The Role of Uterine Natural Killer Cells on Recurrent Miscarriage and Recurrent Implantation Failure: From Pathophysiology to Treatment
Abstract
1. Introduction
2. Material and Methods
3. The Role of uNK Cells in Reproductive Physiology and Pathophysiology
3.1. The Origin and Localization of uNK
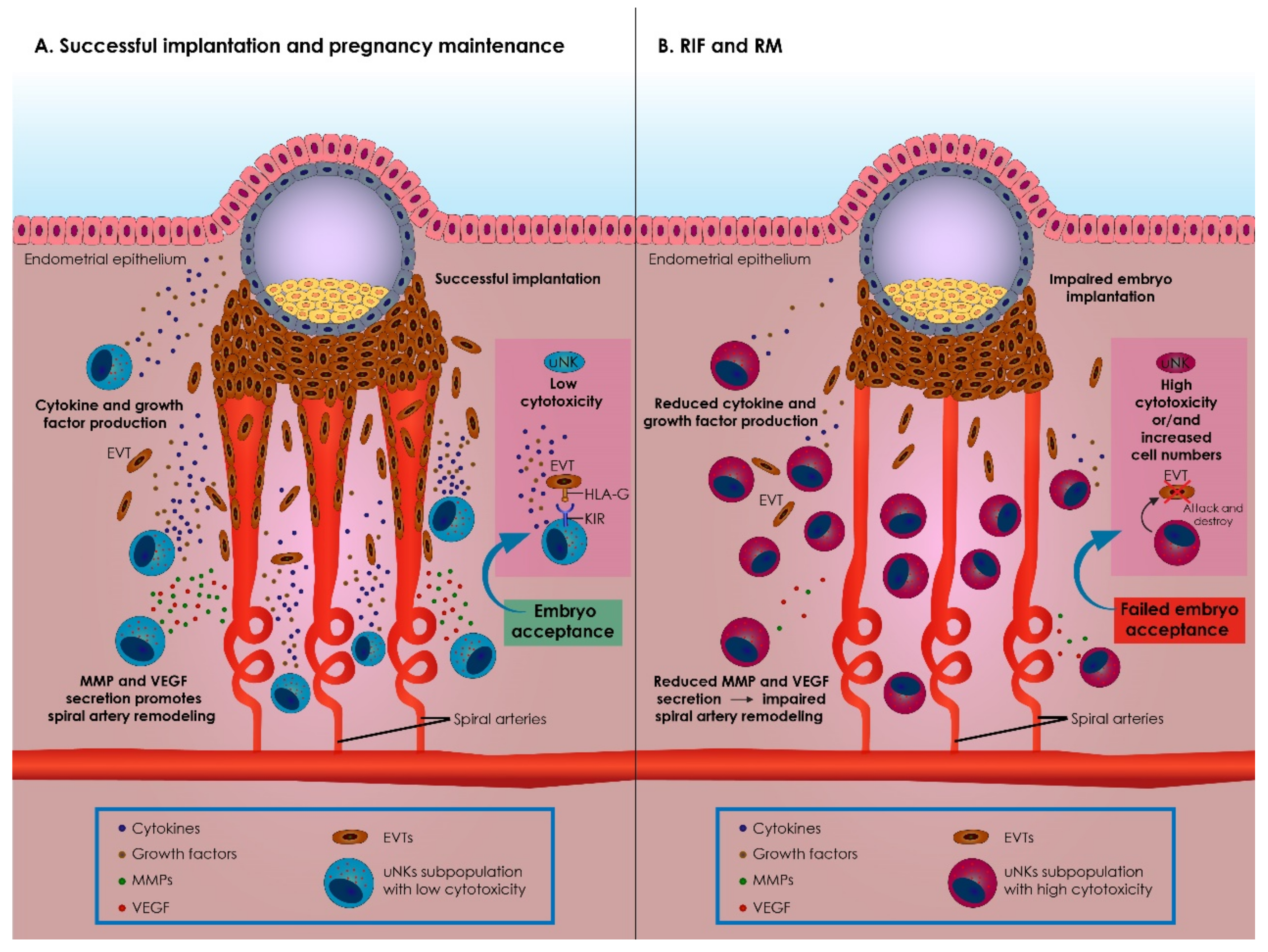
3.2. The Role of uNK Cells in Implantation and Pregnancy
3.3. Immunological Alterations Prompted by uNK during Implantation and Pregnancy
3.4. uNK Cells in RIF and RM
3.4.1. The Case of RIF Patients
3.4.2. The Case of RM Patients
3.4.3. Considerations Emerging while Critically Assessing Literature
4. Immunotherapy Options for uNK Related RIF and RM
4.1. Glucocorticoids
4.2. Intralipid Therapy
4.3. Immunoglobulin
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sojka, D.K.; Yang, L.; Yokoyama, W.M. Uterine natural killer cells. Front. Immunol. 2019, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Long, E.O.; Kim, H.S.; Liu, D.; Peterson, M.E.; Rajagopalan, S. Controlling NK cell responses: Integration of signals for activation and inhibition. Annu. Rev. Immunol. 2013, 31, 4531–4534. [Google Scholar] [CrossRef] [PubMed]
- Kalkunte, S.; Chichester, C.O.; Gotsch, F.; Sentman, C.L.; Romero, R.; Sharma, S. Evolution of non-cytotoxic uterine natural killer (UNK) cells. Am. J. Reprod. Immunol. 2008, 59, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, J.N.; Lash, G.E. Uterine natural killer cells: Time for a re-appraisal? F1000Research 2019, 8, F1000 Faculty Rev-199. [Google Scholar] [CrossRef] [PubMed]
- Vacca, P.; Vitale, C.; Munari, E.; Cassatella, M.A.; Mingari, M.C.; Moretta, L. Human innate lymphoid cells: Their functional and cellular interactions in decidua. Front. Immunol. 2018, 9, 1897. [Google Scholar] [CrossRef]
- Timeva, T.; Shterev, A.; Kyurkchiev, S. Recurrent implantation failure: The role of the endometrium. J. Reprod. Infertil. 2014, 15, 173–183. [Google Scholar]
- Acar, N.; Ustunel, I.; Demir, R. Uterine Natural Killer (UNK) cells and their missions during pregnancy: A review. Acta Histochem. 2011, 113, 82–91. [Google Scholar] [CrossRef]
- Sojka, D.K. Uterine natural killer cell heterogeneity: Lessons from mouse models. Front. Immunol. 2020, 11, 290. [Google Scholar] [CrossRef]
- Simon, A.; Laufer, N. Repeated implantation failure: Clinical approach. Fertil. Steril. 2012, 97, 1039–1043. [Google Scholar] [CrossRef]
- Christiansen, O.B.; Nielsen, H.S.; Kolte, A.M. Future directions of failed implantation and recurrent miscarriage research. Reprod. Biomed. Online 2006, 13, 71–83. [Google Scholar] [CrossRef]
- Garrido-Gimenez, C.; Alijotas-Reig, J. Recurrent miscarriage: Causes, evaluation and management. Postgrad. Med. J. 2015, 91, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Homer, H.A. Modern management of recurrent miscarriage. Aust. N. Z. J. Obstet. Gynaecol. 2019, 59, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Hakim, R.B.; Gray, R.H.; Zacur, H. Infertility and early pregnancy loss. Am. J. Obstet. Gynecol. 1995, 172, 1510–1517. [Google Scholar] [CrossRef]
- Cauchi, M.N.; Coulam, C.B.; Cowchock, S.; Ho, H.N.; Gatenby, P.; Johnson, P.M.; Lubs, M.L.; McIntyre, J.A.; Ramsden, G.H.; Smith, J.B. Predictive factors in recurrent spontaneous aborters—A multicenter study. Am. J. Reprod. Immunol. 1995, 33, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Stern, C.; Chamley, L.; Hale, L.; Kloss, M.; Speirs, A.; Baker, H.W. Antibodies to Beta2 glycoprotein I are associated with in vitro fertilization implantation failure as well as recurrent miscarriage: Results of a prevalence study. Fertil. Steril. 1998, 70, 938–944. [Google Scholar] [CrossRef]
- Matsubayashi, H.; Arai, T.; Izumi, S.; Sugi, T.; McIntyre, J.A.; Makino, T. Anti-annexin V antibodies in patients with early pregnancy loss or implantation failures. Fertil. Steril. 2001, 76, 694–699. [Google Scholar] [CrossRef]
- Lédée-Bataille, N.; Bonnet-Chea, K.; Hosny, G.; Dubanchet, S.; Frydman, R.; Chaouat, G. Role of the endometrial tripod interleukin-18, -15, and -12 in inadequate uterine receptivity in patients with a history of repeated in vitro fertilization-embryo transfer failure. Fertil. Steril. 2005, 83, 598–605. [Google Scholar] [CrossRef]
- Laird, S.; Tuckerman, E.; Li, T.-C. Cytokine expression in the endometrium of women with implantation failure and recurrent miscarriage. Reprod. Biomed. Online 2006, 13, 13–23. [Google Scholar] [CrossRef]
- Quenby, S.; Farquharson, R. Uterine natural killer cells, implantation failure and recurrent miscarriage. Reprod. Biomed. Online 2006, 13, 24–28. [Google Scholar] [CrossRef]
- Lanier, L.L.; Le, A.M.; Civin, C.I.; Loken, M.R.; Phillips, J.H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J. Immunol. 1986, 136, 4480–4486. [Google Scholar] [PubMed]
- Henderson, T.A.; Saunders, P.T.K.; Moffett-King, A.; Groome, N.P.; Critchley, H.O.D. Steroid receptor expression in uterine natural killer cells. J. Clin. Endocrinol. Metab. 2003, 88, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Trundley, A.; Moffett, A. Human uterine leukocytes and pregnancy. Tissue Antigens 2004, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, J.N.; Lash, G.E. Human uterine natural killer cells: A reappraisal. Mol. Immunol. 2005, 42, 511–521. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Loke, Y.W. On the nature and function of human uterine granular lymphocytes. Immunol. Today 1991, 12, 432–435. [Google Scholar] [CrossRef]
- Chantakru, S.; Miller, C.; Roach, L.E.; Kuziel, W.A.; Maeda, N.; Wang, W.-C.; Evans, S.S.; Croy, B.A. Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J. Immunol. 2002, 168, 22–28. [Google Scholar] [CrossRef]
- Lobo, S.C.; Huang, S.-T.J.; Germeyer, A.; Dosiou, C.; Vo, K.C.; Tulac, S.; Nayak, N.R.; Giudice, L.C. The immune environment in human endometrium during the window of implantation. Am. J. Reprod. Immunol. 2004, 52, 244–251. [Google Scholar] [CrossRef]
- Lynch, L.; Golden-Mason, L.; Eogan, M.; O′Herlihy, C.; O′Farrelly, C. Cells with haematopoietic stem cell phenotype in adult human endometrium: Relevance to infertility? Hum. Reprod. 2007, 22, 919–926. [Google Scholar] [CrossRef]
- Keskin, D.B.; Allan, D.S.J.; Rybalov, B.; Andzelm, M.M.; Stern, J.N.H.; Kopcow, H.D.; Koopman, L.A.; Strominger, J.L. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. Proc. Natl. Acad. Sci. USA 2007, 104, 3378–3383. [Google Scholar] [CrossRef]
- Vacca, P.; Vitale, C.; Montaldo, E.; Conte, R.; Cantoni, C.; Fulcheri, E.; Darretta, V.; Moretta, L.; Mingari, M.C. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc. Natl. Acad. Sci. USA 2011, 108, 2402–2407. [Google Scholar] [CrossRef]
- Szereday, L.; Miko, E.; Meggyes, M.; Barakonyi, A.; Farkas, B.; Varnagy, A.; Bodis, J.; Lynch, L.; O′Farrelly, C.; Szekeres-Bartho, J. Commitment of decidual haematopoietic progenitor cells in first trimester pregnancy. Am. J. Reprod. Immunol. 2012, 67, 9–16. [Google Scholar] [CrossRef]
- Matsuura-Sawada, R.; Murakami, T.; Ozawa, Y.; Nabeshima, H.; Akahira, J.-I.; Sato, Y.; Koyanagi, Y.; Ito, M.; Terada, Y.; Okamura, K. Reproduction of menstrual changes in transplanted human endometrial tissue in immunodeficient mice. Hum. Reprod. 2005, 20, 1477–1484. [Google Scholar] [CrossRef][Green Version]
- Male, V.; Hughes, T.; McClory, S.; Colucci, F.; Caligiuri, M.A.; Moffett, A. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J. Immunol. 2010, 185, 3913–3918. [Google Scholar] [CrossRef] [PubMed]
- Cerdeira, A.S.; Rajakumar, A.; Royle, C.M.; Lo, A.; Husain, Z.; Thadhani, R.I.; Sukhatme, V.P.; Karumanchi, S.A.; Kopcow, H.D. Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors. J. Immunol. 2013, 190, 3939–3948. [Google Scholar] [CrossRef] [PubMed]
- Moffett-King, A.; Entrican, G.; Ellis, S.; Hutchinson, J.; Bainbridge, D. Natural killer cells and reproduction. Trends Immunol. 2002, 23, 332–333. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef] [PubMed]
- Laird, S. The Role of Natural Killer Cells in Human Fertility. Available online: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/the-role-of-natural-killer-cells-in-human-fertility-scientific-impact-paper-no.-53/ (accessed on 20 February 2021).
- Smith, S.D.; Dunk, C.E.; Aplin, J.D.; Harris, L.K.; Jones, R.L. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am. J. Pathol. 2009, 174, 1959–1971. [Google Scholar] [CrossRef]
- Robson, A.; Harris, L.K.; Innes, B.A.; Lash, G.E.; Aljunaidy, M.M.; Aplin, J.D.; Baker, P.N.; Robson, S.C.; Bulmer, J.N. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J. 2012, 26, 4876–4885. [Google Scholar] [CrossRef]
- Gaynor, L.M.; Colucci, F. Uterine natural killer cells: Functional distinctions and influence on pregnancy in humans and mice. Front. Immunol. 2017, 8, 467. [Google Scholar] [CrossRef]
- Chakraborty, D.; Rumi, M.A.K.; Konno, T.; Soares, M.J. Natural killer cells direct hemochorial placentation by regulating hypoxia-inducible factor dependent trophoblast lineage decisions. Proc. Natl. Acad. Sci. USA 2011, 108, 16295–16300. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Hatta, K.; Lima, P.D.A.; Yadi, H.; Colucci, F.; Yamada, A.T.; Croy, B.A. DBA-lectin reactivity defines mouse uterine natural killer cell subsets with biased gene expression. Biol. Reprod. 2012, 87, 1–9. [Google Scholar] [CrossRef]
- Wang, C.; Umesaki, N.; Nakamura, H.; Tanaka, T.; Nakatani, K.; Sakaguchi, I.; Ogita, S.; Kaneda, K. Expression of vascular endothelial growth factor by granulated metrial gland cells in pregnant murine uteri. Cell Tissue Res. 2000, 300, 285–293. [Google Scholar] [CrossRef]
- Tayade, C.; Hilchie, D.; He, H.; Fang, Y.; Moons, L.; Carmeliet, P.; Foster, R.A.; Croy, B.A. Genetic deletion of placenta growth factor in mice alters uterine NK cells. J. Immunol. 2007, 178, 4267–4275. [Google Scholar] [CrossRef]
- Lima, P.D.; Zhang, J.; Dunk, C.; Lye, S.J.; Anne Croy, B. Leukocyte driven-decidual angiogenesis in early pregnancy. Cell. Mol. Immunol. 2014, 11, 522–537. [Google Scholar] [CrossRef]
- Winterhager, E.; Gellhaus, A.; Blois, S.M.; Hill, L.A.; Barr, K.J.; Kidder, G.M. Decidual angiogenesis and placental orientation are altered in mice heterozygous for a dominant loss-of-function Gja1 (Connexin43) mutation. Biol. Reprod. 2013, 89, 1–12. [Google Scholar] [CrossRef]
- Quenby, S.; Nik, H.; Innes, B.; Lash, G.; Turner, M.; Drury, J.; Bulmer, J. Uterine natural killer cells and angiogenesis in recurrent reproductive failure. Hum. Reprod. 2009, 24, 45–54. [Google Scholar] [CrossRef]
- Gupta, S.; Agarwal, A.; Banerjee, J.; Alvarez, J.G. The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: A systematic review. Obstet. Gynecol. Surv. 2007, 62, 335–347, quiz 353–354. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-C.; Chen, C.-H.; Chen, M.-J.; Chang, C.-W.; Chen, P.-H.; Yu, M.-H.; Chen, Y.-J.; Tsai, E.-M.; Yang, P.-S.; Lin, S.-Y.; et al. Killer cell Immunoglobulin-like Receptors (KIR) and Human Leukocyte Antigen-C (HLA-C) allorecognition patterns in women with endometriosis. Sci. Rep. 2020, 10, 4897. [Google Scholar] [CrossRef]
- Hu, Y.; Dutz, J.P.; MacCalman, C.D.; Yong, P.; Tan, R.; Dadelszen, P. NK cells alter in vitro first trimester extravillous cytotrophoblast migration: A role for IFN-γ. J. Immunol. 2006, 177, 8522–8530. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Llano, M.; Carretero, M.; Ishitani, A.; Navarro, F.; López-Botet, M.; Geraghty, D.E. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc. Natl. Acad. Sci. USA 1998, 95, 5199–5204. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Allan, D.S.; Bowen, M.; Powis, S.J.; Joseph, S.; Verma, S.; Hiby, S.E.; McMichael, A.J.; Loke, Y.W.; Braud, V.M. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK Cells. Eur. J. Immunol. 2000, 30, 1623–1631. [Google Scholar] [CrossRef]
- Kämmerer, U.; Eggert, A.O.; Kapp, M.; McLellan, A.D.; Geijtenbeek, T.B.H.; Dietl, J.; van Kooyk, Y.; Kämpgen, E. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am. J. Pathol. 2003, 162, 887–896. [Google Scholar] [CrossRef]
- Apps, R.; Gardner, L.; Moffett, A. A critical look at HLA-G. Trends Immunol. 2008, 29, 313–321. [Google Scholar] [CrossRef]
- Lédée-Bataille, N.; Dubanchet, S.; Coulomb-L′hermine, A.; Durand-Gasselin, I.; Frydman, R.; Chaouat, G. A new role for natural killer cells, interleukin (IL)-12, and IL-18 in repeated implantation failure after in vitro fertilization. Fertil. Steril. 2004, 81, 59–65. [Google Scholar] [CrossRef]
- Jokhi, P.P.; King, A.; Loke, Y.W. Cytokine production and cytokine receptor expression by cells of the human first trimester placental-uterine interface. Cytokine 1997, 9, 126–137. [Google Scholar] [CrossRef]
- Li, X.F.; Charnock-Jones, D.S.; Zhang, E.; Hiby, S.; Malik, S.; Day, K.; Licence, D.; Bowen, J.M.; Gardner, L.; King, A.; et al. Angiogenic growth factor messenger ribonucleic acids in uterine natural killer cells. J. Clin. Endocrinol. Metab. 2001, 86, 1823–1834. [Google Scholar] [CrossRef]
- Lash, G.E.; Schiessl, B.; Kirkley, M.; Innes, B.A.; Cooper, A.; Searle, R.F.; Robson, S.C.; Bulmer, J.N. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J. Leukoc. Biol. 2006, 80, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Naruse, K.; Lash, G.E.; Innes, B.A.; Otun, H.A.; Searle, R.F.; Robson, S.C.; Bulmer, J.N. Localization of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitors for MMPs (TIMPs) in uterine natural killer cells in early human pregnancy. Hum. Reprod. 2009, 24, 553–561. [Google Scholar] [CrossRef]
- Tuckerman, E.; Mariee, N.; Prakash, A.; Li, T.C.; Laird, S. Uterine natural killer cells in peri-implantation endometrium from women with repeated implantation failure after IVF. J. Reprod. Immunol. 2010, 87, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Xu, H. Obesity and metabolic inflammation. Drug Discov. Today Dis. Mech. 2013, 10, e21–e25. [Google Scholar] [CrossRef]
- Baltayeva, J.; Castellana, B.; Mara, D.L.; Christians, J.K.; Beristain, A.G. A mouse model of maternal obesity leads to uterine natural killer (uNK) cell activation and uterine artery remodeling defects. bioRxiv 2018, 275503. [Google Scholar] [CrossRef]
- Seijkens, T.; Kusters, P.; Chatzigeorgiou, A.; Chavakis, T.; Lutgens, E. Immune cell crosstalk in obesity: A key role for costimulation? Diabetes 2014, 63, 3982–3991. [Google Scholar] [CrossRef]
- Parker, V.J.; Solano, M.E.; Arck, P.C.; Douglas, A.J. Diet-induced obesity may affect the uterine immune environment in early–mid pregnancy, reducing NK-cell activity and potentially compromising uterine vascularization. Int. J. Obes. 2014, 38, 766–774. [Google Scholar] [CrossRef]
- Matteo, M.G.; Greco, P.; Rosenberg, P.; Mestice, A.; Baldini, D.; Falagario, T.; Martino, V.; Santodirocco, M.; Massenzio, F.; Castellana, L.; et al. Normal percentage of CD56bright natural killer cells in young patients with a history of repeated unexplained implantation failure after in vitro fertilization cycles. Fertil. Steril. 2007, 88, 990–993. [Google Scholar] [CrossRef]
- Tang, A.W.; Alfirevic, Z.; Quenby, S. Natural killer cells and pregnancy outcomes in women with recurrent miscarriage and infertility: A systematic review. Hum. Reprod. 2011, 26, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Sunkara, S.K. Natural killer cells in female infertility and recurrent miscarriage: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 429–438. [Google Scholar] [CrossRef]
- Karami, N.; Boroujerdnia, M.G.; Nikbakht, R.; Khodadadi, A. Enhancement of peripheral blood CD56(Dim) cell and NK cell cytotoxicity in women with recurrent spontaneous abortion or in vitro fertilization failure. J. Reprod. Immunol. 2012, 95, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Lachapelle, M.H.; Miron, P.; Hemmings, R.; Roy, D.C. Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcome. J. Immunol. 1996, 156, 4027–4034. [Google Scholar] [PubMed]
- Fukui, A.; Fujii, S.; Yamaguchi, E.; Kimura, H.; Sato, S.; Saito, Y. Natural killer cell subpopulations and cytotoxicity for infertile patients undergoing in vitro fertilization. Am. J. Reprod. Immunol. 1999, 41, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Quenby, S.; Vince, G.; Farquharson, R.; Aplin, J. Recurrent miscarriage: A defect in nature′s quality control? Hum. Reprod. 2002, 17, 1959–1963. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Takahashi, Y.; Kase, N.; Mori, H. Role of decidual natural killer (NK) cells in patients with missed abortion: Differences between cases with normal and abnormal chromosome. Clin. Exp. Immunol. 1999, 116, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Clifford, K.; Flanagan, A.M.; Regan, L. Endometrial CD56+ natural killer cells in women with recurrent miscarriage: A histomorphometric study. Hum. Reprod. 1999, 14, 2727–2730. [Google Scholar] [CrossRef] [PubMed]
- Quenby, S.; Kalumbi, C.; Bates, M.; Farquharson, R.; Vince, G. Prednisolone reduces preconceptual endometrial natural killer cells in women with recurrent miscarriage. Fertil. Steril. 2005, 84, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Tuckerman, E.; Laird, S.M.; Prakash, A.; Li, T.C. Prognostic value of the measurement of uterine natural killer cells in the endometrium of women with recurrent miscarriage. Hum. Reprod. 2007, 22, 2208–2213. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.-W.; Alfirevic, Z.; Turner, M.A.; Drury, J.; Quenby, S. Prednisolone trial: Study protocol for a randomised controlled trial of prednisolone for women with idiopathic recurrent miscarriage and raised levels of uterine natural killer (uNK) cells in the endometrium. Trials 2009, 10, 102. [Google Scholar] [CrossRef]
- Tang, A.-W.; Alfirevic, Z.; Turner, M.A.; Drury, J.A.; Small, R.; Quenby, S. A feasibility trial of screening women with idiopathic recurrent miscarriage for high uterine natural killer cell density and randomizing to prednisolone or placebo when pregnant. Hum. Reprod. 2013, 28, 1743–1752. [Google Scholar] [CrossRef]
- Cooper, S.; Laird, S.M.; Mariee, N.; Li, T.C.; Metwally, M. The effect of prednisolone on endometrial uterine NK cell concentrations and pregnancy outcome in women with reproductive failure. A retrospective cohort study. J. Reprod. Immunol. 2019, 131, 1–6. [Google Scholar] [CrossRef]
- Michimata, T.; Ogasawara, M.S.; Tsuda, H.; Suzumori, K.; Aoki, K.; Sakai, M.; Fujimura, M.; Nagata, K.; Nakamura, M.; Saito, S. Distributions of endometrial NK cells, B cells, T cells, and Th2/Tc2 cells fail to predict pregnancy outcome following recurrent abortion. Am. J. Reprod. Immunol. 2002, 47, 196–202. [Google Scholar] [CrossRef]
- Quenby, S.; Bates, M.; Doig, T.; Brewster, J.; Lewis-Jones, D.I.; Johnson, P.M.; Vince, G. Pre-implantation endometrial leukocytes in women with recurrent miscarriage. Hum. Reprod. 1999, 14, 2386–2391. [Google Scholar] [CrossRef]
- Fukui, A.; Funamizu, A.; Fukuhara, R.; Shibahara, H. Expression of natural cytotoxicity receptors and cytokine production on endometrial natural killer cells in women with recurrent pregnancy loss or implantation failure, and the expression of natural cytotoxicity receptors on peripheral blood natural killer cells in pregnant women with a history of recurrent pregnancy loss. J. Obstet. Gynaecol. Res. 2017, 43, 1678–1686. [Google Scholar] [CrossRef]
- Lash, G.E.; Bulmer, J.N.; Li, T.C.; Innes, B.A.; Mariee, N.; Patel, G.; Sanderson, J.; Quenby, S.; Laird, S.M. Standardisation of uterine natural killer (UNK) cell measurements in the endometrium of women with recurrent reproductive failure. J. Reprod. Immunol. 2016, 116, 50–59. [Google Scholar] [CrossRef]
- Ogasawara, M.; Aoki, K. Successful uterine steroid therapy in a case with a history of ten miscarriages. Am. J. Reprod. Immunol. 2000, 44, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Ubaldi, F.; Rienzi, L.; Ferrero, S.; Anniballo, R.; Iacobelli, M.; Cobellis, L.; Greco, E. Low dose prednisolone administration in routine ICSI patients does not improve pregnancy and implantation rates. Hum. Reprod. 2002, 17, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- Dan, S.; Wei, W.; Yichao, S.; Hongbo, C.; Shenmin, Y.; Jiaxiong, W.; Hong, L. Effect of prednisolone administration on patients with unexplained recurrent miscarriage and in routine intracytoplasmic sperm injection: A meta-analysis. Am. J. Reprod. Immunol. 2015, 74, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Thakur, S.; Agrawal, S. Lymphocyte immunotherapy and its probable mechanism in the maintenance of pregnancy in women with recurrent spontaneous abortion. Arch. Gynecol. Obstet. 2004, 269, 161–172. [Google Scholar] [CrossRef]
- Gur, C.; Diav-Citrin, O.; Shechtman, S.; Arnon, J.; Ornoy, A. Pregnancy outcome after first trimester exposure to corticosteroids: A prospective controlled study. Reprod. Toxicol. 2004, 18, 93–101. [Google Scholar] [CrossRef]
- Roussev, R.G.; Acacio, B.; Ng, S.C.; Coulam, C.B. Duration of intralipid′s suppressive effect on NK cell′s functional activity. Am. J. Reprod. Immunol. 2008, 60, 258–263. [Google Scholar] [CrossRef]
- Niavarani, S.R.; Lawson, C.; Bakos, O.; Boudaud, M.; Batenchuk, C.; Rouleau, S.; Tai, L.-H. Lipid accumulation impairs natural killer cell cytotoxicity and tumor control in the postoperative period. BMC Cancer 2019, 19, 823. [Google Scholar] [CrossRef]
- Coulam, C.B. Intralipid treatment for women with reproductive failures. Am. J. Reprod. Immunol. 2020, 85, e13290. [Google Scholar] [CrossRef]
- Khan, L.; Qureshi, V.F.; Jabeen, T.; Qureshi, S. Use of intralipid in the management of recurrent implantation failure: An overview. J. Nat. Sci. Biol. Med. 2018, 9, 111. [Google Scholar] [CrossRef]
- Granato, D.; Blum, S.; Rössle, C.; Le Boucher, J.; Malnoë, A.; Dutot, G. Effects of parenteral lipid emulsions with different fatty acid composition on immune cell functions in vitro. JPEN J. Parenter. Enter. Nutr. 2000, 24, 113–118. [Google Scholar] [CrossRef]
- Dakhly, D.M.R.; Bayoumi, Y.A.; Sharkawy, M.; Gad Allah, S.H.; Hassan, M.A.; Gouda, H.M.; Hashem, A.T.; Hatem, D.L.; Ahmed, M.F.; El-Khayat, W. Intralipid supplementation in women with recurrent spontaneous abortion and elevated levels of natural killer cells. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2016, 135, 324–327. [Google Scholar] [CrossRef]
- Shreeve, N.; Sadek, K. Intralipid therapy for recurrent implantation failure: New hope or false dawn? J. Reprod. Immunol. 2012, 93, 38–40. [Google Scholar] [CrossRef]
- Check, J.H.; Check, D.L. Intravenous intralipid therapy is not beneficial in having a live delivery in women aged 40–42 years with a previous history of miscarriage or failure to conceive despite embryo transfer undergoing in vitro fertilization-embryo transfer. Clin. Exp. Obstet. Gynecol. 2016, 43, 14–15. [Google Scholar] [PubMed]
- Martini, A.E.; Jasulaitis, S.; Fogg, L.F.; Uhler, M.L.; Hirshfeld-Cytron, J.E. Evaluating the utility of intralipid infusion to improve live birth rates in patients with recurrent pregnancy loss or recurrent implantation failure. J. Hum. Reprod. Sci. 2018, 11, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, R.; Hull, M.L.; Walkley, J.; Sacks, G. Intralipid immunotherapy for repeated IVF failure. Fertil. Reprod. 2019, 1, 154–160. [Google Scholar] [CrossRef]
- Jacobi, C.; Claus, M.; Wildemann, B.; Wingert, S.; Korporal, M.; Römisch, J.; Meuer, S.; Watzl, C.; Giese, T. Exposure of NK cells to intravenous immunoglobulin induces IFN gamma release and degranulation but inhibits their cytotoxic activity. Clin. Immunol. 2009, 133, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Jolles, S.; Sewell, W.; Misbah, S. Clinical uses of intravenous immunoglobulin. Clin. Exp. Immunol. 2005, 142, 1–11. [Google Scholar] [CrossRef]
- Bhat, R.; Watzl, C. Serial killing of tumor cells by human natural killer cells—Enhancement by therapeutic antibodies. PLoS ONE 2007, 2, e326. [Google Scholar] [CrossRef]
- Moffett, A.; Shreeve, N. First do no harm: Uterine natural killer (NK) cells in assisted reproduction. Hum. Reprod. 2015, 30, 1519–1525. [Google Scholar] [CrossRef]
- Rachid, R.; Bonilla, F.A. The role of anti-IgA antibodies in causing adverse reactions to gamma globulin infusion in immunodeficient patients: A comprehensive review of the literature. J. Allergy Clin. Immunol. 2012, 129, 628–634. [Google Scholar] [CrossRef]
- Sung, N.; Han, A.R.; Park, C.W.; Park, D.W.; Park, J.C.; Kim, N.Y.; Lim, K.S.; Shin, J.E.; Joo, C.W.; Lee, S.E.; et al. Intravenous immunoglobulin G in women with reproductive failure: The Korean society for reproductive immunology practice guidelines. Clin. Exp. Reprod. Med. 2017, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tian, X.; Wang, X.; Xiao, Z. Adverse effects of immunoglobulin therapy. Front. Immunol. 2018, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Daya, S.; Gunby, J.; Clark, D.A. Intravenous immunoglobulin therapy for recurrent spontaneous abortion: A meta-analysis. Am. J. Reprod. Immunol. 1998, 39, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.F.; LaCoursiere, Y.; Scott, J.R. Immunotherapy for recurrent miscarriage. Cochrane Database Syst. Rev. 2006, 10, CD000112. [Google Scholar] [CrossRef]
- Winger, E.E.; Reed, J.L.; Ashoush, S.; El-Toukhy, T.; Ahuja, S.; Taranissi, M. Elevated preconception CD56+ 16+ and/or Th1:Th2 levels predict benefit from IVIG therapy in subfertile women undergoing IVF. Am. J. Reprod. Immunol. 2011, 66, 394–403. [Google Scholar] [CrossRef]
- Moraru, M.; Carbone, J.; Alecsandru, D.; Castillo-Rama, M.; García-Segovia, A.; Gil, J.; Alonso, B.; Aguarón, A.; Ramos-Medina, R.; Martínez de María, J.; et al. Intravenous immunoglobulin treatment increased live birth rate in a Spanish cohort of women with recurrent reproductive failure and expanded CD56+ cells. Am. J. Reprod. Immunol. 2012, 68, 75–84. [Google Scholar] [CrossRef]
- Vaquero, E.; Lazzarin, N.; Caserta, D.; Valensise, H.; Baldi, M.; Moscarini, M.; Arduini, D. Diagnostic evaluation of women experiencing repeated in vitro fertilization failure. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 125, 79–84. [Google Scholar] [CrossRef]
- Macklon, N.S.; Geraedts, J.P.M.; Fauser, B.C.J.M. Conception to ongoing pregnancy: The “black box” of early pregnancy loss. Hum. Reprod. Update 2002, 8, 333–343. [Google Scholar] [CrossRef]
- Society for Assisted Reproductive Technology. American Society for Reproductive Medicine. Assisted Reproductive Technology in the United States. 2000 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology registry. Fertil. Steril. 2004, 81, 1207–1220. [Google Scholar] [CrossRef]
- Sojka, D.K.; Yang, L.; Yokoyama, W.M. Uterine natural killer cells: To protect and to nurture. Birth Defects Res. 2018, 110, 1531–1538. [Google Scholar] [CrossRef]
- Mincheva-Nilsson, L.; Kling, M.; Hammarström, S.; Nagaeva, O.; Sundqvist, K.G.; Hammarström, M.L.; Baranov, V. Gamma delta T cells of human early pregnancy decidua: Evidence for local proliferation, phenotypic heterogeneity, and extrathymic differentiation. J. Immunol. 1997, 159, 3266–3277. [Google Scholar]
- Koopman, L.A.; Kopcow, H.D.; Rybalov, B.; Boyson, J.E.; Orange, J.S.; Schatz, F.; Masch, R.; Lockwood, C.J.; Schachter, A.D.; Park, P.J.; et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J. Exp. Med. 2003, 198, 1201–1212. [Google Scholar] [CrossRef]
- Middleton, D.; Gonzelez, F. The extensive polymorphism of KIR genes. Immunology 2010, 129, 8–19. [Google Scholar] [CrossRef]
- Witt, C.S.; Goodridge, J.; Gerbase-DeLima, M.G.; Daher, S.; Christiansen, F.T. Maternal KIR repertoire is not associated with recurrent spontaneous abortion. Hum. Reprod. 2004, 19, 2653–2657. [Google Scholar] [CrossRef] [PubMed]
- Freitag, N.; Pour, S.J.; Fehm, T.N.; Toth, B.; Markert, U.R.; Weber, M.; Togawa, R.; Kruessel, J.-S.; Baston-Buest, D.M.; Bielfeld, A.P. Are uterine natural killer and plasma cells in infertility patients associated with endometriosis, repeated implantation failure, or recurrent pregnancy loss? Arch. Gynecol. Obstet. 2020, 302, 1487–1494. [Google Scholar] [CrossRef]
- Donoghue, J.F.; Paiva, P.; Teh, W.T.; Cann, L.M.; Nowell, C.; Rees, H.; Bittinger, S.; Obers, V.; Bulmer, J.N.; Stern, C.; et al. Endometrial UNK cell counts do not predict successful implantation in an IVF population. Hum. Reprod. 2019, 34, 2456–2466. [Google Scholar] [CrossRef] [PubMed]
- Coulam, C.B.; Roussev, R.G. Correlation of NK cell activation and inhibition markers with NK cytoxicity among women experiencing immunologic implantation failure after in vitro fertilization and embryo transfer. J. Assist. Reprod. Genet. 2003, 20, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, J.P.; Paiva, A.E.; Prazeres, P.H.D.M.; Guerra, D.A.P.; Silva, W.N.; Vaz, R.S.; Mintz, A.; Birbrair, A. The role of natural killer cells in the uterine microenvironment during pregnancy. Cell. Mol. Immunol. 2018, 15, 941–943. [Google Scholar] [CrossRef]
- Díaz-Hernández, I.; Alecsandru, D.; García-Velasco, J.A.; Domínguez, F. Uterine natural killer cells: From foe to friend in reproduction. Hum. Reprod. Update 2021, 27, 720–746. [Google Scholar] [CrossRef]
- Huhn, O.; Zhao, X.; Esposito, L.; Moffett, A.; Colucci, F.; Sharkey, A.M. How do uterine natural killer and innate lymphoid cells contribute to successful pregnancy? Front. Immunol. 2021, 12, 607669. [Google Scholar] [CrossRef]
| Publication | Study Design | Study Group | Control Group | Interventions | Examined Parameters | Major Findings |
|---|---|---|---|---|---|---|
| [54] | Controlled clinical study | 35 women with RIF after ET in IVF | 12 fertile women | Ultrasound evaluation and endometrial biopsy on day 20 | The balance between IL-12 and IL-18; the number of NK cells; and the vascular status | Distinct IL-12 and IL-18 patterns; significantly higher number of CD56 bright cells in patients with RIF |
| [17] | Pilot study | 37 women with unexplained RIF following ET in IVF | 8 fertile women | Ultrasonic evaluation and endometrial biopsy in luteal phase | Uterine artery Doppler; count of uterine CD56 bright cells/field; and quantification by real-time PCR to monitor IL-12 family, the IL-18 system (IL-18, IL-18R, IL18BP), and the IL-15 mRNA ratios. | Higher number of uNK CD56 bright cells/field in the preimplantation endometrium in RIF group; distinct IL-12/-15/-18 immune related mechanisms |
| [64] | Uncontrolled pilot study | 10 young (30–35 years old) women with unexplained RIF following ET in IVF | Data obtained from the literature | Endometrial biopsy >6 months following the last IVF cycle | The number of CD56bright uNK cells | The percentage of the uNK subpopulation of CD56+CD16– and CD56bright CD16– cells did not differ between RIF patients and normal human endometrium |
| [59] | Prospective observational study | 40 women with RIF | 15 women with no history of infertility | Endometrial biopsy | The number of CD56+, CD16+, and CD69+ cells in the unstimulated endometrium of women with RIF | CD56+ cell density was significantly higher in the RIF group; there was no significant difference in the densities of CD16+ and CD69+ cells |
| [67] | Case-control study | 20 women with IVF failure | Healthy control women: 36 normal multiparous women and 7 women with successful IVF | Peripheral blood sample collection; NK cell cytotoxicity level assessment via lactate dehydrogenase (LDH) release assay | compare the percentage of peripheral blood CD56(+) (CD56(dim) and CD56(bright)) cells and the level of NK cell cytotoxicity | The percentage of CD56(dim) cells and the level of peripheral blood NK cell cytotoxicity in women with IVF failure were significantly higher compared with the control group |
| Publication | Study Design | Study Group | Control Group | Interventions | Examined Parameters | Major Findings |
|---|---|---|---|---|---|---|
| [68] | Prospective study | Recurrent aborters | Normal | Endometrial biopsy in the secretory phase; assessment of endometrial leucocyte via two-color flow cytometric analysis | Immunophenotypic characteristics of endometrial leukocytes from nonpregnant recurrent aborters | Recurrent abortion group: lower levels of CD8+ T lymphocytes; increased CD4:CD8 ratio; increased proportion of CD20+ B leucocytes; increased CD16+CD56 dim uNK cells; and decreased CD16-CD56 bright uNK cell |
| [69] | Case-control | Abortion following IVF | Delivery following IVF | Peripheral blood samples to assess the expression of CD3, CD4, CD8, CD16, and CD56 using FACScan; analysis of NK cytotoxicity in blood sample via 51Cr Assay; endometrial samples to analyze the expression of CD16 and CD56 via FACScan | Expression levels of CD3, CD4, CD8, CD16, and CD56 in the peripheral blood; NK cytotoxicity in peripheral blood; expression levels of CD16 and CD56 in endometrial samples | Abortion group: higher levels of CD56+ and CD16+CD56+ cells in the peripheral blood on the day of ET; increased levels of CD16+CD56dim uNK cells and decreased levels of CD56bright uNK cells in endometrial samples |
| [71] | Case-control | Chromosomally normal abortion; chromosomally abnormal abortion | Selective termination of normal pregnancy | Peripheral blood samples as well as villi and decidual samples were samples | NK cell profile in peripheral blood samples as well as in decidual samples | Chromosomally normal abortions: lower levels of the decidual CD56+16-uNK cells; no difference regarding decidual CD56+16+ uNK cells |
| [72] | Case-control | 29 women with recurrent miscarriages | 10 parous women | Endometrial biopsies obtained in the luteal phase between days 7–10 following mid-cycle | Evaluation of the endometrial CD56+ cells | Increased mean numbers of CD56+ cells were documented in the endometrium of women with early RM |
| [79] | Prospective observational study | 22 patients with idiopathic recurrent miscarriage | 9 women with normal obstetric history | Mid-luteal phase endometrial biopsies | Profiling of endometrial leucocyte sub-populations | Higher number of CD4(+), CD8(+), CD14(+), CD16(+), and CD56(+) leukocytes in the RM group |
| [78] | Prospective observational study | 17 women with RM | 15 cases with male factor infertility | Endometrial sample collection during the peri-implantation period before subsequent pregnancy | Evaluation of natural killer (NK) cell markers, CD56 and CD16, a B-cell marker CD20, T-cell markers CD3 and CD8, and a specific T-helper (Th)2 and T-cytotoxic (Tc)2 marker | No significant difference in lymphocyte subset numbers or ratios was noted between the groups |
| [19] | A before and after study | 29 women with RM | 18 women attending for sterilization | Endometrial samples obtained on day 21+/-2; 20 mg oral prednisolone daily from day 1 to 21 of their menstrual cycle | Comparison of the percentage of stromal cells that were uNK between the groups prior and following prednisolone treatment of the RM group | Women with RM had significantly more uNK than the controls; prednisolone treatment significantly reduced the number of CD56 cells in the endometrium |
| [74] | Retrospective study | 87 women with unexplained RM | 10 normal control women | Biopsies obtained on days LH + 7 to LH + 9 | Comparison of uNK cell number between the two groups; comparison of uNK cell numbers between RM individuals achieving live-birth vs. experiencing miscarriage in a subsequent pregnancy | The number of uNK cells in the RM group was significantly higher than in the control women; no difference was observed in uNK numbers between 19 women who miscarried and 32 women who had a live-birth in a subsequent pregnancy |
| [7] | Prospective study | 28 women with recurrent pregnancy loss (RPL), 34 women with previous implantation failure | 74 healthy women | Endometrial uNK cells were obtained from the mid-secretory endometrium prior to infertility treatment; blood sampled collected at 12, 20, 28, and 36 gestational weeks (GW) from pregnant women with and without a history of RPL | Expression levels of natural cytotoxicity receptors (NCRs) (NKp46, NKp44, and NKp30) and cytokine production in NK cells derived from the uterine endometrium of women with RPL; expression levels of NCRs in peripheral blood NK cells in pregnant women with and without a history of RPL | The percentages of NKp46+ NK cells were significantly lower in both women with RPL and pregnant women with a history of RPL; the percentages of tumor necrosis factor-α- and/or interferon-γ-producing uterine endometrial NK cells were significantly lower in women with RPL compared with controls |
| Immunotherapies | Mechanisms of Action | Outcomes | Adverse Effects |
|---|---|---|---|
| Glucocorticoids | Regulate uNK cells’ proliferation and functionality via uNK cells’ glucocorticoid receptors | Decreased uNK cell numbers in endometrium | No pregnancy complications have been reported |
| Reduce abnormal high uNK cell numbers | Positive effect on endometrial immunological profile | In utero exposure to glucocorticoids may be associated with a higher incidence of preterm births and low birth weight | |
| Reduce abnormal high uNK cytotoxicity | No established beneficial effect on pregnancy outcomes | Mood alterations, headache, nausea | |
| Intralipid therapy | Intralipid molecules act as ligands for the G-protein-coupled receptor that results in activating the cAMP signaling pathway that is associated with the NFkB pathway | Modulates abnormal uNK activity | No side effects have been reported in cases of young women with reproductive failure |
| NFkB pathway ultimately modulates transcription of DNA and controls essential immune responses | Trigger uNK cells’ cytokine secretion | Reduced risk of teratogenesis and congenital abnormalities has been reported | |
| Reduce abnormal high uNK cytotoxicity | No established beneficial effect on pregnancy outcomes | Risk for thrombophlebitis, dyspnea, nausea, hyperlipemia, and allergic reactions | |
| Intravenous immunoglobulin (IVIg) | IVIgs mitigate the function of NK cells and promote alterations in the cytokine production | IVIgs decrease elevated NK levels in peripheral blood | Data advocating safe use of IVIg prior to or during pregnancy are still missing |
| IVIgs inhibit cytotoxic activity of NK cells both in vitro and in vivo | No evidence concerning implementation of IVIg therapy in mitigating the effects of uNK cells has been reported | Anaphylactic reactions and renal insufficiency have been reported in high-dose IVIg treatments | |
| IVIgs induce spontaneous degranulation of NK cells and promote IFNγ production that results in the exhaustion of the NK cell cytotoxic machinery | No significant effect of IVIgs in patients with RM in terms of live birth rate has been reported | Fever, myalgia, headache, fatigue constitute some of the mild side effects. In extremely severe cases, myocardial infarction, alopecia, thrombosis, hemolytic anemia, and aseptic meningitis have been reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfakianoudis, K.; Rapani, A.; Grigoriadis, S.; Pantou, A.; Maziotis, E.; Kokkini, G.; Tsirligkani, C.; Bolaris, S.; Nikolettos, K.; Chronopoulou, M.; et al. The Role of Uterine Natural Killer Cells on Recurrent Miscarriage and Recurrent Implantation Failure: From Pathophysiology to Treatment. Biomedicines 2021, 9, 1425. https://doi.org/10.3390/biomedicines9101425
Sfakianoudis K, Rapani A, Grigoriadis S, Pantou A, Maziotis E, Kokkini G, Tsirligkani C, Bolaris S, Nikolettos K, Chronopoulou M, et al. The Role of Uterine Natural Killer Cells on Recurrent Miscarriage and Recurrent Implantation Failure: From Pathophysiology to Treatment. Biomedicines. 2021; 9(10):1425. https://doi.org/10.3390/biomedicines9101425
Chicago/Turabian StyleSfakianoudis, Konstantinos, Anna Rapani, Sokratis Grigoriadis, Agni Pantou, Evangelos Maziotis, Georgia Kokkini, Chrysanthi Tsirligkani, Stamatis Bolaris, Konstantinos Nikolettos, Margarita Chronopoulou, and et al. 2021. "The Role of Uterine Natural Killer Cells on Recurrent Miscarriage and Recurrent Implantation Failure: From Pathophysiology to Treatment" Biomedicines 9, no. 10: 1425. https://doi.org/10.3390/biomedicines9101425
APA StyleSfakianoudis, K., Rapani, A., Grigoriadis, S., Pantou, A., Maziotis, E., Kokkini, G., Tsirligkani, C., Bolaris, S., Nikolettos, K., Chronopoulou, M., Pantos, K., & Simopoulou, M. (2021). The Role of Uterine Natural Killer Cells on Recurrent Miscarriage and Recurrent Implantation Failure: From Pathophysiology to Treatment. Biomedicines, 9(10), 1425. https://doi.org/10.3390/biomedicines9101425








