Crăciun, A. M. (2021). Sirtuin 3 (SIRT3) Pathways in Age-Related Cardiovascular and Neurodegenerative Diseases. Biomedicines, 9(11), 1574. https://doi.org/10.3390/biomedicines9111574






