The Role of Perivascular Adipose Tissue in Early Changes in Arterial Function during High-Fat Diet and Its Combination with High-Fructose Intake in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Functional Studies on Isolated Arteries
2.3. Data Analysis
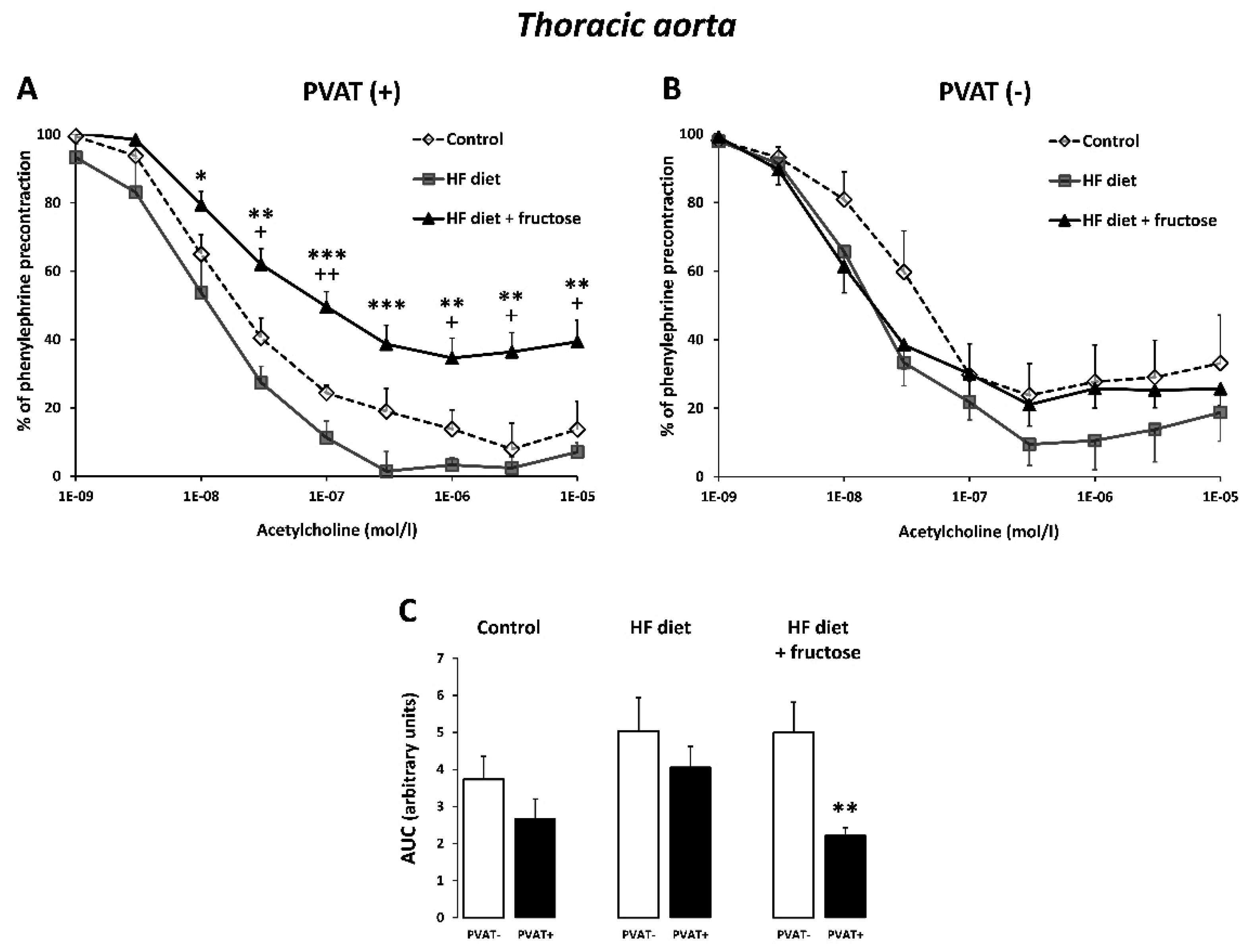
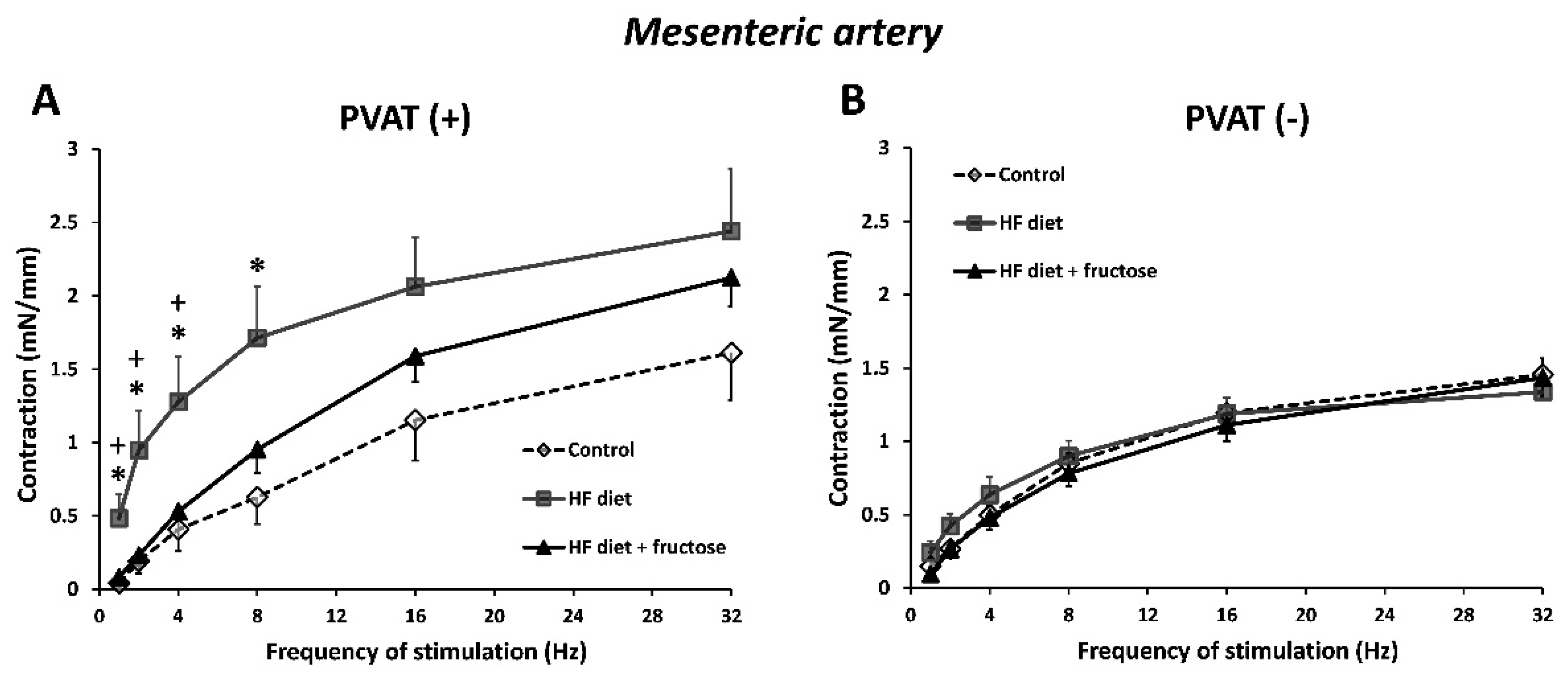
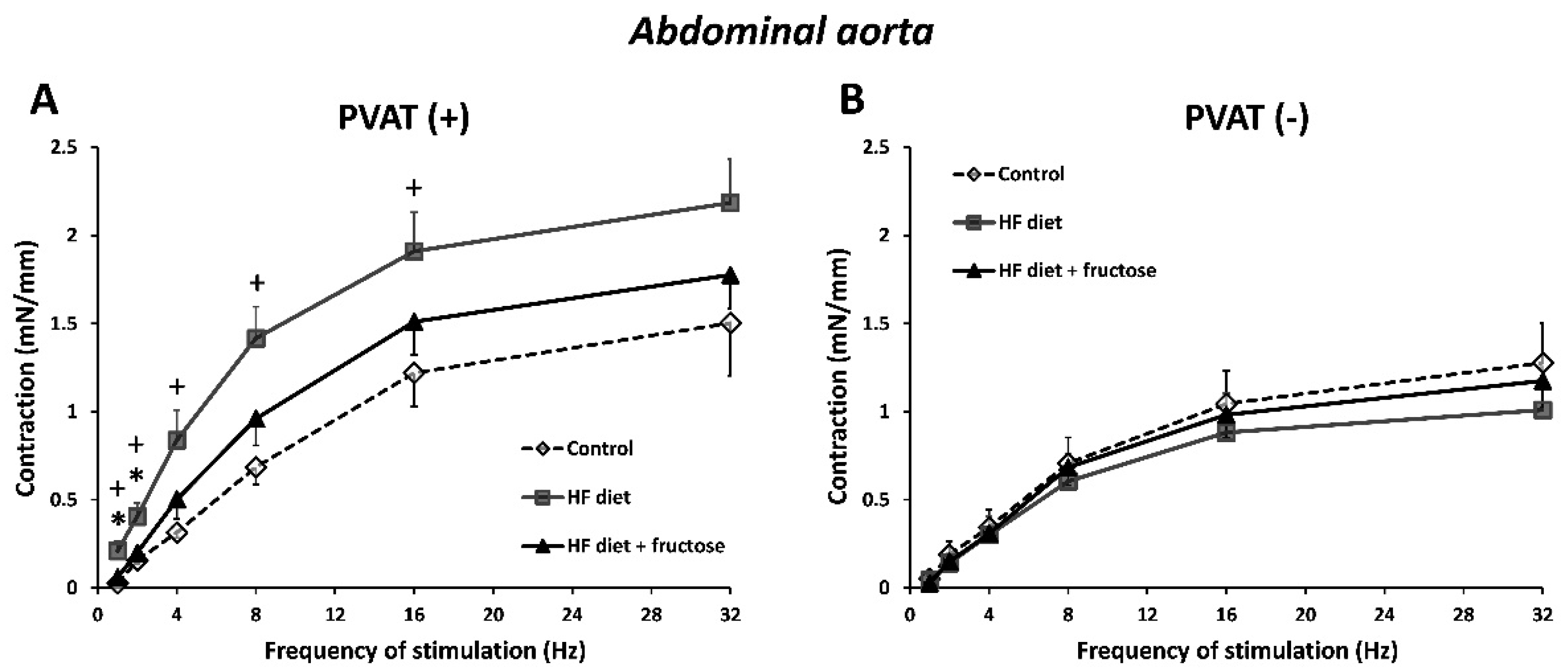
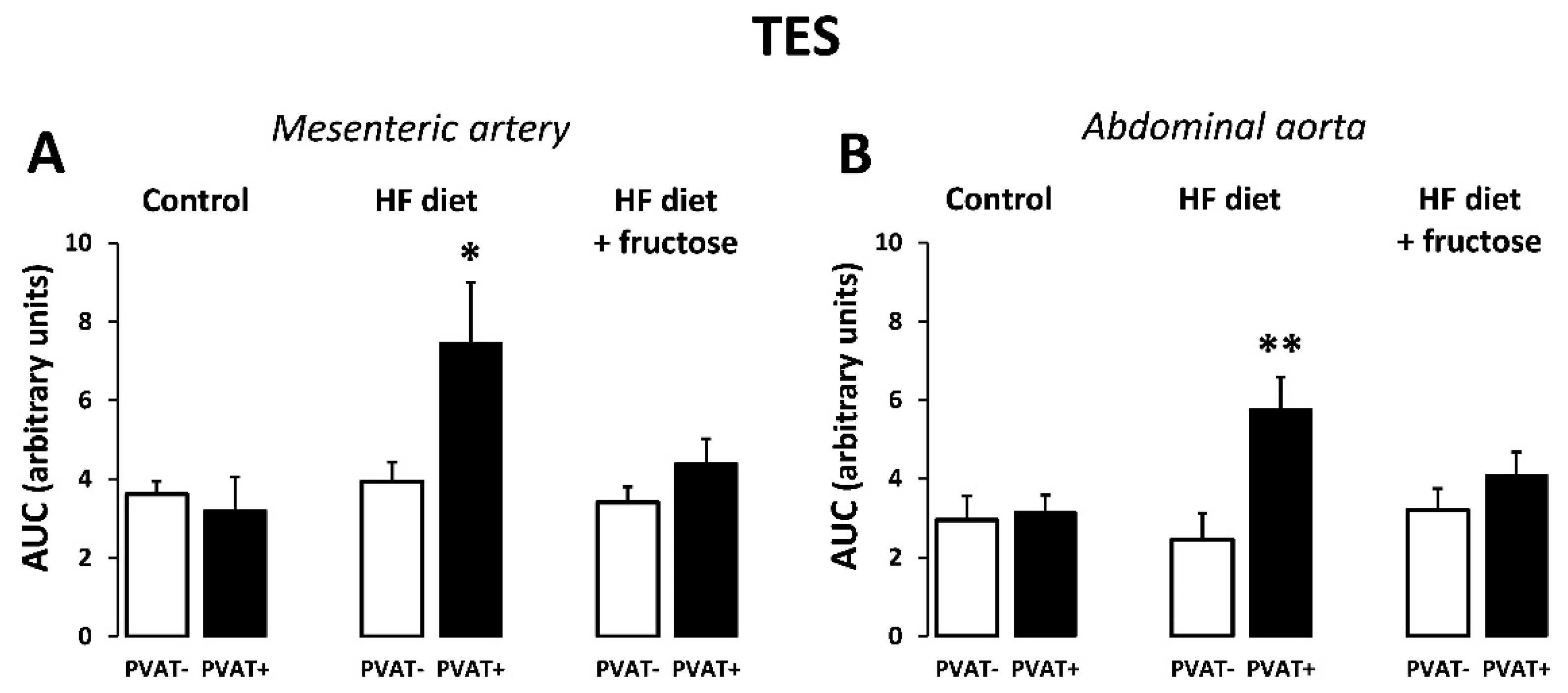
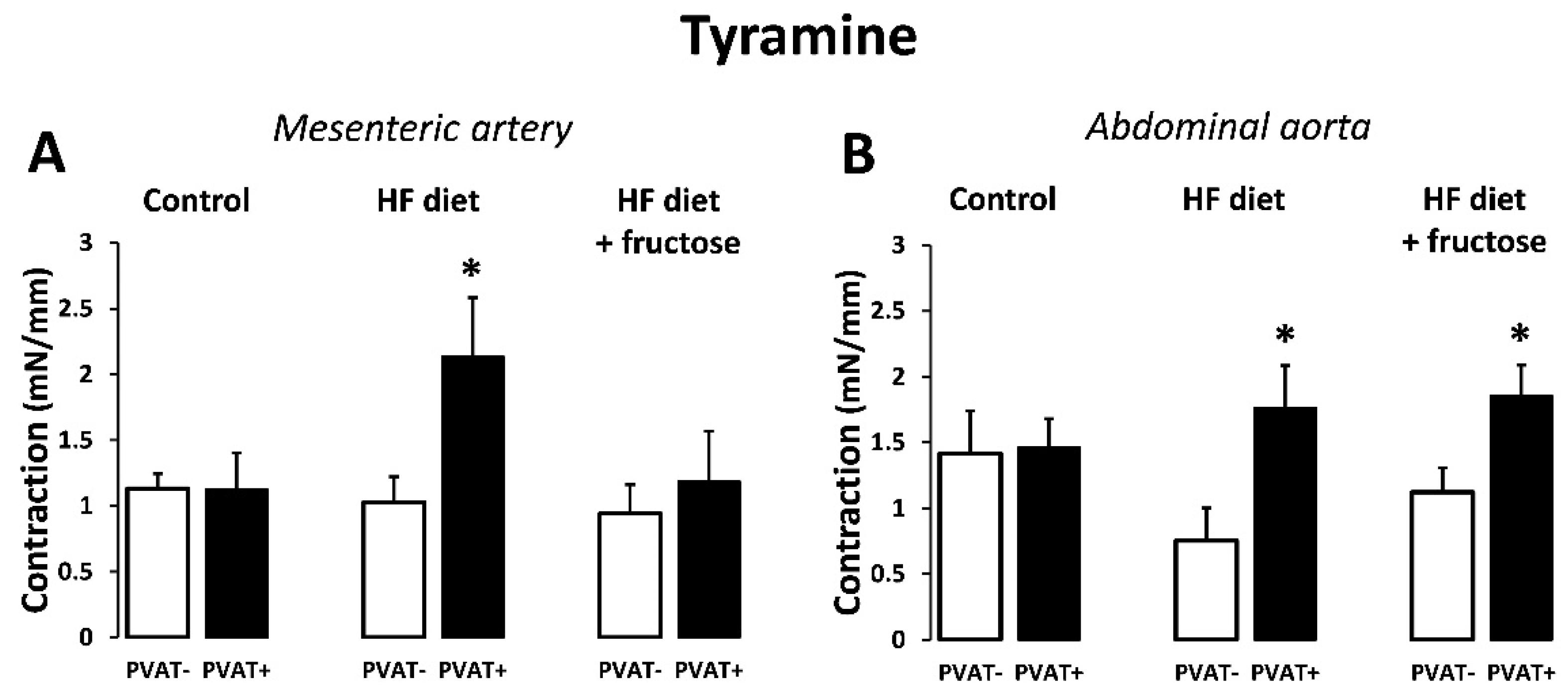
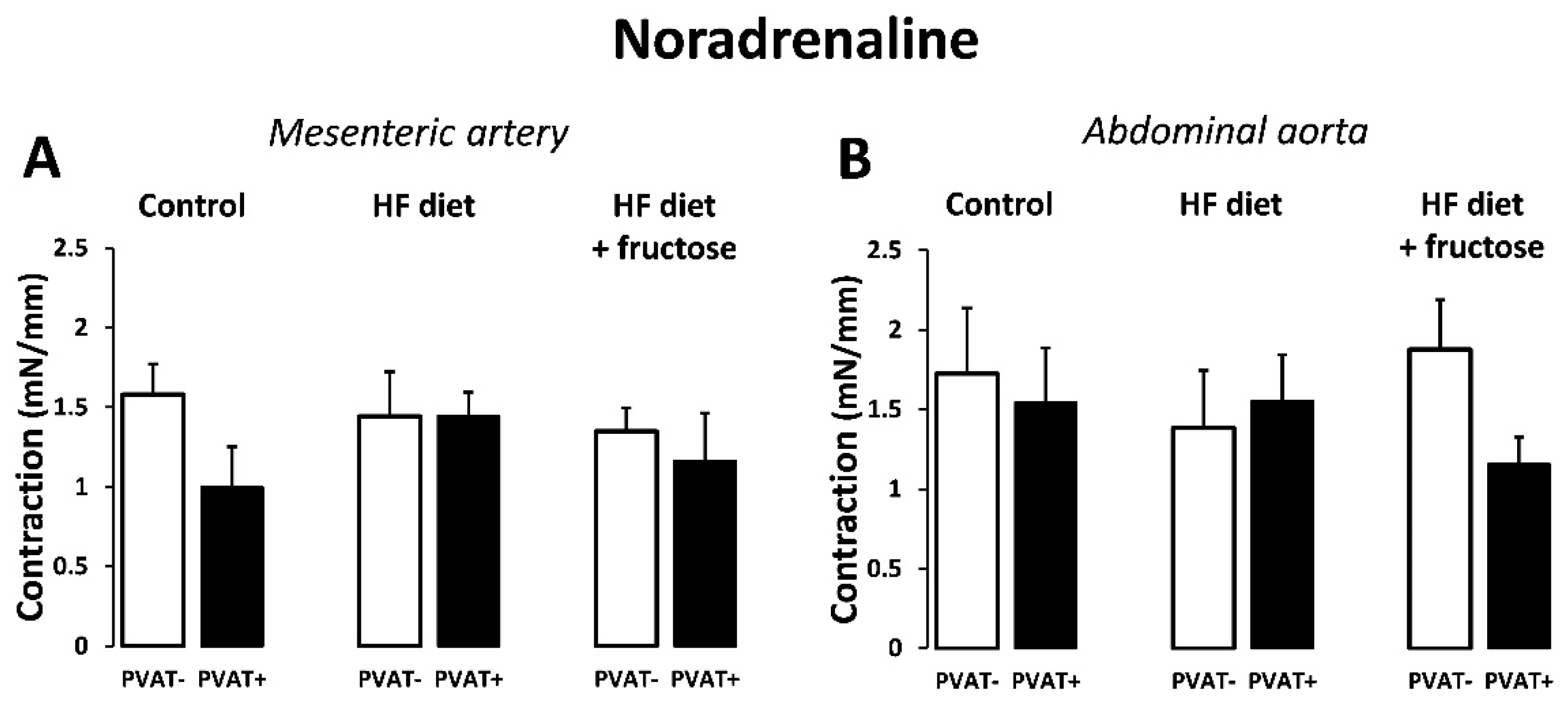
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gami, A.S.; Witt, B.J.; Howard, D.E.; Erwin, P.J.; Gami, L.A.; Somers, V.K.; Montori, V.M. Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J. Am. Coll Cardiol. 2007, 49, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Hess, P.L.; Al-Khalidi, H.R.; Friedman, D.J.; Mulder, H.; Kucharska-Newton, A.; Rosamond, W.R.; Lopes, R.D.; Gersh, B.J.; Mark, D.B.; Curtis, L.H.; et al. The Metabolic Syndrome and Risk of Sudden Cardiac Death: The Atherosclerosis Risk in Communities Study. J. Am. Heart Assoc. 2017, 6, e006103. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Height and body-mass index trajectories of school-aged children and adolescents from 1985 to 2019 in 200 countries and territories: A pooled analysis of 2181 population-based studies with 65 million participants. Lancet 2020, 396, 1511–1524. [Google Scholar] [CrossRef]
- Pinhas-Hamiel, O.; Zeitler, P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet 2007, 369, 1823–1831. [Google Scholar] [CrossRef]
- Gardner, M.; Gardner, D.W.; Sowers, J.R. The cardiometabolic syndrome in the adolescent. Pediatr. Endocrinol. Rev. 2008, 5 (Suppl. S4), 964–968. [Google Scholar]
- Panchal, S.K.; Brown, L. Rodent models for metabolic syndrome research. J. Biomed. Biotechnol. 2011, 2011, 351982. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Fairus, A.; Ima-Nirwana, S. Animal models of metabolic syndrome: A review. Nutr. Metab. 2016, 13, 65. [Google Scholar] [CrossRef]
- Dekker, M.J.; Su, Q.; Baker, C.; Rutledge, A.C.; Adeli, K. Fructose: A highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E685–E694. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Lê, K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef]
- Tran, L.T.; Yuen, V.G.; McNeill, J.H. The fructose-fed rat: A review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol. Cell Biochem. 2009, 332, 145–159. [Google Scholar] [CrossRef]
- Klein, A.V.; Kiat, H. The mechanisms underlying fructose-induced hypertension: A review. J. Hypertens. 2015, 33, 912–920. [Google Scholar] [CrossRef] [PubMed]
- De Moura, R.F.; Ribeiro, C.; de Oliveira, J.A.; Stevanato, E.; de Mello, M.A. Metabolic syndrome signs in Wistar rats submitted to different high-fructose ingestion protocols. Br. J. Nutr. 2009, 101, 1178–1184. [Google Scholar] [CrossRef]
- Toop, C.R.; Gentili, S. Fructose Beverage Consumption Induces a Metabolic Syndrome Phenotype in the Rat: A Systematic Review and Meta-Analysis. Nutrients 2016, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Zemančíková, A.; Török, J. Effect of perivascular adipose tissue on arterial adrenergic contractions in normotensive and hypertensive rats with high fructose intake. Physiol. Res. 2017, 66, S537–S544. [Google Scholar] [CrossRef]
- Levanovich, P.E.; Chung, C.S.; Komnenov, D.; Rossi, N.F. Fructose plus High-Salt Diet in Early Life Results in Salt-Sensitive Cardiovascular Changes in Mature Male Sprague Dawley Rats. Nutrients 2021, 13, 3129. [Google Scholar] [CrossRef]
- Ma, L.; Ma, S.; He, H.; Yang, D.; Chen, X.; Luo, Z.; Liu, D.; Zhu, Z. Perivascular fat-mediated vascular dysfunction and remodeling through the AMPK/mTOR pathway in high-fat diet-induced obese rats. Hypertens. Res. 2010, 33, 446–453. [Google Scholar] [CrossRef]
- Xia, N.; Li, H. The role of perivascular adipose tissue in obesity-induced vascular dysfunction. Br. J. Pharmacol. 2017, 174, 3425–3442. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Yin, H.; Huang, J.; Hu, M. Dysfunction of perivascular adipose tissue in mesenteric artery is restored by aerobic exercise in high-fat diet induced obesity. Clin. Exp. Pharmacol. Physiol. 2021, 48, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Löhn, M.; Dubrovska, G.; Lauterbach, B.; Luft, F.C.; Gollasch, M.; Sharma, A.M. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002, 16, 1057–1063. [Google Scholar] [CrossRef]
- Gollasch, M. Vasodilator signals from perivascular adipose tissue. Br. J. Pharmacol. 2012, 165, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Szasz, T.; Webb, R.C. Perivascular adipose tissue: More than just structural support. Clin. Sci. 2012, 122, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, A.S.; Khavandi, K.; Withers, S.B.; Sonoyama, K.; Clancy, O.; Jeziorska, M.; Laing, I.; Yates, A.P.; Pemberton, P.W.; Malik, R.A.; et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 2009, 119, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.K.; Stoll, L.L.; Denning, G.M.; Harrelson, A.; Blomkalns, A.L.; Idelman, G.; Rothenberg, F.G.; Neltner, B.; Romig-Martin, S.A.; Dickson, E.W.; et al. Proinflammatory phenotype of perivascular adipocytes: Influence of high-fat feeding. Circ Res. 2009, 104, 541–549. [Google Scholar] [CrossRef]
- Ketonen, J.; Shi, J.; Martonen, E.; Mervaala, E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circ J. 2010, 74, 1479–1487. [Google Scholar] [CrossRef]
- Aghamohammadzadeh, R.; Unwin, R.D.; Greenstein, A.S.; Heagerty, A.M. Effects of Obesity on Perivascular Adipose Tissue Vasorelaxant Function: Nitric Oxide, Inflammation and Elevated Systemic Blood Pressure. J. Vasc. Res. 2015, 52, 299–305. [Google Scholar] [CrossRef]
- Litterio, M.C.; Vazquez Prieto, M.A.; Adamo, A.M.; Elesgaray, R.; Oteiza, P.I.; Galleano, M.; Fraga, C.G. (−)-Epicatechin reduces blood pressure increase in high-fructose-fed rats: Effects on the determinants of nitric oxide bioavailability. J. Nutr. Biochem. 2015, 26, 745–751. [Google Scholar] [CrossRef]
- Sepehri, H.; Hojati, A.; Safari, R. Effect of Bitter Melon on Spatial Memory of Rats Receiving a High-Fat Diet. J. Exp. Pharmacol. 2019, 11, 115–119. [Google Scholar] [CrossRef][Green Version]
- Cartland, S.P.; Tamer, N.; Patil, M.S.; Di Bartolo, B.A.; Kavurma, M.M. A “Western Diet” promotes symptoms of hepatic steatosis in spontaneously hypertensive rats. Int. J. Exp. Pathol. 2020, 101, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.Q.; Leray, V.; Le Bloc’h, J.; Thorin, C.; Ouguerram, K.; Nguyen, P. Lipid profile and insulin sensitivity in rats fed with high-fat or high-fructose diets. Br. J. Nutr. 2011, 106 (Suppl. S1), S206–S210. [Google Scholar] [CrossRef]
- Zicha, J.; Kunes, J. Ontogenetic aspects of hypertension development: Analysis in the rat. Physiol. Rev. 1999, 79, 1227–1282. [Google Scholar] [CrossRef]
- Vaněčková, I.; Maletínská, L.; Behuliak, M.; Nagelová, V.; Zicha, J.; Kuneš, J. Obesity-related hypertension: Possible pathophysiological mechanisms. J. Endocrinol. 2014, 223, R63–R78. [Google Scholar] [CrossRef] [PubMed]
- Lozano, I.; Van der Werf, R.; Bietiger, W.; Seyfritz, E.; Peronet, C.; Pinget, M.; Jeandidier, N.; Maillard, E.; Marchioni, E.; Sigrist, S.; et al. High-fructose and high-fat diet-induced disorders in rats: Impact on diabetes risk, hepatic and vascular complications. Nutr. Metab. 2016, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Zemančíková, A.; Török, J. Comparison of high fructose-induced cardiometabolic impairments in two different rat hypertensive models. Curr. Top. Toxicol. 2016, 12, 25–32. [Google Scholar]
- Huang, B.W.; Chiang, M.T.; Yao, H.T.; Chiang, W. The effect of high-fat and high-fructose diets on glucose tolerance and plasma lipid and leptin levels in rats. Diabetes Obes. Metab. 2004, 6, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Handjieva-Darlenska, T.; Boyadjieva, N. The effect of high-fat diet on plasma ghrelin and leptin levels in rats. J. Physiol. Biochem. 2009, 65, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Simonds, S.E.; Cowley, M.A.; Enriori, P.J. Leptin increasing sympathetic nerve outflow in obesity: A cure for obesity or a potential contributor to metabolic syndrome? Adipocyte 2012, 1, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.B.; Rahmouni, K. Leptin as a Mediator of Obesity-Induced Hypertension. Curr. Obes. Rep. 2016, 5, 397–404. [Google Scholar] [CrossRef]
- Lu, S.C.; Akanji, A.O. Leptin, Obesity, and Hypertension: A Review of Pathogenetic Mechanisms. Metab. Syndr. Relat. Disord. 2020, 18, 399–405. [Google Scholar] [CrossRef]
- Zemancikova, A.; Torok, J.; Balis, P.; Valovic, P.; Ulicna, O.; Chomova, M. Modulation of sympathoadrenergic contractions by perivascular adipose tissue in mesenteric arteries of rats with different level of body adiposity. J. Physiol. Pharmacol. 2020, 71, 589–596. [Google Scholar] [CrossRef]
- Ferenczyova, K.; Kalocayova, B.; Kindernay, L.; Jelemensky, M.; Balis, P.; Berenyiova, A.; Zemancikova, A.; Farkasova, V.; Sykora, M.; Tothova, L.; et al. Quercetin Exerts Age-Dependent Beneficial Effects on Blood Pressure and Vascular Function, But Is Inefficient in Preventing Myocardial Ischemia-Reperfusion Injury in Zucker Diabetic Fatty Rats. Molecules 2020, 25, 187. [Google Scholar] [CrossRef]
- Jiang, J.; Tran, L.; Vasudevan, H.; Xia, Z.; Yuen, V.G.; McNeill, J.H. Endothelin-1 blockade prevents COX2 induction and TXA2 production in the fructose hypertensive rat. Can. J. Physiol. Pharmacol. 2007, 85, 422–429. [Google Scholar] [CrossRef]
- Saygin, M.; Asci, H.; Cankara, F.N.; Bayram, D.; Yesilot, S.; Candan, I.A.; Alp, H.H. The impact of high fructose on cardiovascular system: Role of α-lipoic acid. Hum. Exp. Toxicol. 2016, 35, 194–204. [Google Scholar] [CrossRef]
- Berenyiova, A.; Golas, S.; Drobna, M.; Cebova, M.; Cacanyiova, S. Fructose Intake Impairs the Synergistic Vasomotor Manifestation of Nitric Oxide and Hydrogen Sulfide in Rat Aorta. Int. J. Mol. Sci. 2021, 22, 4749. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Chen, S.J.; Tsao, C.M.; Wu, C.C. Perivascular adipose tissue inhibits endothelial function of rat aortas via caveolin-1. PLoS ONE 2014, 9, e99947. [Google Scholar] [CrossRef]
- Abdulla, M.H.; Sattar, M.A.; Johns, E.J.; Abdullah, N.A.; Hye Khan, M.A.; Rathore, H.A. High-fructose feeding impacts on the adrenergic control of renal haemodynamics in the rat. Br. J. Nutr. 2012, 107, 218–228. [Google Scholar] [CrossRef]
- Török, J.; Zemančíková, A. Relaxation of rat main pulmonary artery to electrical stimulation: Role of nitric oxide. Act. Nerv. Super Rediviva. 2010, 52, 151–156. [Google Scholar]
- Abu Bakar, H.; Robert Dunn, W.; Daly, C.; Ralevic, V. Sensory innervation of perivascular adipose tissue: A crucial role in artery vasodilatation and leptin release. Cardiovasc. Res. 2017, 113, 962–972. [Google Scholar] [CrossRef]
- Aalkjær, C.; Nilsson, H.; De Mey, J.G.R. Sympathetic and Sensory-Motor Nerves in Peripheral Small Arteries. Physiol. Rev. 2021, 101, 495–544. [Google Scholar] [CrossRef] [PubMed]
- Bulloch, J.M.; Daly, C.J. Autonomic nerves and perivascular fat: Interactive mechanisms. Pharmacol. Ther. 2014, 143, 61–73. [Google Scholar] [CrossRef]
- Török, J.; Zemančíková, A.; Kocianová, Z. Interaction of perivascular adipose tissue and sympathetic nerves in arteries from normotensive and hypertensive rats. Physiol. Res. 2016, 65 (Suppl. S3), S391–S399. [Google Scholar] [CrossRef]
- Loesch, A.; Dashwood, M.R. Nerve-perivascular fat communication as a potential influence on the performance of blood vessels used as coronary artery bypass grafts. J. Cell Commun. Signal. 2018, 12, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Saxton, S.N.; Withers, S.B.; Heagerty, A.M. Emerging Roles of Sympathetic Nerves and Inflammation in Perivascular Adipose Tissue. Cardiovasc. Drugs Ther. 2019, 33, 245–259. [Google Scholar] [CrossRef] [PubMed]
| Control | HF Diet | HF Diet + Fructose | |
|---|---|---|---|
| Body weight (g) | 273.2 ± 9.1 | 301.7 ± 13.8 | 279.6 ± 12.2 |
| Systolic blood pressure (mm Hg) | 114.8 ± 2.6 | 120.1 ± 1.7 | 123.7 ± 2.7 * |
| Heart rate (bpm) | 412.3 ± 5.0 | 440.5 ± 8.1 * | 439.5 ± 8.8 * |
| Heart weight/tibia length (mg/mm) | 28.2 ± 1.2 | 30.2 ± 1.0 | 29.1 ± 0.6 |
| Kidney weight/tibia length (mg/mm) | 31.1 ± 0.6 | 31.1 ± 1.2 | 30.2 ± 0.4 |
| Liver weight/tibia length (mg/mm) | 184.8 ± 5.7 | 192.9 ± 7.4 | 202.6 ± 3.3 * |
| RP fat weight/tibia length (mg/mm) | 34.4 ± 7.6 | 91.5 ± 13.2 * | 63.6 ± 4.0 * |
| ED fat weight/tibia length (mg/mm) | 46.7 ± 4.5 | 75.1 ± 8.1 * | 54.2 ± 1.9 |
| Glucose (mmol/L) | 5.2 ± 0.2 | 5.4 ± 0.2 | 5.1 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torok, J.; Zemancikova, A.; Valaskova, Z.; Balis, P. The Role of Perivascular Adipose Tissue in Early Changes in Arterial Function during High-Fat Diet and Its Combination with High-Fructose Intake in Rats. Biomedicines 2021, 9, 1552. https://doi.org/10.3390/biomedicines9111552
Torok J, Zemancikova A, Valaskova Z, Balis P. The Role of Perivascular Adipose Tissue in Early Changes in Arterial Function during High-Fat Diet and Its Combination with High-Fructose Intake in Rats. Biomedicines. 2021; 9(11):1552. https://doi.org/10.3390/biomedicines9111552
Chicago/Turabian StyleTorok, Jozef, Anna Zemancikova, Zuzana Valaskova, and Peter Balis. 2021. "The Role of Perivascular Adipose Tissue in Early Changes in Arterial Function during High-Fat Diet and Its Combination with High-Fructose Intake in Rats" Biomedicines 9, no. 11: 1552. https://doi.org/10.3390/biomedicines9111552
APA StyleTorok, J., Zemancikova, A., Valaskova, Z., & Balis, P. (2021). The Role of Perivascular Adipose Tissue in Early Changes in Arterial Function during High-Fat Diet and Its Combination with High-Fructose Intake in Rats. Biomedicines, 9(11), 1552. https://doi.org/10.3390/biomedicines9111552






