Evaluation of Nanoparticle Penetration in the Tumor Spheroid Using Two-Photon Microscopy
Abstract
1. Introduction
2. Experimental Methods
2.1. Synthesis of MSNs with Different Physicochemical Properties
2.2. The Characterization of Physicochemical Properties of MSNs
2.3. Generation of MG-63 Spheroids
2.4. Evaluation of the Penetration of MSNs in a Spheroid
2.5. Statistical Analysis
3. Result
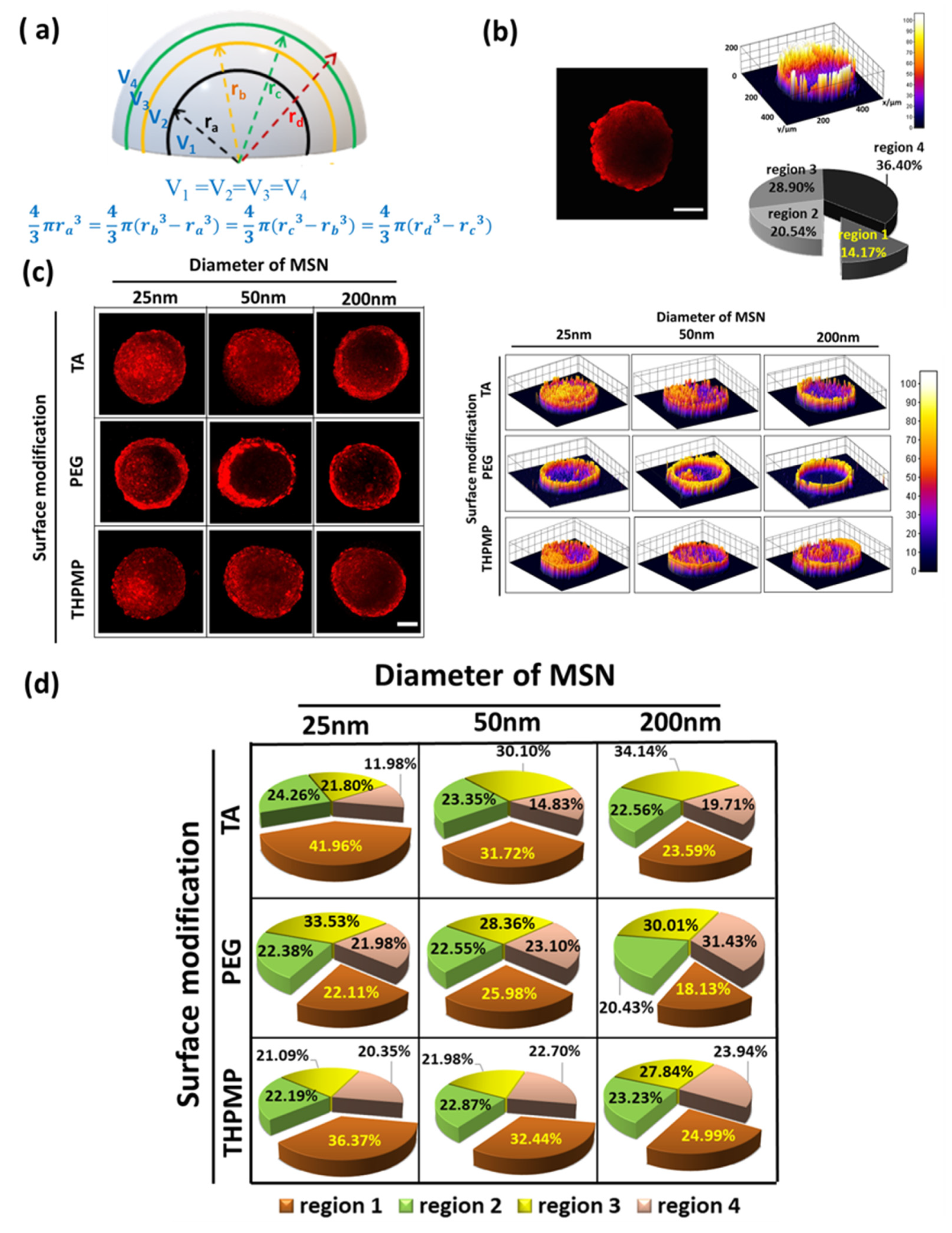
3.1. Effect of MSNs’ Properties on the Total Penetration and Accumulation in the Spheroids
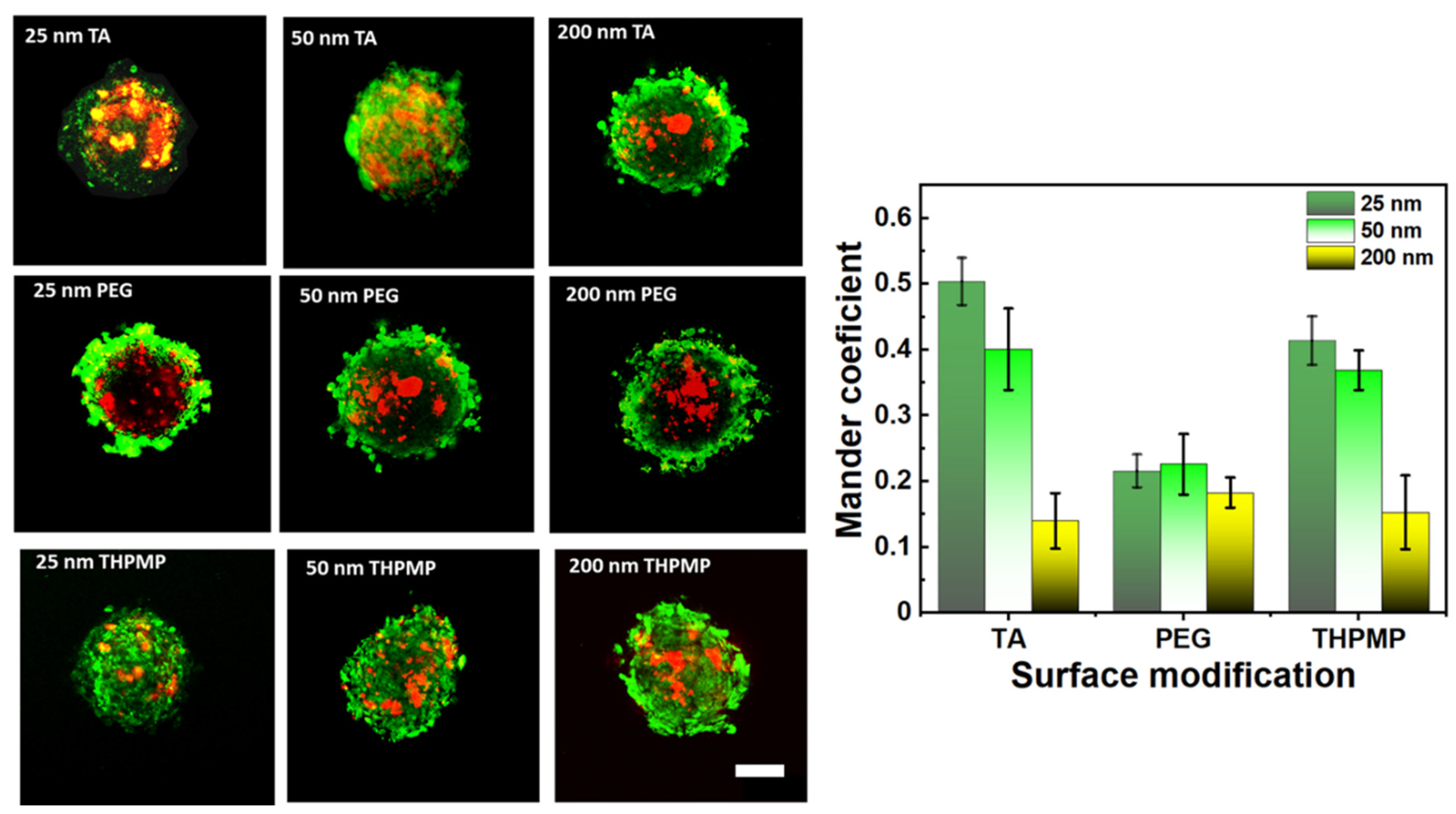
3.2. Effect of MSNs’ Properties on Diffusive Motion in the Spheroids
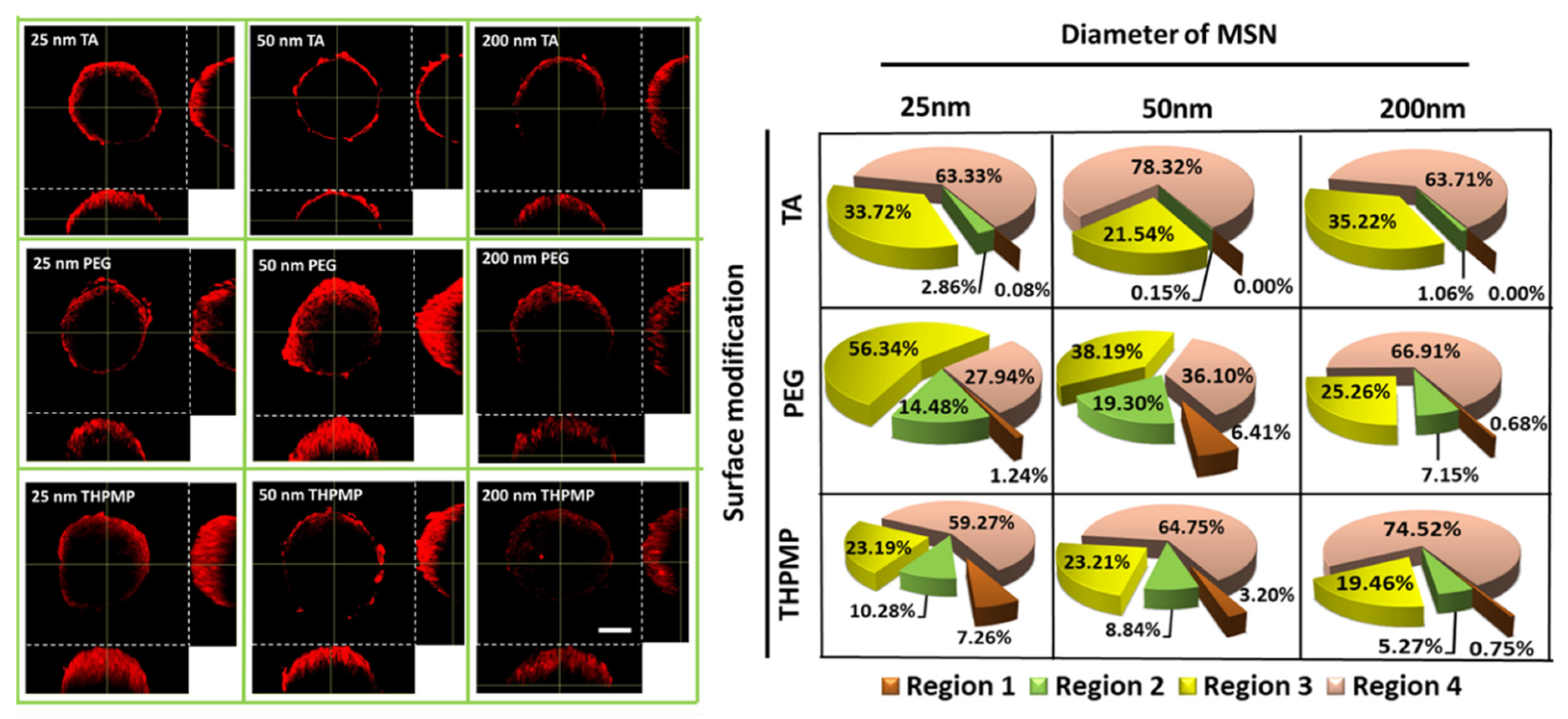
3.3. Accumulation of MSNs in the Hypoxic Region of the Spheroid
3.4. Profiling the Multihierarchical Physicochemical Properties of MSNs in Their Transport
3.5. The Influence of Serum on MSN Penetration in the Spheroid
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Sun, S.; Zhang, Z.; Shi, D. Nanomaterials for Cancer Precision Medicine. Adv. Mater. 2018, 30, e1705660. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Zan, X.; Hao, P.; Yang, D.; Feng, S.; Peng, B.; Appelhans, D.; Zhang, T.; Zan, X. Designing nanoparticles with improved tumor penetration: Surface properties from the molecular architecture viewpoint. J. Mater. Chem. B 2019, 7, 953–964. [Google Scholar] [CrossRef]
- Lu, H.; Utama, R.H.; Kitiyotsawat, U.; Babiuch, K.; Jiang, Y.; Barner-Kowollik, C. Enhanced transcellular penetration and drug delivery by crosslinked polymeric micelles into pancreatic multicellular tumor spheroids. Biomater. Sci. 2015, 3, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Greenwald, D.R.; Ruoslahti, E. Coadministration of a Tumor-Penetrating Peptide Enhances the Efficacy of Cancer Drugs. Science 2010, 328, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Li, Y.; Lo, P.-C.; Lee, H.; Choi, Y. Fucoidan-Based Theranostic Nanogel for Enhancing Imaging and Photodynamic Therapy of Cancer. Nano-Micro Lett. 2020, 12, 47. [Google Scholar] [CrossRef]
- Lin, Y.S.; Tsai, C.P.; Huang, H.Y.; Kuo, C.T.; Hung, Y.; Huang, D.M.; Chen, A.Y.C.; Mou, C.Y. Well-Ordered Mesoporous Silica Nanoparticles as Cell Markers. Chem. Mater. 2005, 17, 4570–4573. [Google Scholar] [CrossRef]
- Chen, Y.P.; Chen, H.A.; Hung, Y.; Chien, F.C.; Chen, P.; Mou, C.Y. Surface charge effect in intracellular localization of mesoporous silicananoparticles as probed by fluorescent ratiometric pH imaging. RSC Adv. 2012, 2, 968–973. [Google Scholar] [CrossRef]
- Liu, T.P.; Wu, S.H.; Chen, Y.P.; Chou, C.M.; Chen, C.T. Biosafety evaluations of well-dispersed mesoporous silica nanoparticles: Towards in vivo-relevant conditions. Nanoscale 2015, 7, 6471–6480. [Google Scholar] [CrossRef]
- Pratiwi, F.W.; Kuo, C.W.; Wu, S.-H.; Chen, Y.-P.; Mou, C.Y.; Chen, P. The Bioimaging Applications of Mesoporous Silica Nanoparticles. Enzymes 2018, 43, 123–153. [Google Scholar] [CrossRef]
- Pratiwi, F.W.; Kuo, C.W.; Chen, B.-C.; Chen, P. Recent advances in the use of fluorescent nanoparticles for bioimaging. Nanomedicine 2019, 14, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour, S.; Walsh, L.J.; Xu, C. Biomedical application of mesoporous silica nanoparticles as delivery systems: A biological safety perspective. J. Mater. Chem. B 2020, 8, 9863–9876. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Yang, D.; Huang, W.; Hao, P.; Feng, S.; Appelhans, D.; Zhang, T.; Zan, X. Shape Effect of Nanoparticles on Tumor Penetration in Monolayers Versus Spheroids. Mol. Pharm. 2019, 16, 2902–2911. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, R.; Miyagawa, S.; Fukushima, S.; Goto, T.; Harada, A.; Shimozaki, Y.; Yamaki, K.; Sanami, S.; Kikuta, J.; Ishii, M.; et al. Intravital imaging with two-photon microscopy reveals cellular dynamics in the ischeamia-reperfused rat heart. Sci. Rep. 2018, 8, 15991. [Google Scholar] [CrossRef] [PubMed]
- Tchoryk, A.; Taresco, V.; Argent, R.H.; Ashford, M.B.; Gellert, P.R.; Stolnik, S.; Grabowska, A.M.; Garnett, M.C. Penetration and Uptake of Nanoparticles in 3D Tumor Spheroids. Bioconj. Chem. 2019, 30, 1371–1384. [Google Scholar] [CrossRef]
- Takechi-Haraya, Y.; Goda, Y.; Sakai-Kato, K. Control of Liposomal Penetration into Three-Dimensional Multicellular Tumor Spheroids by Modulating Liposomal Membrane Rigidity. Mol. Pharm. 2017, 14, 2158–2165. [Google Scholar] [CrossRef]
- Huang, K.; Ma, H.; Liu, J.; Huo, S.; Kumar, A.; Wei, T.; Zhang, X.; Jin, S.; Gan, Y.; Wang, P.C.; et al. Size-Dependent Localization and Penetration of Ultrasmall Gold Nanoparticles in Cancer Cells, Multicellular Spheroids, and Tumors in Vivo. ACS Nano 2012, 6, 4483–4493. [Google Scholar] [CrossRef]
- Hui, Y.; Yi, X.; Hou, F.; Wibowo, D.; Zhang, F.; Zhao, D.; Gao, H.; Zhao, C.-X. Role of Nanoparticle Mechanical Properties in Cancer Drug Delivery. ACS Nano 2019, 13, 7410–7424. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv. Drug Deliv. Rev. 2019, 143, 3–21. [Google Scholar] [CrossRef]
- Mapanao, A.K.; Voliani, V. Three-dimensional tumor models: Promoting breakthroughs in nanotheranostics translational research. Appl. Mater. Today 2020, 19, 100552. [Google Scholar] [CrossRef]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef] [PubMed]
- Ooft, S.N.; Weeber, F.; Dijkstra, K.K.; McLean, C.; Kaing, S.; Van Werkhoven, E.D.; Schipper, L.; Hoes, L.; Vis, D.J.; Van De Haar, J.; et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci. Transl. Med. 2019, 11, eaay2574. [Google Scholar] [CrossRef] [PubMed]
- Muraca, F.; AlAhmari, A.; Giannone, V.A.; Adumeau, L.; Yan, Y.; McCafferty, M.M.; Dawson, K.A. A Three-Dimensional Cell Culture Platform for Long Time-Scale Observations of Bio–Nano Interactions. ACS Nano 2019, 13, 13524–13536. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kumacheva, E. Hydrogel microenvironments for cancer spheroid growth and drug screening. Sci. Adv. 2018, 4, eaas8998. [Google Scholar] [CrossRef] [PubMed]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Ban, Z.; Yuan, P.; Yu, F.; Peng, T.; Zhou, Q.; Hu, X. Machine learning predicts the functional composition of the protein corona and the cellular recognition of nanoparticles. Proc. Natl. Acad. Sci. USA 2020, 117, 10492–10499. [Google Scholar] [CrossRef]
- Cai, X.; Dong, J.; Liu, J.; Zheng, H.; Kaweeteerawat, C.; Wang, F.; Ji, Z.; Li, R. Multi-hierarchical profiling the structure-activity relationships of engineered nanomaterials at nano-bio interfaces. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Silvestri, A.; Di Silvio, D.; Llarena, I.; Murray, R.A.; Marelli, M.; Lay, L.; Polito, L.; Moya, S. Influence of surface coating on the intracellular behaviour of gold nanoparticles: A fluorescence correlation spectroscopy study. Nanoscale 2017, 9, 14730–14739. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Nederman, T.; Norling, B.; Glimelius, B.; Carlsson, J.; Brunk, U. Demonstration of an extracellular matrix in multicellular tumor spheroids. Cancer Res. 1984, 44, 3090–3097. [Google Scholar]
- Knight, E.; Przyborski, S. Advances in 3D cell culture technologies enabling tissue-like structures to be createdin vitro. J. Anat. 2015, 227, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Peng, C.C.; Kuo, C.W.; Chueh, D.Y.; Wu, H.M.; Liu, Y.H.; Chen, P.; Tung, Y.C. Study of oxygen tension variation within live tumor spheroids using microfluidic devices and multi-photon laser scanning microscopy. RSC Adv. 2018, 8, 30320–30329. [Google Scholar] [CrossRef]
- Wang, H.X.; Zuo, Z.Q.; Du, J.Z.; Wang, Y.; Sun, R.; Cao, Z.T.; Ye, X.D.; Wang, J.L.; Leong, K.W.; Wang, J. Surface charge critically affects tumor penetration and therapeutic efficacy of cancer nanomedicines. Nano Today 2016, 11, 133–144. [Google Scholar] [CrossRef]
- Himmelstoß, S.F.; Hirsch, T. Long-Term Colloidal and Chemical Stability in Aqueous Media of NaYF 4 -Type Upconversion Nanoparticles Modified by Ligand-Exchange. Part. Part. Syst. Charact. 2019, 36, 1900235. [Google Scholar] [CrossRef]
- Chang, R.L.; Pratiwi, F.; Chen, B.C.; Chen, P.; Wu, S.H.; Mou, C.Y. Simultaneous Single-particle Tracking and Dynamic pH Sensing Reveal Lysosome-targetable Mesoporous Silica Nanoparticles Pathways. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; Macmillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef]
- Godet, I.; Shin, Y.J.; Ju, J.A.; Ye, I.C.; Wang, G.; Gilkes, D.M. Fate-mapping post-hypoxic tumor cells reveals a ROS-resistant phenotype that promotes metastasis. Nat. Commun. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Huang, X.; Zhuang, J.; Chung, S.W.; Huang, B.; Halpert, G.; Negron, K.; Sun, X.; Yang, J.; Oh, Y.; Hwang, P.M.; et al. Hypoxia-tropic Protein Nanocages for Modulation of Tumor- and Chemotherapy-Associated Hypoxia. ACS Nano 2019, 13, 236–247. [Google Scholar] [CrossRef]
- Park, C.C.; Georgescu, W.; Polyzos, A.; Pham, C.; Ahmed, K.M.; Zhang, H.; Costes, S.V. Rapid and automated multidimensional fluorescence microscopy profiling of 3D human breast cultures. Integr. Biol. 2013, 5, 681–691. [Google Scholar] [CrossRef][Green Version]
- Lynch, I.; Weiss, C.; Valsami-Jones, E. A strategy for grouping of nanomaterials based on key physico-chemical descriptors as a basis for safer-by-design NMs. Nano Today 2014, 9, 266–270. [Google Scholar] [CrossRef]
- Xu, M.; Soliman, M.G.; Sun, X.; Pelaz, B.; Feliu, N.; Parak, W.J.; Liu, S. How Entanglement of Different Physicochemical Properties Complicates the Prediction of in Vitro and in Vivo Interactions of Gold Nanoparticles. ACS Nano 2018, 12, 10104–10113. [Google Scholar] [CrossRef] [PubMed]
- Sujai, P.T.; Joseph, M.M.; Saranya, G.; Nair, J.B.; Murali, V.P.; Maiti, K.K. Surface charge modulates the internalization vs. penetration of gold nanoparticles: Comprehensive scrutiny on monolayer cancer cells, multicellular spheroids and solid tumors by SERS modality. Nanoscale 2020, 12, 6971–6975. [Google Scholar] [CrossRef] [PubMed]
- Harush-Frenkel, O.; Rozentur, E.; Benita, S.; Altschuler, Y. Surface Charge of Nanoparticles Determines Their Endocytic and Transcytotic Pathway in Polarized MDCK Cells. Biomacromolecules 2008, 9, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Lam, A.K.; Sykes, E.A.; Rocheleau, J.V.; Chan, W.C.W. Tumour-on-a-chip provides an optical window into nanoparticle tissue transport. Nat. Commun. 2013, 4, 2718. [Google Scholar] [CrossRef] [PubMed]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef]
- Chen, D.; Ganesh, S.; Wang, W.; Amiji, M. Protein Corona-Enabled Systemic Delivery and Targeting of Nanoparticles. AAPS J. 2020, 22, 1–9. [Google Scholar] [CrossRef]
- Chou, C.C.; Chen, W.; Hung, Y.; Mou, C.Y. Molecular Elucidation of Biological Response to Mesoporous Silica Nanoparticles in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 22235–22251. [Google Scholar] [CrossRef]
- Valente, K.P.; Suleman, A.; Brolo, A.G. Exploring Diffusion and Cellular Uptake: Charged Gold Nanoparticles in an in Vitro Breast Cancer Model. ACS Appl. Bio Mater. 2020, 3, 6992–7002. [Google Scholar] [CrossRef]
- Tsou, C.J.; Hsia, C.H.; Chu, J.Y.; Hung, Y.; Chen, Y.P.; Chien, F.C.; Chou, K.C.; Chen, P.; Mou, C.Y. Local pH tracking in living cells. Nanoscale 2015, 7, 4217–4225. [Google Scholar] [CrossRef]
- Adair, J.H.; Suvaci, E.; Sindel, J. Surface and Colloid Chemistry. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 3, pp. 1–10. [Google Scholar] [CrossRef]
- Verwey, E.J.W. Theory of the Stability of Lyophobic Colloids. J. Phys. Chem. 1947, 51, 631–636. [Google Scholar] [CrossRef]
- Derjaguin, B.; Landau, L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Prog. Surf. Sci. 1993, 43, 30–59. [Google Scholar] [CrossRef]
- Mulvihill, M.J.; Habas, S.E.; Plante, I.J.L.; Wan, J.; Mokari, T. Influence of Size, Shape, and Surface Coating on the Stability of Aqueous Suspensions of CdSe Nanoparticles. Chem. Mater. 2010, 22, 5251–5257. [Google Scholar] [CrossRef]
- Aldana, J.; Lavelle, N.; Wang, A.Y.; Peng, X. Size-Dependent Dissociation pH of Thiolate Ligands from Cadmium Chalcogenide Nanocrystals. J. Am. Chem. Soc. 2005, 127, 2496–2504. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Kim, H.S.; Palanikumar, L.; Go, E.M.; Jana, B.; Park, S.A.; Kim, H.Y.; Kim, K.; Seo, J.K.; Kwak, S.K.; et al. Cloaking nanoparticles with protein corona shield for targeted drug delivery. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Huang, K.; Boerhan, R.; Liu, C.; Jiang, G. Nanoparticles Penetrate into the Multicellular Spheroid-on-Chip: Effect of Surface Charge, Protein Corona, and Exterior Flow. Mol. Pharm. 2017, 14, 4618–4627. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pratiwi, F.W.; Peng, C.-C.; Wu, S.-H.; Kuo, C.W.; Mou, C.-Y.; Tung, Y.-C.; Chen, P. Evaluation of Nanoparticle Penetration in the Tumor Spheroid Using Two-Photon Microscopy. Biomedicines 2021, 9, 10. https://doi.org/10.3390/biomedicines9010010
Pratiwi FW, Peng C-C, Wu S-H, Kuo CW, Mou C-Y, Tung Y-C, Chen P. Evaluation of Nanoparticle Penetration in the Tumor Spheroid Using Two-Photon Microscopy. Biomedicines. 2021; 9(1):10. https://doi.org/10.3390/biomedicines9010010
Chicago/Turabian StylePratiwi, Feby Wijaya, Chien-Chung Peng, Si-Han Wu, Chiung Wen Kuo, Chung-Yuan Mou, Yi-Chung Tung, and Peilin Chen. 2021. "Evaluation of Nanoparticle Penetration in the Tumor Spheroid Using Two-Photon Microscopy" Biomedicines 9, no. 1: 10. https://doi.org/10.3390/biomedicines9010010
APA StylePratiwi, F. W., Peng, C.-C., Wu, S.-H., Kuo, C. W., Mou, C.-Y., Tung, Y.-C., & Chen, P. (2021). Evaluation of Nanoparticle Penetration in the Tumor Spheroid Using Two-Photon Microscopy. Biomedicines, 9(1), 10. https://doi.org/10.3390/biomedicines9010010




