Association of Galectin 9 Expression with Immune Cell Infiltration, Programmed Cell Death Ligand-1 Expression, and Patient’s Clinical Outcome in Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of TNBC Patients and Tissue Microarray Construction
2.2. Evaluation of Stromal Tumor-Infiltrating Lymphocytes
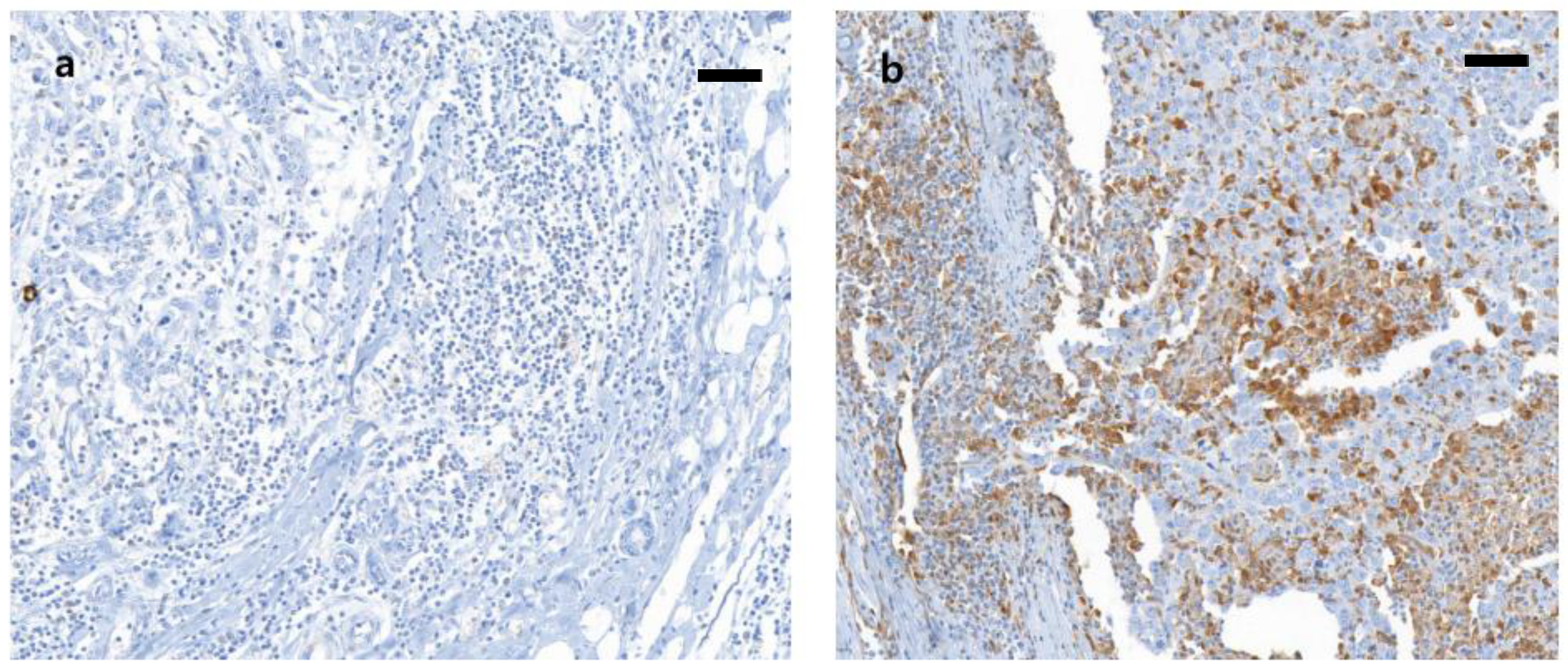
2.3. Immunohistochemistry and Scoring
2.4. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics
3.2. Galectin-9 Expression and its Correlation with Clinical and Pathologic Characteristics and Immune Status
3.3. Correlation between Galectin-9 Expression and PD-L1 Expression and sTIL Level
3.4. Prognostic Significance of Galectin-9 Expression and its Association with Immune Status in TNBC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dobosz, P.; Dzieciątkowski, T. The intriguing history of cancer immunotherapy. Front. Immunol. 2019, 10, 2965. [Google Scholar] [CrossRef]
- Dighe, A.S.; Richards, E.; Old, L.J.; Schreiber, R.D. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity 1994, 1, 447–456. [Google Scholar] [CrossRef]
- Smyth, M.J.; Thia, K.Y.; Street, S.E.; MacGregor, D.; Godfrey, D.I.; Trapani, J.A. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J. Exp. Med. 2000, 192, 755–760. [Google Scholar] [CrossRef]
- Shankaran, V.; Ikeda, H.; Bruce, A.T.; White, J.M.; Swanson, P.E.; Old, L.J.; Schreiber, R.D. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001, 410, 1107–1111. [Google Scholar] [CrossRef]
- Stanton, S.E.; Adams, S.; Disis, M.L. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: A systematic review. JAMA Oncol. 2016, 2, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.A.; Dees, E.C.; Sawyer, L.; Gatti, L.; Moore, D.T.; Collichio, F.; Ollila, D.W.; Sartor, C.I.; Graham, M.L.; Perou, C.M. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin. Cancer Res. 2007, 13, 2329–2334. [Google Scholar] [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Denkert, C.; Liedtke, C.; Tutt, A.; von Minckwitz, G. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet 2017, 389, 2430–2442. [Google Scholar] [CrossRef]
- Kieber-Emmons, T.; Kohler, H. Evolutionary origin of autoreactive determinants (autogens). Proc. Natl. Acad. Sci. USA 1986, 83, 2521–2525. [Google Scholar] [CrossRef]
- Kurozumi, S.; Matsumoto, H.; Kurosumi, M.; Inoue, K.; Fujii, T.; Horiguchi, J.; Shirabe, K.; Oyama, T.; Kuwano, H. Prognostic significance of tumour-infiltrating lymphocytes for oestrogen receptor-negative breast cancer without lymph node metastasis. Oncol. Lett. 2019, 17, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.Z.; Sarian, L.O.; Derchain, S.F.; Pinto, G.A.; Vassallo, J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum. Pathol. 2016, 47, 78–84. [Google Scholar] [CrossRef]
- Li, Z.; Dong, P.; Ren, M.; Song, Y.; Qian, X.; Yang, Y.; Li, S.; Zhang, X.; Liu, F. PD-L1 expression is associated with tumor FOXP3+ regulatory T-cell infiltration of breast cancer and poor prognosis of patient. J. Cancer 2016, 7, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Oner, G.; Onder, S.; Karatay, H.; Tukenmez, M.; Muslumanoglu, M.; Igci, A.; Dinccag, A.; Ozmen, V.; Aydiner, A.; Yavuz, E.; et al. High expression of pdl-1 in patients with triple negative breast cancer with residual tumor burden after neoadjuvant chemotherapy. J. Clin. Oncol. 2018, 36, e24136. [Google Scholar] [CrossRef]
- Makhoul, I.; Atiq, M.; Alwbari, A.; Kieber-Emmons, T. Breast cancer immunotherapy: An update. Breast Cancer 2018, 12, 1178223418774802. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Approves Atezolizumab for PD-L1 Positive Unresectable Locally Advanced or Metastatic Triple-Negative Breast Cancer. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative (accessed on 8 March 2019).
- Merani, S.; Chen, W.; Elahi, S. The bitter side of sweet: The role of Galectin-9 in immunopathogenesis of viral infections. Rev. Med. Virol. 2015, 25, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Elahi, S.; Dinges, W.L.; Lejarcegui, N.; Laing, K.J.; Collier, A.C.; Koelle, D.M.; McElrath, M.J.; Horton, H. Protective HIV-specific CD8+ T cells evade Treg cell suppression. Nat. Med. 2011, 17, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, S.; Dunsmore, G.; Koleva, P.; Xu, L.; Houston, S.; Elahi, S. Galectin-9 and VISTA expression define terminally exhausted T cells in HIV-1 infection. J. Immunol. 2020, 204, 2474–2491. [Google Scholar] [CrossRef]
- Kageshita, T.; Kashio, Y.; Yamauchi, A.; Seki, M.; Abedin, M.J.; Nishi, N.; Shoji, H.; Nakamura, T.; Ono, T.; Hirashima, M. Possible role of galectin-9 in cell aggregation and apoptosis of human melanoma cell lines and its clinical significance. Int. J. Cancer 2002, 99, 809–816. [Google Scholar] [CrossRef]
- Jiang, J.; Jin, M.S.; Kong, F.; Cao, D.; Ma, H.X.; Jia, Z.; Wang, Y.P.; Suo, J.; Cao, X. Decreased Galectin-9 and increased Tim-3 expression are related to poor prognosis in gastric cancer. PLoS ONE 2013, 8, e81799. [Google Scholar] [CrossRef]
- Sideras, K.; Biermann, K.; Verheij, J.; Takkenberg, B.R.; Mancham, S.; Hansen, B.E.; Schutz, H.M.; De Man, R.A.; Sprengers, D.; Buschow, S.I.; et al. PD-L1, Galectin-9 and CD8+ tumor-infiltrating lymphocytes are associated with survival in hepatocellular carcinoma. Oncoimmunology 2017, 6, e1273309. [Google Scholar] [CrossRef]
- Ohue, Y.; Kurose, K.; Nozawa, R.; Isobe, M.; Nishio, Y.; Tanaka, T.; Doki, Y.; Hori, T.; Fukuoka, J.; Oka, M.; et al. Survival of lung adenocarcinoma patients predicted from expression of PD-L1, Galectin-9, and XAGE1 (GAGED2a) on tumor cells and tumor-infiltrating T cells. Cancer Immunol. Res. 2016, 4, 1049–1060. [Google Scholar] [CrossRef]
- Hamada, T.; Kawano, Y.; Szczecinska, W.; Wozniak, K.; Yasumoto, S.; Kowalewski, C.; Hashimoto, T. Novel keratin 5 and 14 gene mutations in patients with epidermolysis bullosa simplex from Poland. Arch. Dermatol. Res. 2005, 296, 577–579. [Google Scholar] [CrossRef]
- Clayton, K.L.; Haaland, M.S.; Douglas-Vail, M.B.; Mujib, S.; Chew, G.M.; Ndhlovu, L.C.; Ostrowski, M.A. T cell Ig and mucin domain-containing protein 3 is recruited to the immune synapse, disrupts stable synapse formation, and associates with receptor phosphatases. J. Immunol. 2014, 192, 782–791. [Google Scholar] [CrossRef]
- Byun, K.D.; Hwang, H.J.; Park, K.J.; Kim, M.C.; Cho, S.H.; Ju, M.H.; Lee, J.H.; Jeong, J.S. T-cell immunoglobulin mucin 3 expression on tumor infiltrating lymphocytes as a positive prognosticator in triple-negative breast cancer. J. Breast Cancer 2018, 21, 406–414. [Google Scholar] [CrossRef]
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch. Pathol. Lab. Med. 2010, 134, 907–922. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American society of clinical oncology/college of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch. Pathol. Lab. Med. 2007, 131, 18–43. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. Arch. Pathol. Lab. Med. 2018, 142, 1364–1382. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.R.; Kim, H.J.; Jang, M.H.; Park, S.Y. Prognostic value of tumor infiltrating lymphocyte subsets in breast cancer depends on hormone receptor status. Breast Cancer Res. Treat. 2017, 161, 409–420. [Google Scholar] [CrossRef]
- Grosset, A.A.; Labrie, M.; Vladoiu, M.C.; Yousef, E.M.; Gaboury, L.; St-Pierre, Y. Galectin signatures contribute to the heterogeneity of breast cancer and provide new prognostic information and therapeutic targets. Oncotarget 2016, 7, 18183–18203. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.I.; Seo, K.W.; Kook, M.C.; Kim, C.G.; Kim, Y.W.; Cho, S.J. Prognostic value of tumoral expression of Galectin-9 in gastric cancer. Turk. J. Gastroenterol. 2017, 28, 166–170. [Google Scholar] [CrossRef]
- Punt, S.; Thijssen, V.L.; Vrolijk, J.; De Kroon, C.D.; Gorter, A.; Jordanova, E.S. Galectin-1, -3 and -9 expression and clinical significance in squamous cervical cancer. PLoS ONE 2015, 10, e0129119. [Google Scholar] [CrossRef]
- Erber, R.; Hartmann, A. Understanding PD-L1 testing in breast cancer: A practical approach. Breast Care 2020, 15, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Kwa, M.J.; Adams, S. Checkpoint inhibitors in triple-negative breast cancer (TNBC): Where to go from here. Cancer 2018, 124, 2086–2103. [Google Scholar] [CrossRef]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef]
- Yang, R.Y.; Rabinovich, G.A.; Liu, F.T. Galectins: Structure, function and therapeutic potential. Expert Rev. Mol. Med. 2008, 10, e17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Huang, D.J.; Baloche, V.; Zhang, L.; Xu, J.X.; Li, B.W.; Zhao, X.R.; He, J.; Mai, H.Q.; Chen, Q.Y.; et al. Galectin-9 promotes a suppressive microenvironment in human cancer by enhancing STING degradation. Oncogenesis 2020, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, S.; Reddy, P.B.; Rajasagi, N.; Suryawanshi, A.; Hirashima, M.; Rouse, B.T. Galectin-9/TIM-3 interaction regulates virus-specific primary and memory CD8 T cell response. PLoS Pathog. 2010, 6, e1000882. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, A.; Tsukada, J.; Mizobe, T.; Higashi, T.; Mouri, F.; Tanikawa, R.; Yamauchi, A.; Hirashima, M.; Tanaka, Y. Intracellular Galectin-9 activates inflammatory cytokines in monocytes. Genes Cells 2009, 14, 511–521. [Google Scholar] [CrossRef]
- Yamauchi, A.; Dai, S.Y.; Nakagawa, R.; Kashio, Y.; Abe, H.; Katoh, S.; Kontani, K.; Hirashima, M. Galectin-9 induces maturation of human monocyte-derived dendritic cells. Nihon Rinsho Meneki Gakkai Kaishi 2005, 28, 381–388. [Google Scholar] [CrossRef][Green Version]
- Irie, A.; Yamauchi, A.; Kontani, K.; Kihara, M.; Liu, D.; Shirato, Y.; Seki, M.; Nishi, N.; Nakamura, T.; Yokomise, H.; et al. Galectin-9 as a prognostic factor with antimetastatic potential in breast cancer. Clin. Cancer Res. 2005, 11, 2962–2968. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Dong, J.H.; Chen, Y.W.; Wang, X.Q.; Li, C.H.; Wang, J.; Wang, G.Q.; Li, H.L.; Wang, X.D. Galectin-9 acts as a prognostic factor with antimetastatic potential in hepatocellular carcinoma. Asian Pac. J. Cancer Prev. 2012, 13, 2503–2509. [Google Scholar] [CrossRef] [PubMed]
- Soran, A.; Ozmen, V.; Ozbas, S.; Karanlik, H.; Muslumanoglu, M.; Igci, A.; Canturk, Z.; Utkan, Z.; Ozaslan, C.; Evrensel, T.; et al. Randomized trial comparing resection of primary tumor with no surgery in stage IV breast cancer at presentation: Protocol MF07-01. Ann. Surg. Oncol. 2018, 25, 3141–3149. [Google Scholar] [CrossRef]
- Mori, H.; Kubo, M.; Yamaguchi, R.; Nishimura, R.; Osako, T.; Arima, N.; Okumura, Y.; Okido, M.; Yamada, M.; Kai, M.; et al. The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget 2017, 8, 15584–15592. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Hu-Lieskovan, S. What does PD-L1 positive or negative mean? J. Exp. Med. 2016, 213, 2835–2840. [Google Scholar] [CrossRef]
- Knudsen, A.M.; Rudkjobing, S.J.; Sorensen, M.D.; Dahlrot, R.H.; Kristensen, B.W. Expression and prognostic value of the immune checkpoints Galectin-9 and PD-L1 in glioblastomas. J. Neuropathol. Exp. Neurol. 2021, 80, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, J.; Ma, C.; Gao, W.; Song, B.; Xue, H.; Chen, W.; Chen, X.; Zhang, Y.; Shao, Q.; et al. Reduced expression of Galectin-9 contributes to a poor outcome in colon cancer by inhibiting NK cell chemotaxis partially through the Rho/ROCK1 signaling pathway. PLoS ONE 2016, 11, e0152599. [Google Scholar] [CrossRef]
- Liang, S.C.; Latchman, Y.E.; Buhlmann, J.E.; Tomczak, M.F.; Horwitz, B.H.; Freeman, G.J.; Sharpe, A.H. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur. J. Immunol 2003, 33, 2706–2716. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple. negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]

| Clinicopathologic Characteristics | Number (%) |
|---|---|
| Age (years) | |
| Median (range) | 50 (30–74) |
| T stage | |
| T1 | 47 (40.5) |
| T2 | 55 (50.5) |
| T3 | 7 (6.4) |
| N stage | |
| N0 | 74 (67.9) |
| N1 | 16 (14.7) |
| N2 | 6 (5.5) |
| N3 | 13 (11.9) |
| Histologic grade | |
| 1 | 4 (3.7) |
| 2 | 21 (19.3) |
| 3 | 84 (77.1) |
| EIC | |
| Absent | 96 (88.1) |
| Present | 13 (11.9) |
| Lymphovascular invasion | |
| Absent | 83 (76.1) |
| Present | 26 (23.9) |
| Ki-67 labeling index | |
| <20% | 28 (25.7) |
| ≥20% | 81 (74.3) |
| Stromal TIL percentage | |
| 0–10% | 18 (16.5) |
| 11–40% | 44 (40.4) |
| ≥40% | 47 (43.1) |
| PD-L1 expression in tumor cells | |
| <1% | 54 (49.5) |
| ≥1% | 55 (50.5) |
| PD-L1 expression in lymphocytes | |
| <1% | 67 (61.5) |
| ≥1% | 42 (38.5) |
| P53 overexpression | |
| Absent | 46 (42.2) |
| Present | 63 (57.8) |
| Adjuvant therapy | |
| Chemotherapy | 107 (99.1) |
| Radiation | 82 (75.2) |
| Variable | Gal-9 Expression | p Value | |
|---|---|---|---|
| Low | High | ||
| Age | |||
| <50 years | 11 (50%) | 42 (48.3) | 0.885 |
| ≥50 years | 11 (50%) | 45 (51.7) | |
| pT stage | |||
| T1 | 5 (22.7) | 42 (48.3) | 0.031 |
| T2–3 | 17 (77.3) | 45 (51.7) | |
| pN stage | |||
| N0 | 13 (59.1) | 61 (70.1) | 0.322 |
| N1–3 | 9 (40.9) | 26 (29.9) | |
| Histologic grade | |||
| 1–2 | 6 (27.3) | 19 (21.8) | 0.588 |
| 3 | 16 (72.7) | 68 (78.2) | |
| Ki-67 labeling index | 0.067 | ||
| <20% | 9 (40.9) | 19 (21.8) | |
| ≥20% | 13 (59.1) | 68 (78.2) | |
| Extensive intraductal component | 0.081 | ||
| Absent | 17 (77.3) | 79 (90.8) | |
| Present | 5 (22.7) | 8 (9.2) | |
| Lymphovascular invasion | 0.008 | ||
| Absent | 12 (54.5) | 71 (81.6) | |
| Present | 10 (45.5) | 16 (18.4) | |
| P53 overexpression | 0.270 | ||
| Absent | 7 (31.8) | 39 (44.8) | |
| Present | 15 (68.2) | 48 (55.2) | |
| Variable | Gal-9 Expression | p Value | |
|---|---|---|---|
| Low | High | ||
| Level of sTIL | 0.011 | ||
| Low | 7 (31.8) | 11 (12.6) | |
| Intermediate | 11 (50.0) | 33 (37.9) | |
| High | 4 (18.2) | 43 (49.4) | |
| PD-L1 expression in lymphocytes | 0.001 | ||
| <1% | 20 (90.9) | 47 (54.0) | |
| ≥1% | 2 (9.1) | 40 (46.0) | |
| PD-L1 expression in tumor cells | 0.004 | ||
| <1% | 17 (77.3) | 37 (42.5) | |
| ≥1% | 5 (22.7) | 50 (57.5) | |
| Disease-Free Survival | Univariate Analysis | Multivariate Analysis | |||
| Variable | Category | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Age | ≥50 years vs. <50 years | 0.564–2.556 | 0.634 | ||
| T stage | T2-3 vs. T1 | 1.072–6.002 | 0.034 | 1.107–6.970 | 0.024 |
| N stage | N1-N3 vs. N0 | 1.111–5.034 | 0.026 | 1.042–5.749 | 0.011 |
| Histologic grade | III vs. I and II | 0.442–2.716 | 0.842 | ||
| LVI | Present vs. absent | 0.677–3.537 | 0.301 | ||
| sTIL level | High vs. low to intermediate | 0.339–1.618 | 0.451 | ||
| PD-L1 expression in tumor cells | Positive vs. negative | 0.410–1.854 | 0.721 | ||
| PD-L1 expression in lymphocytes | Positive vs. negative | 0.502–2.330 | 0.842 | ||
| Ki-67 labeling index | High vs. low | 1.055–10.610 | 0.041 | 1.055–10.410 | 0.047 |
| Gal-9 expression | High vs. low | 0.276–1.544 | 0.332 | ||
| Overall Survival | Univariate Analysis | Multivariate Analysis | |||
| Variable | Category | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Age | 50 years vs. <50 years | 0.486–2.602 | 0.784 | ||
| T stage | T2-3 vs. T1 | 2.071–37.944 | 0.003 | 2.144–37.279 | 0.004 |
| N stage | N1-N3 vs. N0 | 1.551–8.508 | 0.003 | 1.783–9.503 | 0.001 |
| Histologic grade | III vs. I and II | 0.471–4.113 | 0.551 | ||
| LVI | Present vs. absent | 0.711–4.285 | 0.224 | ||
| sTIL level | High vs. low to intermediate | 0.702–4.224 | 0.235 | ||
| PD-L1 expression in tumor cells | Positive vs. negative | 0.472–2.510 | 0.843 | ||
| PD-L1 expression in lymphocytes | Positive vs. negative | 0.285–1.717 | 0.436 | ||
| Ki-67 labeling index | High vs. low | 0.699–7.981 | 0.167 | 1.020–11.986 | 0.043 |
| Gal-9 expression | High vs. low | 0.241–1.577 | 0.313 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, M.-H.; Byun, K.-D.; Park, E.-H.; Lee, J.-H.; Han, S.-H. Association of Galectin 9 Expression with Immune Cell Infiltration, Programmed Cell Death Ligand-1 Expression, and Patient’s Clinical Outcome in Triple-Negative Breast Cancer. Biomedicines 2021, 9, 1383. https://doi.org/10.3390/biomedicines9101383
Ju M-H, Byun K-D, Park E-H, Lee J-H, Han S-H. Association of Galectin 9 Expression with Immune Cell Infiltration, Programmed Cell Death Ligand-1 Expression, and Patient’s Clinical Outcome in Triple-Negative Breast Cancer. Biomedicines. 2021; 9(10):1383. https://doi.org/10.3390/biomedicines9101383
Chicago/Turabian StyleJu, Mi-Ha, Kyung-Do Byun, Eun-Hwa Park, Jin-Hwa Lee, and Song-Hee Han. 2021. "Association of Galectin 9 Expression with Immune Cell Infiltration, Programmed Cell Death Ligand-1 Expression, and Patient’s Clinical Outcome in Triple-Negative Breast Cancer" Biomedicines 9, no. 10: 1383. https://doi.org/10.3390/biomedicines9101383
APA StyleJu, M.-H., Byun, K.-D., Park, E.-H., Lee, J.-H., & Han, S.-H. (2021). Association of Galectin 9 Expression with Immune Cell Infiltration, Programmed Cell Death Ligand-1 Expression, and Patient’s Clinical Outcome in Triple-Negative Breast Cancer. Biomedicines, 9(10), 1383. https://doi.org/10.3390/biomedicines9101383






