Pharmacotherapy in COVID 19: Potential Impact of Targeting the Complement System
Abstract
1. Introduction
2. Search Strategy and Selection Criteria
3. Coronaviruses
4. The Complement System
5. COVID-19 Clinical Presentation and Progression
6. Current Treatments under Investigation
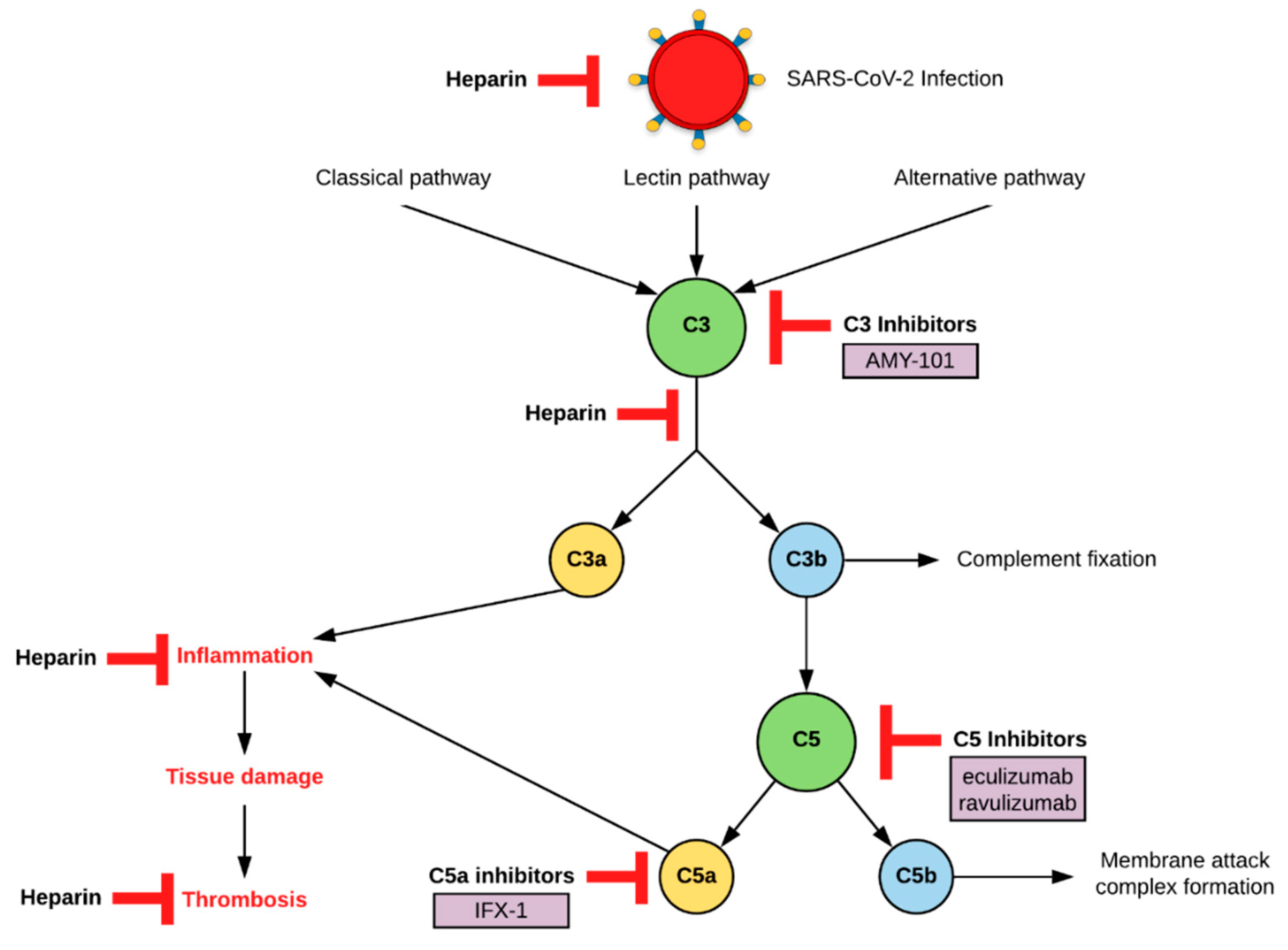
7. Complement as a Therapeutic Target
7.1. C3 Inhibition
7.2. C5 Inhibition
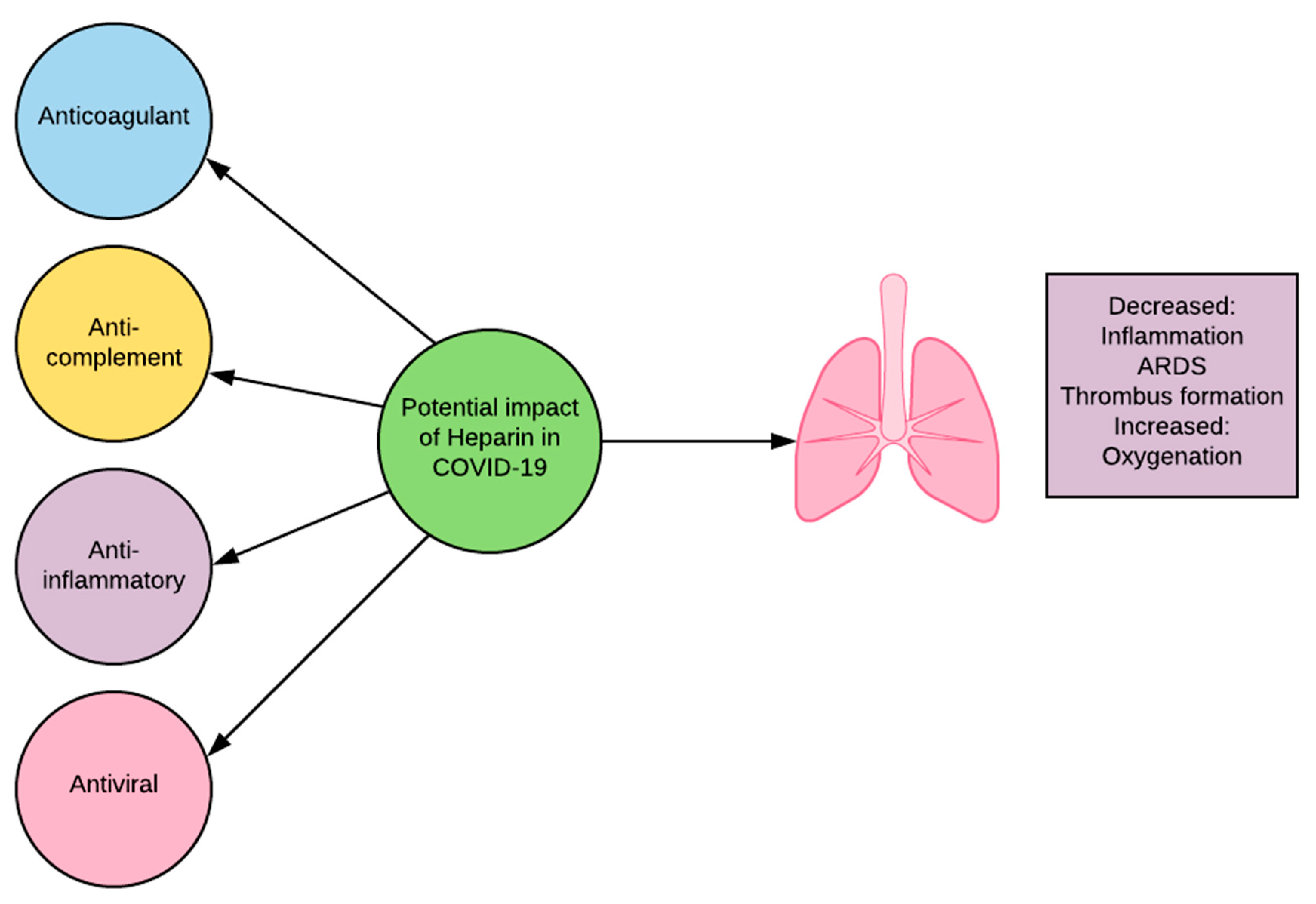
7.3. Heparin
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 17 July 2020).
- World Health Organization. Clinical Management of COVID-19: Intermin Guidance. Available online: https://www.who.int/publications/i/item/clinical-management-of-covid-19 (accessed on 18 June 2020).
- Coronavirus Cases Worldometer. Available online: https://www.worldometers.info/coronavirus/?utm_campaign=homeAdUOA?Si (accessed on 4 September 2020).
- Zafar, H. The Microbiology of Coronaviruses. J. Pak. Med. Assoc. 2020, 70, S44–S47. [Google Scholar] [CrossRef] [PubMed]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Parham, P. The Immune System, 4th ed.; Garland Science: New York, NY, USA, 2014. [Google Scholar]
- Riedl, M.; Fakhouri, F.; Le Quintrec, M.; Noone, D.G.; Jungraithmayr, T.C.; Fremeaux-Bacchi, V.; Licht, C. Abstract: Spectrum of complement-mediated thrombotic microangiopathies: Pathogenetic insights identifying novel treatment approaches. Semin. Thromb. Hemost. 2014, 40, 444–464. [Google Scholar] [CrossRef] [PubMed]
- Risitano, A.M.; Mastellos, D.C.; Huber-Lang, M.; Yancopoulou, D.; Garlanda, C.; Ciceri, F.; Lambris, J.D. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020, 20, 343–344. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yuan, X.; Chen, H.; Chaturvedi, S.; Braunstein, E.M.; Brodsky, R.A. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor d inhibition. Blood 2020, 136, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Gralinski, L.E.; Sheahan, T.P.; Morrison, T.E.; Menachery, V.D.; Jensen, K.; Leist, S.R.; Whitmore, A.; Heise, M.T.; Baric, R.S. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. MBio 2018, 9, e01753-18. [Google Scholar] [CrossRef]
- Gao, T.; Hu, M.; Zhang, X.; Li, H.; Zhu, L.; Liu, H.; Dong, Q.; Zhang, Z.; Wang, Z.; Hu, Y. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxiv 2020. [Google Scholar] [CrossRef]
- Mastaglio, S.; Ruggeri, A.; Risitano, A.M.; Angelillo, P.; Yancopoulou, D.; Mastellos, D.C.; Huber-Lang, M.; Piemontese, S.; Assanelli, A.; Garlanda, C.; et al. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin. Immunol. 2020, 215, 108450. [Google Scholar] [CrossRef]
- Prompetchara, E.; Ketloy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1–9. [Google Scholar]
- Centers for Disease Control. Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html (accessed on 18 June 2020).
- Gao, J.; Tian, Z.; Yang, X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends 2020, 14, 72–73. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.J.; Bergeron, E.; Benjannet, S.; Erickson, B.R.; Rollin, P.E.; Ksiazek, T.G.; Seidah, N.G.; Nichol, S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Borba, M.G.S.; Val, F.F.A.; Sampaio, V.S.; Alexandre, M.A.A.; Melo, G.C.; Brito, M.; Mourao, M.P.G.; Brito-Sousa, J.D.; Baia-da-Silva, D.; Guerra, M.V.F.; et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: A randomized clinical trial. JAMA Netw. Open 2020, 3, e208857. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Tang, T.; Pang, P.; Li, M.; Ma, R.; Lu, J.; Shu, J.; You, Y.; Chen, B.; Liang, J.; et al. Treating COVID-19 with chloroquine. J. Mol. Cell Biol. 2020, 12, 322–325. [Google Scholar] [CrossRef]
- Magagnoli, J.; Narendran, S.; Pereira, F.; Cummings, T.; Hardin, J.W.; Sutton, S.S.; Ambati, J. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Tang, W.; Cao, Z.; Han, M.; Wang, Z.; Chen, J.; Sun, W.; Wu, Y.; Xiao, W.; Liu, S.; Chen, E.; et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: Open label, randomised controlled trial. BMJ 2020, 369, m1849. [Google Scholar] [CrossRef]
- Mahevas, M.; Tran, V.T.; Roumier, M.; Chabrol, A.; Paule, R.; Guillaud, C.; Fois, E.; Lepeule, R.; Szwebel, T.A.; Lescure, F.X.; et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: Observational comparative study using routine care data. BMJ 2020, 369, m1844. [Google Scholar] [CrossRef]
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic efficacy of the small molecule GS-5734 against ebola virus in rhesus monkeys. Nature 2016, 531, 381–385. [Google Scholar] [CrossRef]
- National Institutes of Health. NIH Clinical Trial Shows Remdesivir Accelerates Recovery from Advanced COVID-19. Available online: https://www.nih.gov/news-events/news-releases/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19 (accessed on 17 July 2020).
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.X.; et al. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef]
- Marccuci, C.; Sandson, N.; Gierl, B.T.; Nicholson, W.T. Favipiravir and remdesivir Appear to Be Relatively Unencumbered with Drug-Drug Interactions. Available online: https://www.apsf.org/article/favipiravir-and-remdesivir-appear-to-be-relatively-unencumbered-with-drug-drug-interactions/ (accessed on 18 June 2020).
- Coomes, E.A.; Haghbayan, H. Favipiravir, an antiviral for COVID-19? J. Antimicrob. Chemother. 2020, 75, 2013–2014. [Google Scholar] [CrossRef]
- Cai, Q.; Yang, M.; Liu, D.; Chen, J.; Shu, D.; Xia, J.; Liao, X.; Gu, Y.; Cai, Q.; Yang, Y.; et al. Experimental treatment with favipiravir for COVID-19: An open-label control study. Engineering 2020, 6, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Nukoolkarn, V.; Lee, V.S.; Malaisree, M.; Aruksakulwong, O.; Hannongbua, S. Molecular dynamic simulations analysis of ritonavir and lopinavir as SARS-CoV 3CL(pro) inhibitors. J. Theor. Biol. 2008, 254, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, X.; Xin, Q.; Pan, Y.; Hu, Y.; Li, J.; Chu, Y.; Feng, Y.; Wang, Q. Neutralizing antibodies responses to SARS-CoV-2 in COVID-19 inpatients and convalescent patients. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Narick, C.; Triulzi, D.J.; Yazer, M.H. Transfusion-associated circulatory overload after plasma transfusion. Transfusion 2012, 52, 160–165. [Google Scholar] [CrossRef]
- Shao, Z.; Feng, Y.; Zhong, L.; Xie, Q.; Lei, M.; Liu, Z.; Wang, C.; Ji, J.; Li, W.; Liu, H. Clinical efficacy of intravenous immunoglobulin therapy in critical patients with COVID-19: A multicenter retrospective cohort study. medRxiv 2020. [Google Scholar] [CrossRef]
- Spiegel, M.; Pichlmair, A.; Mühlberger, E.; Haller, O.; Weber, F. The antiviral effect of interferon-beta against SARS-coronavirus is not mediated by MxA protein. J. Clin. Virol. 2004, 30, 211–213. [Google Scholar] [CrossRef]
- Food and Drug Administration. Interferon alfa-2b (INTRON A). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103132Orig1s5199lbl.pdf (accessed on 17 July 2020).
- Food and Drug Administration. Peginterferon alfa-2a (PEGASYS). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/103964s5270lbl.pdf (accessed on 17 July 2020).
- Haji Abdolvahab, M.; Mofrad, M.R.; Schellekens, H. Interferon beta: From molecular level to therapeutic effects. Int. Rev. Cell. Mol. Biol. 2016, 326, 343–372. [Google Scholar]
- Food and Drug Administration. Interferon beta-1a (REBIF). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/103780s5204lbl.pdf (accessed on 17 July 2020).
- Shalhoub, S.; Farahat, F.; Al-Jiffri, A.; Simhairi, R.; Shamma, O.; Siddiqi, N.; Mushtaq, A. IFN-alpha2a or IFN-beta1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: A retrospective study. J. Antimicrob. Chemother. 2015, 70, 2129–2132. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Asiri, A.Y.; Assiri, A.M.; Aziz Jokhdar, H.A.; Alothman, A.; Balkhy, H.H.; Al Johani, S.; Al Harbi, S.; Kojan, S.; Al Jeraisy, M.; et al. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-beta1b (MIRACLE trial): Statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials 2020, 21, 8. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Shalhoub, S.; Mandourah, Y.; Al-Hameed, F.; Al-Omari, A.; Al Qasim, E.; Jose, J.; Alraddadi, B.; Almotairi, A.; Al Khatib, K.; et al. Ribavirin and interferon therapy for critically ill patients with Middle East respiratory syndrome: A multicenter observational study. Clin. Infect. Dis. 2020, 70, 1837–1844. [Google Scholar] [CrossRef]
- Zumla, A.; Chan, J.F.; Azhar, E.I.; Hui, D.S.; Yuen, K.Y. Coronaviruses—Drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016, 15, 327–347. [Google Scholar] [CrossRef]
- Schofield, A. Synairgen to Start Trial of SNG001 in COVID-19. Available online: https://pharmafield.co.uk/pharma_news/synairgen-to-start-trial-ofsng001-in-covid-19/ (accessed on 18 June 2020).
- Davis, N. Trial of Covid-19 Drug Given via Inhaler ‘Very Promising’, Say Scientists. Available online: https://www.theguardian.com/world/2020/jul/20/trial-of-covid-19-coronavirus-drug-given-via-inhaler-sng001-very-promising-say-scientists (accessed on 23 July 2020).
- Food and Drug Administration. Baricitinib (OLUMIANT). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207924s001lbl.pdf (accessed on 17 July 2020).
- Richardson, P.; Griffin, I.; Tucker, C.; Smith, D.; Oechsle, O.; Phelan, A.; Stebbing, J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020, 395, e30–e31. [Google Scholar] [CrossRef]
- Food and Drug Administration. Sarilumab (KEVZARA). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761037s001lbl.pdf. (accessed on 17 July 2020).
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. SYLVANT (Siltuximab). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125496s018lbl.pdf (accessed on 17 July 2020).
- Regeneron and Sanofi Provide Update on U.S. Phase 2/3 adaptive-Designed Trial of KEVZARA® (Sarilumab) in Hospitalized COVID-19 Patients. Available online: https://investor.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-provide-update-us-phase-23-adaptive (accessed on 18 June 2020).
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef] [PubMed]
- Oxford University. Low-cost Dexamethasone Reduces Death by Up to One Third in Hospitalised Patients with Severe Respiratory Complications of COVID-19. Available online: https://www.recoverytrial.net/files/recovery_dexamethasone_statement_160620_v2final.pdf (accessed on 18 June 2020).
- Ricklin, D.; Lambris, J.D. Compstatin: A complement inhibitor on its way to clinical application. In Current Topics in Complement II.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 262–281. [Google Scholar]
- The Sidney Kimmel Comprehensive Cancer Center. Paroxysmal nocturnal hemoglobinuria (PNH). Available online: https://www.hopkinsmedicine.org/kimmel_cancer_center/types_cancer/paroxysmal_nocturnal_hemoglobinuria_PNH.html (accessed on 17 July 2020).
- Dubois, E.A.; Cohen, A.F. Eculizumab. Br. J. Clin. Pharmacol. 2009, 68, 318–319. [Google Scholar] [CrossRef] [PubMed]
- Eculizumab (Soliris) in Covid-19 Infected Patients (SOLID-C19). Available online: https://clinicaltrials.gov/ct2/show/NCT04288713?term=complement&cond=COVID-19&draw=2 (accessed on 4 September 2020).
- Efficacy and Safety Study of IV Ravulizumab in Patients with COVID-19 Severe Pneumonia. Available online: https://clinicaltrials.gov/ct2/show/NCT04369469 (accessed on 17 July 2020).
- Diurno, F.; Numis, F.G.; Porta, G.; Cirillo, F.; Maddaluno, S.; Ragozzino, A.; De Negri, P.; Di Gennaro, C.; Pagano, A.; Allegorico, E.; et al. Eculizumab treatment in patients with COVID-19: Preliminary results from real life ASL Napoli 2 Nord experience. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4040–4047. [Google Scholar]
- Pitts, T. A Preliminary Update to the Soliris to Stop Immune Mediated Death in Covid-19 (SOLID-C19) Compassionate Use Study. Available online: https://hudsonmedical.com/articles/soliris-stop-death-covid-19/ (accessed on 18 June 2020).
- Clinical Trial Arena. Alexion to Study Ultomiris for Covid-19 Treatment. Available online: https://www.clinicaltrialsarena.com/news/alexion-ultomiris-covid-19-trial/ (accessed on 18 June 2020).
- Mastellos, D.C.; Pires da Silva, B.G.P.; Fonseca, B.A.L.; Fonseca, N.P.; Auxiliadora-Martins, M.; Mastaglio, S.; Ruggeri, A.; Sironi, M.; Radermacher, P.; Chrysanthopoulou, A.; et al. Complement C3 vs c5 inhibition in severe COVID-19: Early clinical findings reveal differential biological efficacy. Clin. Immunol. 2020, 220, 108598. [Google Scholar] [CrossRef]
- Clincal Trials Arena. Inflarx Starts Dosing Covid-19 Patients in Europe. Available online: https://www.clinicaltrialsarena.com/news/inflarx-covid-19-dosing-europe/ (accessed on 18 June 2020).
- Open-Label, Randomized Study of IFX-1 in Patients with Severe COVID-19 Pneumonia (PANAMO). Available online: https://clinicaltrials.gov/ct2/show/NCT04333420 (accessed on 18 June 2020).
- Inflarx Reports Encouraging Topline Results from the Exploratory Phase II Part of the Adaptive Randomized Phase II/III Trial of IFX-1 in COVID-19. Available online: https://www.inflarx.de/Home/Investors/Press-Releases/06-2020-InflaRx-Reports-Encouraging-Topline-Results-from-the-Exploratory-Phase-II-Part-of-the-Adaptive-Randomized-Phase-II-III-Trial-of-IFX-1-in-COVID-19.html (accessed on 23 July 2020).
- Innate Pharma. Avdoralimab/IPH5401: Anti-C5AR MAB. Available online: Innate-pharma.com/en/pipeline/avdoralimab/iph5401-anti-c5ar-mab (accessed on 17 December 2020).
- Avdoralimab an anti-C5aR Antibody, in Patients with COVID-19 Severe Pneumonia (FORCE). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04371367 (accessed on 17 December 2020).
- Barrowcliffe, T. History of heparin. In Heparin-a Century of Progress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 3–22. [Google Scholar]
- Hirsh, J.; Anand, S.S.; Halperin, J.L.; Fuster, V. Mechanism of action and pharmacology of unfractionated heparin. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1094–1096. [Google Scholar] [CrossRef]
- Kazatchkine, M.D.; Fearon, D.T.; Metcalfe, D.D.; Rosenberg, R.D.; Austen, K.F. Structural determinants of the capacity of heparin to inhibit the formation of the human amplification C3 convertase. J. Clin. Investig. 1981, 67, 223–228. [Google Scholar] [CrossRef]
- Hippensteel, J.A.; LaRiviere, W.B.; Colbert, J.F.; Langouet-Astrie, C.J.; Schmidt, E.P. Heparin as a therapy for COVID-19: Current evidence and future possibilities. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L211–L217. [Google Scholar] [CrossRef]
- Mycroft-West, C.J.; Su, D.; Elli, S.; Li, Y.; Guimond, S.E.; Miller, G.J.; Turnbull, J.E.; Yates, E.A.; Guerrini, M.; Fernig, D.G. The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 receptor binding domain undergoes conformational change upon heparin binding. BioRxiv 2020. [Google Scholar] [CrossRef]
- Milewska, A.; Zarebski, M.; Nowak, P.; Stozek, K.; Potempa, J.; Pyrc, K. Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells. J. Virol. 2014, 88, 13221–13230. [Google Scholar] [CrossRef] [PubMed]
- de Haan, C.A.; Te Lintelo, E.; Li, Z.; Raaben, M.; Wurdinger, T.; Bosch, B.J.; Rottier, P.J. Cooperative involvement of the S1 and S2 subunits of the murine coronavirus spike protein in receptor binding and extended host range. J. Virol. 2006, 80, 10909–10918. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef]
- Paranjpe, I.; Fuster, V.; Lala, A.; Russak, A.; Glicksberg, B.S.; Levin, M.A.; Charney, A.W.; Narula, J.; Fayad, Z.A.; Bagiella, E.; et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 122–124. [Google Scholar] [CrossRef]
- Ayerbe, L.; Risco, C.; Ayis, S. The association between treatment with heparin and survival in patients with Covid-19. J. Thromb. Thrombolysis 2020, 50, 298–301. [Google Scholar] [CrossRef]
- Ricklin, D.; Lambris, J.D. Complement in immune and inflammatory disorders: Pathophysiological mechanisms. J. Immunol. 2013, 190, 3831–3838. [Google Scholar] [CrossRef]
| Drug | FDA Approved Indication | Proposed COVID-19 MOA | ADEs | Possible DDIs | Clinical Data/ Limitations |
|---|---|---|---|---|---|
| Antiviral Therapies | |||||
| Chloroquine | Malaria | ● Prevents virus/cell binding and fusion [15,16,17] ● Immunomodulatory effects decrease inflammatory cytokines [16] | ● QTc prolongation ● GI effects | ● Other QTc prolonging drugs ● CYP2D6 inhibitor ● P-gp inhibitor | ● No benefit found: ○ High dose vs. low dose CQ [18] ○ CQ vs. LPV [19] ○ HCQ vs. HCQ + AZM vs. no HCQ [20] ○ HCQ vs. SOC [21] ○ HCQ vs. no HCQ [22] ● Problems with study design (e.g., small sample sizes, extremes in patient demographics, differences in standard of care definitions) [1] |
| Hydroxy-chloroquine | Malaria, Lupus, RA | ||||
| Remdesivir (RDV) | COVID-19 | ● Adenosine nucleotide analog prodrug [23] ● Inhibits viral-dependent RNA polymerase [23] | ● Elevated LFTs ● Renal toxicity associated with drug vehicle ● GI symptoms | ● Strong induction of P-gp will reduce levels | ● First to show clinical benefit of a pharmacological treatment for COVID 19 [1] ○ RDV vs. placebo [24] ○ Patients recovered 4 days faster on average [24] ○ Mortality rate 8% (RDV) vs. 11·5% (placebo) [24] ● RDV compassionate use [25] ○ Less oxygen support needed, no control [25] ● Some small sample sizes, uncontrolled cases [1] |
| Favipiravir (FPV) | None [26] | ● Purine nucleoside analogue prodrug [27] ● Competitive inhibitor of RNA-dependent RNA polymerase [27] | ● Diarrhea [28] ● Liver injury [28] ● Poor diet [28] | ● Avoid administration with aldehyde oxidase inhibitors [26] ● CYP2D8 inhibitor [26] | ● FPV vs. LPV [27] ○ Reduction in median time to viral clearance ○ 4 days (FPV) vs. 11 days (LPV) ○ Fewer adverse events than LPV |
| Protease Inhibitors (Lopinavir, Ritonavir) | HIV | ● Possible SARS-CoV-2 protease 3CLpro inhibition [29] | ● GI effects ● Elevated LFTs ● QTc prolongation | ● Lopinavir: CYP3A4 inhibitor and substrate ● Ritonavir: CYP3A4 and 2D6 inhibitor and substrate, UGT1A1 inducer, inducer of CYP1A2, 2C8, 2C9, 2C19 | ● No virologic or clinical benefit found ○ LPV vs. SOC [1] ● Limitations (e.g.,. small sample sizes, unblinded, underpowered) [1] |
| Ivermectin | Parasitic infections | ● Inhibits host nuclear transport proteins, preventing viral mediated antiviral suppression | ● Rash ● Dizziness | ● Few | ● Retrospective analysis ● Non-standardized timing of interventions |
| Immune-Based Therapies | |||||
| Convalescent Plasma | None | ● Contains antibodies against SARS-CoV-2 [30] | ● Treatment-associated acute lung injury and circulatory overload [31] ● Hypersensitivity reactions ● Transfusion risks | ● Dedicated IV line | ● Limited data [1] ○ Small sample sizes ○ Retrospective cohort studies ○ Case series ○ Case reports |
| Immune Globulins: SARS-CoV-2 specific | None | ● Concentrated antibodies against SARS-CoV-2 and/or other pathogens [1] | ● Thrombotic events ● Renal dysfunction ● Infusion reactions | ● May interfere with response to other vaccines | ● SARS-CoV-2 specific: no clinical data [1] ● Non-SARS-CoV-2 specific: Limitations in study design (e.g.,. non-randomized, older patient age, higher population of patients with severe disease) [32] |
| Non-SARS-CoV-2 specific | Immune disorders, prophylaxis of bacterial and viral disorders | ||||
| Mesenchymal Stem Cells | None | ● Hypothesized to reduce acute lung injury and inhibit cell mediated inflammatory response [1] | ● Unpredictability of stem cell activity ● Tumor growth ● Infection ● Thrombus formation ● Site reactions | ● Dedicated IV line | ● Small, non-randomized studies [1] ● No statistical significance [1] |
| Interferons: Alpha | Leukemia, melanoma, lymphoma, HBV, HCV | ● Antiviral ● Antiproliferative ● Immuno-modulatory [33,34,35,36] | ● Flu-like symptoms ● Injection site reactions ● Altered LFTs ● Worsening depression [35,37] | ● Low potential ● CYP1A2 inhibition with IFN-a | ● No clinical data for COVID-19 [1] ● Mixed/limited data in MERS [38,39,40,41] ● Inhaled IFN-b in other conditions [42,43] |
| Beta | Multiple sclerosis | ||||
| JAK Inhibitors (Baricitinib) | RA [44] | ● Inhibition of kinases that regulate endocytosis [1] ● Predicted to interfere with endocytosis in alveolar epithelial cells [45] | ● Lymphoma ● Thrombosis ● GI perforation ● LFT changes ● Herpes simplex ● Herpes zoster | ● Modify dose with strong OAT3 inhibitors | ● No data for COVID 19, SARS, or MERS [1] |
| IL-1 inhibitors (Anakinra) | RA | ● Competitive binding to IL-1 receptor | ● Neutropenia ● Anaphylaxis ● Injection site reactions Infusion reactions | ● Avoid with TNF inhibitors due to risk of infection | ● No data for COVID 19, SARS, or MERS [1] |
| IL-6 inhibitors (Sarilumab, Siltuximab, Tocilizumab) | Sar: RA [46] Sil: Multicentric Castleman Disease Toc: Cytokine release syndrome, RA | ● Monoclonal Antibodies [1] I● L-6 receptor antagonists [47,48] | Neutropenia GI perforation Infusion reactions LFT changes | ● Elevated IL-6 may downregulate CYP | ● Sar, Toc: Underway [49] ● Sil: single center observational study; mixed results [1] ● Toc: uncontrolled retrospective cohort; small sample size; no control; positive results [50] |
| Other | |||||
| Dexamethasone [51] | Asthma, inflammatory disorders | ● Synthetic adrenal corticosteroid ● Anti-inflammatory effects | ● Hypertension ● Hyperglycemia | ● CYP3A4 inducer | ● RECOVERY Trial ○ Reduced death by 1/3 in patients on respirator, by 1/5 in patients on ventilator [51] ○ Positive effects in critically ill patients, not in mild cases [51] ● Delayed viral clearance in SARS and MERS [1] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkoff, C.M.; Mousa, S.A. Pharmacotherapy in COVID 19: Potential Impact of Targeting the Complement System. Biomedicines 2021, 9, 11. https://doi.org/10.3390/biomedicines9010011
Barkoff CM, Mousa SA. Pharmacotherapy in COVID 19: Potential Impact of Targeting the Complement System. Biomedicines. 2021; 9(1):11. https://doi.org/10.3390/biomedicines9010011
Chicago/Turabian StyleBarkoff, Courtney M., and Shaker A. Mousa. 2021. "Pharmacotherapy in COVID 19: Potential Impact of Targeting the Complement System" Biomedicines 9, no. 1: 11. https://doi.org/10.3390/biomedicines9010011
APA StyleBarkoff, C. M., & Mousa, S. A. (2021). Pharmacotherapy in COVID 19: Potential Impact of Targeting the Complement System. Biomedicines, 9(1), 11. https://doi.org/10.3390/biomedicines9010011






