Relevant Biological Effects of Varicocele Embolization with N-Butyl Cyanoacrylate Glue on Semen Parameters in Infertile Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Varicocele Diagnosis
- Stage 1: moderate spontaneous hyperflow, frank reflux the duration of which is less than the time of thrust but longer than two seconds;
- Stage 2: significant spontaneous hyperflow, frank reflux lasting as long as the push;
- Stage 3: strong spontaneous hyperflow, massive reflux not only at the Valsalva maneuver but also at each breath in.
2.3. Semen Analyses
- Total sperm number (TSN): ≥39 × 106/ejaculate;
- Sperm motility (SMot): progressive motility (grades A+B): >32% at 1 h (H1), total motility (grades A+B+C): >40% at 1 h (H1);
- Sperm vitality (SV): ≥58%;
- Sperm morphology (SMor): ≥23%.
2.4. Embolization Technique
2.5. Outcomes
2.6. Statistical Analyses
3. Results
3.1. Study Population
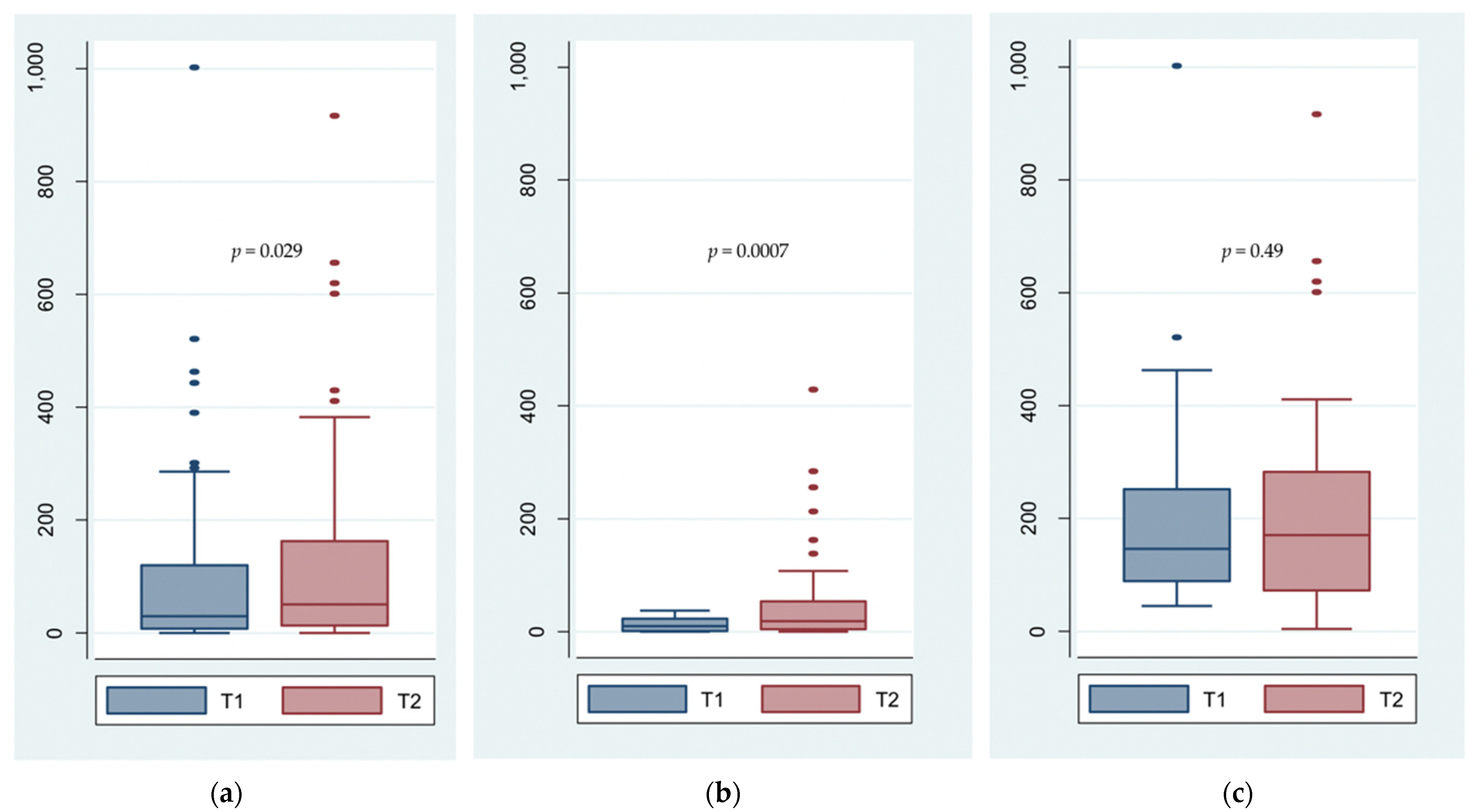
3.2. Total Sperm Number
3.3. Sperm Motility, Vitality and Morphology
3.4. Testicular Volume
3.5. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jungwirth, A.; Giwercman, A.; Tournaye, H.; Diemer, T.; Kopa, Z.; Dohle, G.; Krausz, C.; European Association of Urology Working Group on Male Infertility. European Association of Urology Guidelines on Male Infertility: The 2012 update. Eur. Urol. 2012, 62, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.; Tostain, J. Varicocele and male infertility: AFU 2006 guidelines. Prog. Urol. 2007, 17, 12–17. [Google Scholar] [CrossRef]
- Mali, W.P.; Oei, H.Y.; Arndt, J.W.; Kremer, J.; Coolsaet, B.L.; Schuur, K. Hemodynamics of the varicocele. Part II. Correlation among the results of renocaval pressure measurements, varicocele scintigraphy and phlebography. J. Urol. 1986, 135, 489–493. [Google Scholar] [CrossRef]
- Tulloch, W.S. Varicocele in subfertility: Results of treatment. 1955. J. Urol. 2001, 166, 2032–2033. [Google Scholar] [CrossRef]
- Abdel-Meguid, T.A.; Al-Sayyad, A.; Tayib, A.; Farsi, H.M. Does varicocele repair improve male infertility? An evidence-based perspective from a randomized, controlled trial. Eur. Urol. 2011, 59, 455–461. [Google Scholar] [CrossRef]
- Agarwal, A.; Deepinder, F.; Cocuzza, M.; Agarwal, R.; Short, R.A.; Sabanegh, E.; Marmar, J.L. Efficacy of varicocelectomy in improving semen parameters: New meta-analytical approach. Urology 2007, 70, 532–538. [Google Scholar] [CrossRef]
- Baazeem, A.; Belzile, E.; Ciampi, A.; Dohle, G.; Jarvi, K.; Salonia, A.; Weidner, W.; Zini, A. Varicocele and male factor infertility treatment: A new meta-analysis and review of the role of varicocele repair. Eur. Urol. 2011, 60, 796–808. [Google Scholar] [CrossRef]
- Sigmund, G.; Bähren, W.; Gall, H.; Lenz, M.; Thon, W. Idiopathic varicoceles: Feasibility of percutaneous sclerotherapy. Radiology 1987, 164, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Riedl, P.; Kumpan, W.; Maier, U.; Stackl, W.; Lunglmayr, G. Long-term results after sclerotherapy of the spermatic vein in patients with varicocele. Cardiovasc. Intervent. Radiol. 1985, 8, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Seyferth, W.; Jecht, E.; Zeitler, E. Percutaneous sclerotherapy of varicocele. Radiology 1981, 139, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Dewire, D.M.; Thomas, A.J.; Falk, R.M.; Geisinger, M.A.; Lammert, G.K. Clinical outcome and cost comparison of percutaneous embolization and surgical ligation of varicocele. J. Androl. 1994, 15 (Suppl. S6), 38S–42S. [Google Scholar]
- Jargiello, T.; Drelich-Zbroja, A.; Falkowski, A.; Sojka, M.; Pyra, K.; Szczerbo-Trojanowska, M. Endovascular transcatheter embolization of recurrent postsurgical varicocele: Anatomic reasons for surgical failure. Acta Radiol. 2015, 56, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.M.; Racadio, J.M.; McKinney, D.N.; Racadio, J.M.; Vu, D.N. Varicocele retrograde embolization with boiling contrast medium and gelatin sponges in adolescent subjects: A clinically effective therapeutic alternative. J. Vasc. Interv. Radiol. 2012, 23, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Puche-Sanz, I.; Flores-Martín, J.F.; Vázquez-Alonso, F.; Pardo-Moreno, P.L.; Cózar-Olmo, J.M. Primary treatment of painful varicocoele through percutaneous retrograde embolization with fibred coils. Andrology 2014, 2, 716–720. [Google Scholar] [CrossRef] [Green Version]
- Carmignani, L.; Casellato, S.; Galasso, G.; Bozzini, G.; Spinelli, M.; Dell’Agnola, C.A.; Rocco, F. Sclerotherapy of the pampiniform plexus with modified Marmar technique in children and adolescents. Urol. Int. 2009, 82, 187–190. [Google Scholar] [CrossRef]
- Ali, A.; Wirth, S.; Treitl, K.M.; Treitl, M. Treatment of male varicoceles by transcatheter polidocanol foam sclerotherapy: Evaluation of clinical success, complications, and patients’ satisfaction with regard to alternative techniques. Eur. Radiol. 2015, 25, 2889–2897. [Google Scholar] [CrossRef]
- Kunnen, M. New techniques for embolisation of the internal spermatic vein: Intravenous tissue adhesive (author’s transl). Rofo 1980, 133, 625–629. [Google Scholar] [CrossRef]
- Brothers, M.F.; Kaufmann, J.C.; Fox, A.J.; Deveikis, J.P. N-Butyl 2-cyanoacrylate--substitute for IBCA in interventional neuroradiology: Histopathologic and polymerization time studies. Am. J. Neuroradiol. 1989, 10, 777–786. [Google Scholar] [PubMed]
- Urbano, J.; Cabrera, M.; Alonso-Burgos, A. Sclerosis and varicocele embolization with n-butyl cyanoacrylate: Experience in 41 patients. Acta Radiol. 2014, 55, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Vanlangenhove, P.; De Keukeleire, K.; Everaert, K.; Van Maele, G.; Defreyne, L. Efficacy and safety of two different n-butyl-2-cyanoacrylates for the embolization of varicoceles: A prospective, randomized, blinded study. Cardiovasc. Intervent. Radiol. 2012, 35, 598–606. [Google Scholar] [CrossRef]
- Vanlangenhove, P.; Everaert, K.; Van Maele, G.; Defreyne, L. Tolerance of glue embolization under local anesthesia in varicoceles: A comparative study of two different cyanoacrylates. Eur. J. Radiol. 2014, 83, 559–563. [Google Scholar] [CrossRef]
- Sze, D.Y.; Kao, J.S.; Frisoli, J.K.; McCallum, S.W.; Kennedy, W.A.; Razavi, M.K. Persistent and recurrent postsurgical varicoceles: Venographic anatomy and treatment with n-butyl cyanoacrylate embolization. J. Vasc. Interv. Radiol. 2008, 19, 539–545. [Google Scholar] [CrossRef]
- Favard, N.; Moulin, M.; Fauque, P.; Bertaut, A.; Favelier, S.; Estivalet, L.; Michel, F.; Cormier, L.; Sagot, P.; Loffroy, R. Comparison of three different embolic materials for varicocele embolization: Retrospective study of tolerance, radiation and recurrence rate. Quant. Imaging Med. Surg. 2015, 5, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Loffroy, R.; Guiu, B.; Cercueil, J.-P.; Krausé, D. Endovascular therapeutic embolisation: An overview of occluding agents and their effects on embolised tissues. Curr. Vasc. Pharmacol. 2009, 7, 250–263. [Google Scholar] [CrossRef]
- Levrier, O.; Mekkaoui, C.; Rolland, P.H.; Murphy, K.; Cabrol, P.; Moulin, G.; Bartoli, J.M.; Raybaud, C. Efficacy and low vascular toxicity of embolization with radical versus anionic polymerization of n-butyl-2-cyanoacrylate (NBCA). An experimental study in the swine. J. Neuroradiol. 2003, 30, 95–102. [Google Scholar] [PubMed]
- Heye, S.; Maleux, G.; Wilms, G. Pain experience during internal spermatic vein embolization for varicocele: Comparison of two cyanoacrylate glues. Eur. Radiol. 2006, 16, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The international glossary on infertility and fertility care, 2017. Fertil. Steril. 2017, 108, 393–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubin, L.; Amelar, R.D. Reprint of: Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil. Steril. 2019, 112, e57–e60. [Google Scholar] [CrossRef]
- Battino, J.; Battino, A. Diagnosis of varicocele by Doppler effect. J. Mal. Vasc. 1989, 14, 339–342. [Google Scholar]
- Schiff, J.D.; Li, P.S.; Goldstein, M. Correlation of ultrasonographic and orchidometer measurements of testis volume in adults. BJU Int. 2004, 93, 1015–1017. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; Cambridge University Press: Cambridge, UK, 2010; Available online: https://apps.who.int/iris/handle/10665/44261 (accessed on 7 October 2021).
- Kwak, N.; Siegel, D. Imaging and interventional therapy for varicoceles. Curr. Urol. Rep. 2014, 15, 399. [Google Scholar] [CrossRef] [PubMed]
- Halpern, J.; Mittal, S.; Pereira, K.; Bhatia, S.; Ramasamy, R. Percutaneous embolization of varicocele: Technique, indications, relative contraindications, and complications. Asian J. Androl. 2016, 18, 234–238. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine; Society for Male Reproduction and Urology Report on Varicocele and Infertility: A committee opinion. Fertil. Steril. 2014, 102, 1556–1560. [CrossRef]
- Galfano, A.; Novara, G.; Iafrate, M.; De Marco, V.; Cosentino, M.; D’Elia, C.; Artibani, W.; Ficarra, V. Improvement of seminal parameters and pregnancy rates after antegrade sclerotherapy of internal spermatic veins. Fertil. Steril. 2009, 91, 1085–1089. [Google Scholar] [CrossRef]
- Di Bisceglie, C.; Fornengo, R.; Grosso, M.; Gazzera, C.; Mancini, A.; Andriani, B.; Lanfranco, F.; Brocato, L.; Gandini, G.; Manieri, C. Follow-up of varicocele treated with percutaneous retrograde sclerotherapy: Technical, clinical and seminal aspects. J. Endocrinol. Investig. 2003, 26, 1059–1064. [Google Scholar] [CrossRef]
- Prasivoravong, J.; Marcelli, F.; Lemaître, L.; Pigny, P.; Ramdane, N.; Peers, M.C.; Mitchell, V.; Rigot, J.M. Beneficial effects of varicocele embolization on semen parameters. Basic Clin. Androl. 2014, 24, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinters, H.V.; Galil, K.A.; Lundie, M.J.; Kaufmann, J.C. The histotoxicity of cyanoacrylates. A selective review. Neuroradiology 1985, 27, 279–291. [Google Scholar] [CrossRef]
- Iaccarino, V.; Venetucci, P. Interventional radiology of male varicocele: Current status. Cardiovasc. Intervent. Radiol. 2012, 35, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Jarow, J.P.; Coburn, M.; Sigman, M. Incidence of varicoceles in men with primary and secondary infertility. Urology 1996, 47, 73–76. [Google Scholar] [CrossRef]
- Nevoux, P.; Mitchell, V.; Chevallier, D.; Rigot, J.M.; Marcelli, F. Varicocele repair: Does it still have a role in infertility treatment? Curr. Opin. Obstet. Gynecol. 2011, 23, 151–157. [Google Scholar] [CrossRef]
- Dorfman, S.F. Tobacco and fertility: Our responsibilities. Fertil. Steril. 2008, 89, 502–504. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. World Health Organization. Fertil. Steril. 1992, 57, 1289–1293. [Google Scholar] [CrossRef]
- Sakamoto, H.; Saito, K.; Ogawa, Y.; Yoshida, H. Effects of varicocele repair in adults on ultrasonographically determined testicular volume and on semen profile. Urology 2008, 71, 485–489. [Google Scholar] [CrossRef]
- Sakamoto, H.; Ogawa, Y.; Yoshida, H. Relationship between testicular volume and varicocele in patients with infertility. Urology 2008, 71, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Kroese, A.C.; de Lange, N.M.; Collins, J.; Evers, J.L. Surgery or embolization for varicoceles in subfertile men. Cochrane Database Syst. Rev. 2012, 10, CD000479. [Google Scholar] [CrossRef] [Green Version]
- Auger, J.; Eustache, F.; Ducot, B.; Blandin, T.; Daudin, M.; Diaz, I.; Matribi, S.E.; Gony, B.; Keskes, L.; Kolbezen, M.; et al. Intra- and inter-individual variability in human sperm concentration, motility and vitality assessment during a workshop involving ten laboratories. Hum. Reprod. 2000, 15, 2360–2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
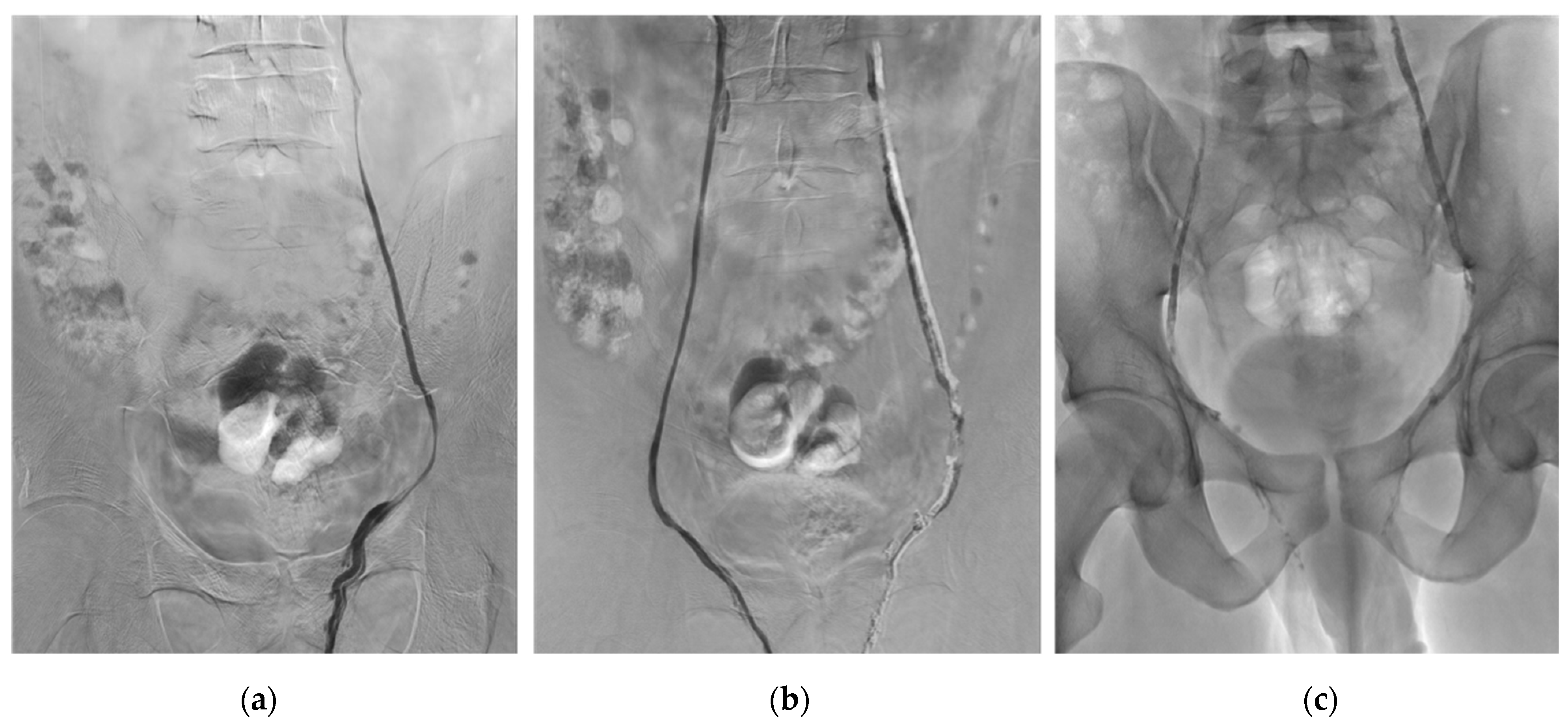
| Characteristics | No. of Patients * | No. (%) ** |
|---|---|---|
| Age (years) | 102 | |
| Mean (95% CI) | 32.3 (31.3–33.3) | |
| Median (IQR) | 32 (29–35) | |
| BMI (kg/m2) | 97 | |
| Mean (95% CI) | 25 (24.1–25.9) | |
| Median (IQR) | 24.8 (22.2–27.0) | |
| Heat exposure | 93 | |
| No | 55 (59.1) | |
| Yes | 38 (40.9) | |
| Toxic exposure | 93 | |
| No | 73 (78.5) | |
| Yes | 20 (21.5) | |
| Tobacco consumption | 98 | |
| Never | 53 (54.1) | |
| Active | 30 (30.6) | |
| Cessation | 15 (15.3) | |
| Infertility type | 102 | |
| Primary | 87 (85.3) | |
| Secondary | 15 (14.7) | |
| Sperm alteration type | 102 | |
| Normal TSN | 42 (41.2) | |
| Oligospermia | 52 (51.0) | |
| Azoospermia | 8 (7.8) | |
| Embolized side | 102 | |
| Left | 92 (90.2) | |
| Right | 3 (2.9) | |
| Both | 7 (6.9) |
| Semen Parameters | No. | Before VE | After VE | p Value * |
|---|---|---|---|---|
| Total sperm number (×106/ejaculate) | 94 | 0.0295 | ||
| Mean ± SD | 95.35 ± 146.24 | 126.24 ± 165.58 | ||
| Median (IQR) | 31.79 (11.10–127.40) | 62.24 (17.90–201.60) | ||
| Progressive sperm motility at H1 (%) | 92 | 0.0003 | ||
| Mean ± SD | 31.49 ± 15.24 | 38.02 ± 14.95 | ||
| Median (IQR) | 30.5 (20.0–43.5) | 38.0 (29.0–48.0) | ||
| Total sperm motility at H1 (%) | 92 | 0.0013 | ||
| Mean ± SD | 40.50 ± 14.67 | 46.14 ± 13.79 | ||
| Median (IQR) | 41.0 (31.0–53.0) | 47.0 (38.0–55.0) | ||
| Sperm vitality (%) | 90 | 0.0356 | ||
| Mean ± SD | 57.87 ± 15.30 | 62.18 ± 15.20 | ||
| Median (IQR) | 57.0 (50.0–70.0) | 62.5 (52.0–72.0) | ||
| Sperm morphology (%) | 64 | 0.0007 | ||
| Mean ± SD | 9.42 ± 8.73 | 14.22 ± 9.83 | ||
| Median (IQR) | 7.0 (3.0–11.5) | 13.0 (6.0–23.0) |
| After VE | ||||
|---|---|---|---|---|
| Total Sperm Number | Abnormal, No. (%) | Normal, No. (%) | Total, No. (%) | |
| Before VE | Abnormal, No. (%) | 33 (35.1) | 19 (20.2) | 52 (55.3) |
| Normal, No. (%) | 6 (6.4) | 36 (38.3) | 42 (44.7) | |
| Total, No. (%) | 39 (41.5) | 55 (58.5) | 94 (100.0) | |
| Characteristics | No. of Patients | Before VE | p Value | After VE | p Value | Comparison Test of Percentage Variation (p Value) |
|---|---|---|---|---|---|---|
| Mean ± SD Median (IQR) | Mean ± SD Median (IQR) | |||||
| Heat exposure | ||||||
| No | 55 | 95.54 ± 160.61 | 0.356 | 113.53 ± 146.30 | 0.794 | 0.361 |
| 35.00 (8.42–120.06) | 62.08 (13.26–162.77) | |||||
| Yes | 38 | 67.95 ± 105.88 | 104.74 ± 176.28 | |||
| 15.81 (3.87–89.4) | 27.27 (8.04–138.6) | |||||
| Toxic exposure | ||||||
| No | 73 | 88.62 ± 153.46 | 0.572 | 115.99 ± 169.90 | 0.484 | 0.341 |
| 28.49 (5.43–108.00) | 50.12 (13.37–138.60) | |||||
| Yes | 20 | 68.38 ± 80.36 | 87.84 ± 106.70 | |||
| 36.34 (10.90–99.03) | 31.00 (5.82–182.19) | |||||
| Tobacco consumption | ||||||
| No | 68 | 88.46 ± 149.88 | 0.900 | 113.56 ± 144.46 | 0.948 | 0.667 |
| 29.85 (7.31–127.23) | 53.93 (13.19–171) | |||||
| Yes | 30 | 84.46 ± 134.14 | 111.27 ± 185.36 | |||
| 27.09 (4.35–97.50) | 31.25 (9.13–162.77) | |||||
| Infertility | ||||||
| Primary | 87 | 92.02 ± 150.65 | 0.482 | 117.73 ± 159.69 | 0.847 | 0.636 |
| 30.12 (8.42–120.06) | 62.08 (12.60–182.00) | |||||
| Secondary | 15 | 63.81 ± 81.91 | 108.96 ± 183.43 | |||
| 23.10 (1.64–97.50) | 36.89 (20.72–81.60) | |||||
| Age | ||||||
| ≤35 years | 78 | 95.94 ± 157.03 | 0.306 | 133.31 ± 178.35 | 0.058 | 0.337 |
| 29.85 (9.24–120.06) | 53.93 (15.96–212.42) | |||||
| >35 years | 24 | 61.67 ± 76.78 | 61.62 ± 72.69 | |||
| 32.20 (3.90–85.29) | 44.40 (5.82–84.50) | |||||
| Body Mass Index | ||||||
| <30 kg/m2 | 84 | 75.20 ± 106.82 | 0.052 | 112.14 ±155.88 | 0.566 | 0.880 |
| 29.10 (7.00–106.90) | 50.56 (13.25–161.39) | |||||
| ≥30 kg/m2 | 18 | 147.01 ± 246.45 | 136.51 ± 193.95 | |||
| 42.18 (7.70–175.68) | 46.53 (8.04–218.30) |
| After VE | ||||
|---|---|---|---|---|
| Progressive Motility * | Abnormal, No. (%) | Normal, No. (%) | Total, No. (%) | |
| Before VE | Abnormal, No. (%) | 21 (22.8) | 27 (29.4) | 48 (52.2) |
| Normal, No. (%) | 7 (7.6) | 37 (40.2) | 44 (47.8) | |
| Total, No. (%) | 28 (30.4) | 64 (69.6) | 92 (100.0) | |
| After VE | ||||
|---|---|---|---|---|
| Total Motility * | Abnormal, No. (%) | Normal, No. (%) | Total, No. (%) | |
| Before VE | Abnormal, No. (%) | 16 (17.4) | 26 (28.2) | 42 (45.6) |
| Normal, No. (%) | 9 (9.8) | 41 (44.6) | 50 (54.4) | |
| Total, No. (%) | 25 (27.2) | 67 (72.8) | 92 (100.0) | |
| After VE | ||||
|---|---|---|---|---|
| Sperm Vitality | Abnormal, No. (%) | Normal, No. (%) | Total, No. (%) | |
| Before VE | Abnormal, No. (%) | 21 (23.3) | 24 (26.7) | 45 (50.0) |
| Normal, No. (%) | 12 (13.3) | 33 (36.7) | 45 (50.0) | |
| Total, No. (%) | 33 (36.6) | 57 (63.4) | 90 (100.0) | |
| After VE | ||||
|---|---|---|---|---|
| Sperm Morphology | Abnormal, No. (%) | Normal, No. (%) | Total, No. (%) | |
| Before VE | Abnormal, No. (%) | 44 (68.8) | 13 (20.3) | 57 (89.1) |
| Normal, No. (%) | 3 (4.7) | 4 (6.3) | 7 (10.9) | |
| Total, No. (%) | 47 (73.4) | 17 (26.6) | 64 (100.0) | |
| Before VE | After VE | p Value | |
|---|---|---|---|
| Embolized side (n = 60) * | |||
| Mean volume ± SD (mL) | 12.30 ± 3.88 | 12.64 ± 4.16 | 0.251 |
| p50 (IQR: p25–p75) | 12.00 (10.05–15.35) | 13.25 (10.00–15.35) | |
| Non-embolized side (n = 53) * | |||
| Mean volume ± SD (mL) | 14.62 ± 4.45 | 14.79 ± 4.27 | 0.630 |
| p50 (IQR: p25–p75) | 15.00 (12.00–17.00) | 15.00 (12.30–18.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chevallier, O.; Fauque, P.; Poncelet, C.; Guillen, K.; Comby, P.-O.; Astruc, K.; Barberet, J.; Falvo, N.; Simon, E.; Loffroy, R. Relevant Biological Effects of Varicocele Embolization with N-Butyl Cyanoacrylate Glue on Semen Parameters in Infertile Men. Biomedicines 2021, 9, 1423. https://doi.org/10.3390/biomedicines9101423
Chevallier O, Fauque P, Poncelet C, Guillen K, Comby P-O, Astruc K, Barberet J, Falvo N, Simon E, Loffroy R. Relevant Biological Effects of Varicocele Embolization with N-Butyl Cyanoacrylate Glue on Semen Parameters in Infertile Men. Biomedicines. 2021; 9(10):1423. https://doi.org/10.3390/biomedicines9101423
Chicago/Turabian StyleChevallier, Olivier, Patricia Fauque, Carole Poncelet, Kévin Guillen, Pierre-Olivier Comby, Karine Astruc, Julie Barberet, Nicolas Falvo, Emmanuel Simon, and Romaric Loffroy. 2021. "Relevant Biological Effects of Varicocele Embolization with N-Butyl Cyanoacrylate Glue on Semen Parameters in Infertile Men" Biomedicines 9, no. 10: 1423. https://doi.org/10.3390/biomedicines9101423
APA StyleChevallier, O., Fauque, P., Poncelet, C., Guillen, K., Comby, P.-O., Astruc, K., Barberet, J., Falvo, N., Simon, E., & Loffroy, R. (2021). Relevant Biological Effects of Varicocele Embolization with N-Butyl Cyanoacrylate Glue on Semen Parameters in Infertile Men. Biomedicines, 9(10), 1423. https://doi.org/10.3390/biomedicines9101423






