Impact of Subclinical Congestion on Outcome of Patients Undergoing Mitral Valve Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasma Volume Equations
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Alkaline Phosphatase Metabolism
3.3. Peri-and Postoperative Characteristics
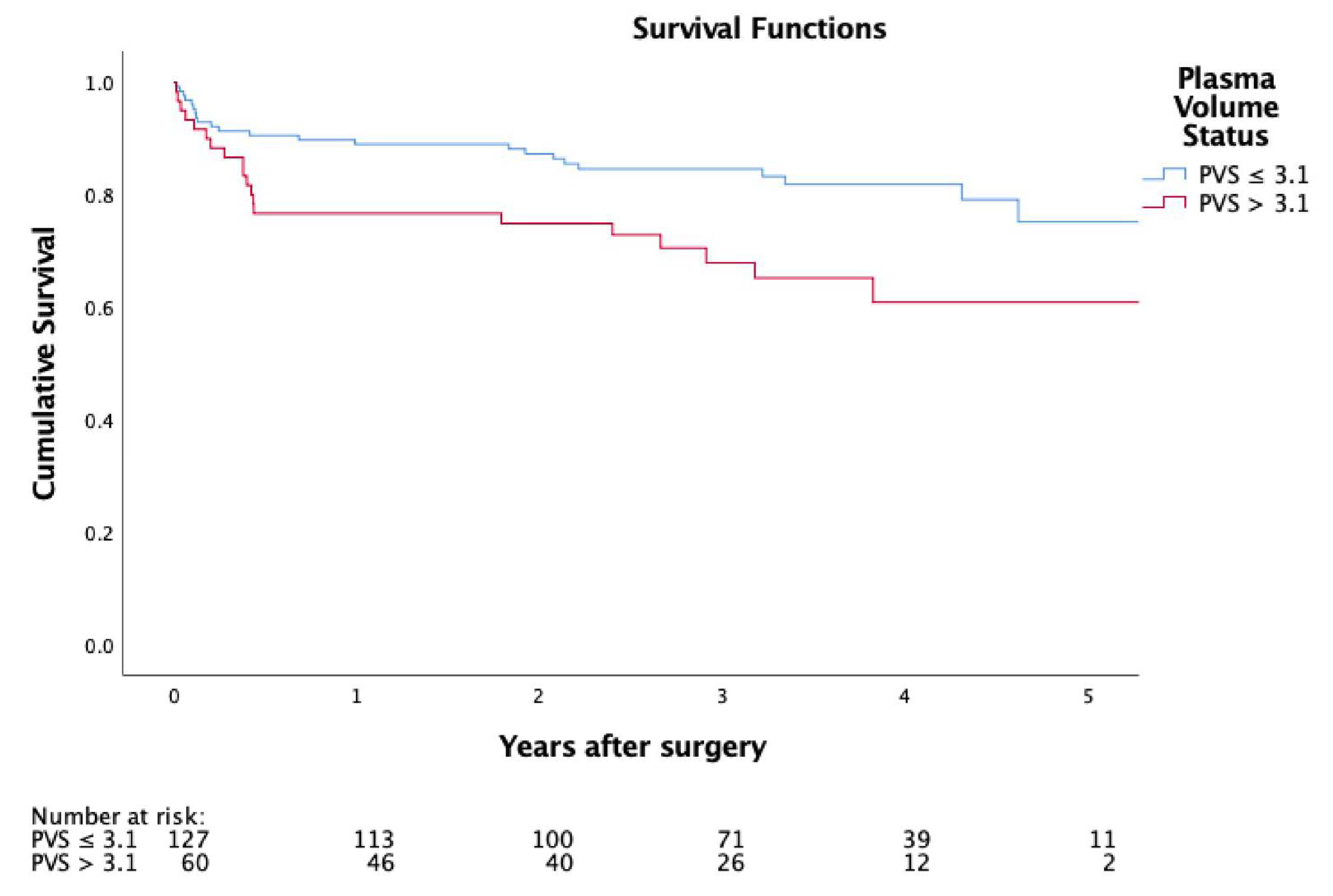
3.4. Adverse Events and Survival
4. Discussion
Limitations
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PVS | Plasma Volume Score |
| ECMO | Extracorporeal Membrane oxygenation |
| CHF | Chronic Heart Failure |
| BMI | Body Mass Index |
| AP | Alkaline Phosphatase |
| CABG | Coronary Artery Bypass Graft |
References
- Acker, M.A. Should moderate or greater mitral regurgitation be repaired in all patients with LVEF <30%? Mitral valve repair in patients with advanced heart failure and severe functional mitral insufficiency reverses left ventricular remodeling and improves symptoms. Circ. Heart Fail. 2008, 1, 281–284. [Google Scholar] [CrossRef]
- Crestanello, J.A. Mitral Valve Surgery for Congestive Heart Failure. Heart Fail. Clin. 2018, 14, 585–600. [Google Scholar] [CrossRef]
- Lavall, D.; Hagendorff, A.; Schirmer, S.H.; Böhm, M.; Borger, M.A.; Laufs, U. Mitral valve interventions in heart failure. ESC Heart Fail. 2018, 5, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, T.G.; Acker, M.A.; Dec, G.W.; Byrne, J.G. Mitral valve surgery in advanced heart failure. J. Am. Coll. Cardiol. 2010, 55, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Setoguchi, S.; Stevenson, L.W.; Schneeweiss, S. Repeated hospitalizations predict mortality in the community population with heart failure. Am. Heart J. 2007, 154, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Yoshihisa, A.; Abe, S.; Sato, Y.; Watanabe, S.; Yokokawa, T.; Miura, S.; Misaka, T.; Sato, T.; Suzuki, S.; Oikawa, M.; et al. Plasma volume status predicts prognosis in patients with acute heart failure syndromes. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.Z.; Flint, J.; Damgaard, M.; Bonfils, P.K.; Cheng, A.S.; Aggarwal, S.; Velmurugan, S.; Mendonca, M.; Rashid, M.; Kang, S.; et al. Calculated plasma volume status and prognosis in chronic heart failure. Eur. J. Heart Fail. 2015, 17, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Maznyczka, A.M.; Barakat, M.F.; Ussen, B.; Kaura, A.; Abu-Own, H.; Jouhra, F.; Jaumdally, H.; Amin-Youssef, G.; Nicou, N.; Baghai, M.; et al. Calculated plasma volume status and outcomes in patients undergoing coronary bypass graft surgery. Heart 2019, 105, 1020–1026. [Google Scholar] [CrossRef]
- Adlbrecht, C.; Piringer, F.; Resar, J.; Watzal, V.; Andreas, M.; Strouhal, A.; Hasan, W.; Geisler, D.; Weiss, G.; Grabenwoger, M.; et al. The impact of subclinical congestion on the outcome of patients undergoing transcatheter aortic valve implantation. Eur. J. Clin. Investig. 2020, 22, e13251. [Google Scholar]
- De Bonis, M.; Bolling, S.F. Mitral valve surgery: Wait and see vs. early operation. Eur. Heart J. 2013, 34, 13–19a. [Google Scholar] [CrossRef][Green Version]
- Stone, G.W.; Adams, D.H.; Abraham, W.T.; Kappetein, A.P.; Généreux, P.; Vranckx, P.; Mehran, R.; Kuck, K.H.; Leon, M.B.; Piazza, N.; et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: Part 2: Endpoint definitions: A consensus document from the Mitral Valve Academic Research Consortium. Eur. Heart J. 2015, 36, 1878–1891. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.; Brown, E.; Daley, C.; Lawrence, A. Oxford Handbook of Dialysis; Oxford University Press: Oxford, UK, 2010. [Google Scholar] [CrossRef]
- Longo, D.; Fauci, A.; Kasper, D.; Hauser, S.; Jameson, J.; Loscalzo, J. Harrisons Manual of Medicine, 18th ed.; McGraw-Hill Professional: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- El Sabbagh, A.; Reddy, Y.N.V.; Nishimura, R.A. Mitral Valve Regurgitation in the Contemporary Era: Insights into Diagnosis, Management, and Future Directions. JACC Cardiovasc. Imaging 2018, 11, 628–643. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Sarano, M. Timing of mitral valve surgery. Heart 2002, 87, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Simonavicius, J.; Sanders van-Wijk, S.; Rickenbacher, P.; Maeder, M.T.; Pfister, O.; Kaufmann, B.A.; Pfisterer, M.; Celutkiene, J.; Puronaite, R.; Knackstedt, C.; et al. Prognostic Significance of Longitudinal Clinical Congestion Pattern in Chronic Heart Failure: Insights From TIME-CHF Trial. Am. J. Med. 2019, 132, e679–e692. [Google Scholar] [CrossRef]
- Kobayashi, M.; Huttin, O.; Donal, E.; Duarte, K.; Hubert, A.; Le Breton, H.; Galli, E.; Fournet, M.; Mabo, P.; Schnell, F.; et al. Association of estimated plasma volume status with hemodynamic and echocardiographic parameters. Clin. Res. Cardiol. 2020, 109, 1060–1069. [Google Scholar] [CrossRef]
- Beale, A.L.; Nanayakkara, S.; Segan, L.; Mariani, J.A.; Maeder, M.T.; van Empel, V.; Vizi, D.; Evans, S.; Lam, C.S.P.; Kaye, D.M. Sex Differences in Heart Failure With Preserved Ejection Fraction Pathophysiology: A Detailed Invasive Hemodynamic and Echocardiographic Analysis. JACC Heart Fail. 2019, 7, 239–249. [Google Scholar] [CrossRef]
- Galderisi, M.; Anderson, K.M.; Wilson, P.W.; Levy, D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study). Am. J. Cardiol. 1991, 68, 85–89. [Google Scholar] [CrossRef]
- Avierinos, J.F.; Inamo, J.; Grigioni, F.; Gersh, B.; Shub, C.; Enriquez-Sarano, M. Sex differences in morphology and outcomes of mitral valve prolapse. Ann. Intern. Med. 2008, 149, 787–795. [Google Scholar] [CrossRef]
- Adlbrecht, C.; Kommata, S.; Hulsmann, M.; Szekeres, T.; Bieglmayer, C.; Strunk, G.; Karanikas, G.; Berger, R.; Mortl, D.; Kletter, K.; et al. Chronic heart failure leads to an expanded plasma volume and pseudoanaemia, but does not lead to a reduction in the body’s red cell volume. Eur. Heart J. 2008, 29, 2343–2350. [Google Scholar] [CrossRef]
- Martinez, F.; Martinez-Ibanez, L.; Pichler, G.; Ruiz, A.; Redon, J. Multimorbidity and acute heart failure in internal medicine. Int. J. Cardiol. 2017, 232, 208–215. [Google Scholar] [CrossRef]
- Schaefer, A.K.; Hutschala, D.; Andreas, M.; Bernardi, M.H.; Brands, R.; Shabanian, S.; Laufer, G.; Wiedemann, D. Decrease in serum alkaline phosphatase and prognostic relevance in adult cardiopulmonary bypass. Interact. Cardiovasc. Thorac. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.; Tong, S.; Hauck, A.; Lawson, D.S.; Jaggers, J.; Kaufman, J.; da Cruz, E. Alkaline phosphatase activity after cardiothoracic surgery in infants and correlation with post-operative support and inflammation: A prospective cohort study. Crit. Care 2012, 16, R160. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.A.; Urban, T.T.; Baird, C.; Tong, S.; Woodruff, A.; Twite, M.; Jaggers, J.; Simoes, E.A.F.; Wischmeyer, P. Alkaline Phosphatase in Infant Cardiopulmonary Bypass: Kinetics and Relationship to Organ Injury and Major Cardiovascular Events. J. Pediatr. 2017, 190, 49–55 e42. [Google Scholar] [CrossRef] [PubMed]
| Overall Cohort (n = 187) | PVS ≤ 3.1 (n = 127) | PVS > 3.1 (n = 60) | p-Value | |
|---|---|---|---|---|
| Female, n (%) | 68 (36.4) | 67 (52.8) | 1 (1.7) | 0.000 |
| Age, median (±IQR) | 67.0 (15) | 66.0 (16) | 69.0 (14) | 0.161 |
| BMI, median (±IQR) | 26.0 (5.5) | 26.8 (6.1) | 24.4 (4.3) | 0.004 |
| Logistic EuroSCORE, median (±IQR) | 10.9 (13.2) | 9.1 (13.1) | 13.7 (13.9) | 0.047 |
| EuroSCORE II, median (±IQR) | 7.6 (10.0) | 6.8 (8.6) | 10.3 (13.8) | 0.004 |
| Active smoker, n (%) | 32 (17.1) | 23 (18.1) | 9 (15.0) | 0.381 |
| Chronic heart failure, n (%) | 90 (48.1) | 58 (45.7) | 32 (53.3) | 0.205 |
| Hypertension, n (%) | 154 (82.4) | 102 (80.3) | 52 (86.7) | 0.258 |
| Dyslipidemia, n (%) | 106 (56.7) | 68 (53.5) | 38 (63.3) | 0.482 |
| Diabetes mellitus, n (%) | 59 (31.6) | 37 (29.1) | 22 (36.7) | 0.193 |
| Diabetes mellitus (IDDM), n (%) | 12 (6.4) | 4 (3.1) | 8 (13.3) | 0.012 |
| Chronic renal insufficiency, n (%) | 39 (20.9) | 19 (15.0) | 20 (33.3) | 0.004 |
| Last preoperative creatinine (mg/dL), median (±IQR) | 1.1 (0.4) | 1.0 (0.3) | 1.2 (0.8) | 0.016 |
| Preoperative Creatinine Clearance (mL/min), median (±IQR) | 67.3 (38.1) | 70.6 (35.3) | 58.0 (41.8) | 0.013 |
| Preoperative dialysis, n (%) | 6 (3.2) | 2 (1.6) | 4 (6.7) | 0.085 |
| Previous vascular stroke, n (%) | 19 (10.2) | 11 (8.7) | 8 (13.3) | 0.230 |
| Neurological disease, n (%) | 8 (4.3) | 5 (3.9) | 3 (5.0) | 0.503 |
| Prior myocardial infarction, n (%) | 75 (40.1) | 45 (35.4) | 30 (50.0) | 0.042 |
| Coronary artery disease, n (%) | 115 (61.5) | 70 (55.1) | 45 (75.0) | 0.007 |
| Prior CABG, n (%) | 15 (8.0) | 7 (5.5) | 8 (13.3) | 0.120 |
| Prior PCI, n (%) | 34 (18.2) | 19 (15.0) | 15 (25.0) | 0.074 |
| Prior valve surgery, n (%) | 22 (11.8) | 13 (10.2) | 9 (15.0) | 0.393 |
| Thoracic aortic surgery n (%) | 7 (3.7) | 4 (3.1) | 3 (5.0) | 0.497 |
| Atrial fibrillation, n (%) | 95 (50.8) | 66 (52.0) | 29 (48.3) | 0.379 |
| AV-Block, n (%) | 6 (3.2) | 4 (3.1) | 2 (3.3) | 0.627 |
| Prior pacemaker, n (%) | 17 (9.1) | 13 (10.2) | 4 (6.7) | 0.309 |
| Prior ICD, n (%) | 9 (4.8) | 5 (3.9) | 4 (6.7) | 0.316 |
| Endocarditis, n (%) | 5 (2.7) | 3 (2.4) | 2 (3.3) | 0.516 |
| Liver cirrhosis, n (%) | 1 (0.5) | 0 (0.0) | 1 (1.7) | 0.321 |
| NYHA class IV, n (%) | 32 (17.1) | 15 (11.8) | 17 (28.3) | 0.001 |
| COPD Gold ≥ II, n (%) | 43 (23.0) | 28 (22.0) | 15 (25.0) | 0.389 |
| Bronchodilators, n (%) | 40 (21.4) | 25 (19.7) | 15 (25.0) | 0.260 |
| Left ventricular function, mean (±SD) | 38.4 (9.3) | 40.0 (8.8) | 36.0 (10.0) | 0.022 |
| Severe mitral regurgitation, n (%) | 161 (86.1) | 110 (86.6) | 51 (85.0) | 0.850 |
| Primary mitral regurgitation, n (%) | 81 (43.3) | 62 (28.8) | 19 (31.7) | |
| Secondary mitral regurgitation, n (%) | 106 (56.7) | 65 (51.2) | 41 (68.3) | |
| Moderate or severe tricuspid regurgitation, n (%) | 65 (34.8) | 44 (34.6) | 21 (35.0) | 0.883 |
| Systolic pulmonary artery pressure in mmHg, median (±IQR) | 60.0 (34) | 60.0 (34.0) | 61.0 (30.0) | 0.449 |
| Hematocrit, mean (±SD) | 37.8 (5.2) | 39.3 (5.1) | 34.8 (4.2) | 0.262 |
| Preoperative alkaline phosphatase (AP) U/L, median (±IQR) | 69.0 (34) | 67.0 (31) | 73.5 (36) | 0.012 |
| AP 1st post-op day U/L, median (±IQR) | 39.0 (20) | 38.0 (21) | 42.5 (18) | 0.178 |
| AP 1st post-op day/preoperative AP %, median (±IQR) | 59.6 (17.2) | 60.3 (17.7) | 56.3 (16.2) | 0.065 |
| Consumption of AP in U/L, median (±IQR) | 27.0 (20) | 27.0 (17) | 33.0 (29) | 0.012 |
| Time between PVS calculation and surgery in d, median (±IQR) | 2.0 (3) | 2.0 (3) | 3.0 (3) | 0.265 |
| Overall Cohort (n = 187) | PVS ≤ 3.1 (n = 127) | PVS > 3.1 (n = 60) | p-Value | |
|---|---|---|---|---|
| Urgent operation, n (%) | 56 (29.9) | 32 (25.2) | 24 (40.0) | 0.089 |
| Cardiogenic shock, n (%) | 5 (2.7) | 2 (1.6) | 3 (5.0) | 0.189 |
| Isolated mitral valve repair, n (%) | 17 (9.1) | 14 (11.0) | 3 (5.0) | 0.520 |
| Combined mitral valve repair and CABG, n (%) | 47 (25.1) | 28 (22.0) | 19 (31.7) | 0.520 |
| Isolated mitral valve replacement, n (%) | 7 (3.7) | 5 (3.9) | 2 (3.3) | 0.520 |
| Combined mitral valve replacement and CABG, n (%) | 10 (5.3) | 7 (5.5) | 3 (5.0) | 0.520 |
| Combined mitral and atrial fibrillation surgery, n (%) | 41 (21.9) | 34 (26.8) | 7 (11.7) | 0.014 |
| Minimal invasive mitral valve procedure, n (%) | 8 (4.3) | 6 (4.7) | 2 (3.3) | 0.497 |
| LV aneurysm surgery, n (%) | 3 (1.6) | 2 (1.6) | 1 (1.7) | 0.678 |
| Cardiopulmonary bypass in min, mean (±SD) | 176.7 (60.4) | 172.5 (57.9) | 185.4 (65.2) | 0.601 |
| Aortic cross clamp time in min, mean (±SD) | 107.4 (35.4) | 107.4 (34.7) | 107.6 (37.2) | 0.847 |
| Intraoperative blood products, n (%) | 120 (64.1) | 75 (59.1) | 45 (75.0) | 0.024 |
| Intraoperative red blood cell units, mean (±SD) | 2.0 (4.3) | 1.7 (4.8) | 2.5 (3.0) | 0.001 |
| Intraoperative fresh frozen plasma units, mean (±SD) | 0.7 (2.2) | 0.5 (1.6) | 1.1 (3.0) | 0.089 |
| Intraoperative platelet units, mean (±SD) | 0.41 (2.3) | 0.41 (2.7) | 0.42 (0.8) | 0.027 |
| Postoperative blood products, n (%) | 55 (29.4) | 35 (27.6) | 20 (33.3) | 0.261 |
| Postoperative red blood cell units, mean (±SD) | 1.0 (3.2) | 0.8 (2.3) | 1.4 (4.6) | 0.552 |
| Postoperative fresh frozen plasma units, mean (±SD) | 0.2 (1.3) | 0.2 (0.8) | 0.4 (2.1) | 0.710 |
| Postoperative platelet units, mean (±SD) | 0.09 (0.6) | 0.04 (2.6) | 0.18 (1.1) | 0.336 |
| Implanted intraaortic balloon pump, n (%) | 1 (0.5) | 0 (0.0) | 1 (1.7) | 0.321 |
| Implanted ECMO, n (%) | 20 (10.7) | 9 (7.1) | 11 (18.3) | 0.018 |
| Reintubation, n (%) | 13 (7.0) | 6 (4.7) | 7 (11.7) | 0.079 |
| Length of stay at ICU (total), median (±IQR) | 5.0 (8.0) | 4.0 (7.0) | 6.0 (11.0) | 0.015 |
| Readmission at ICU, n (%) | 13 (7.0) | 7 (5.5) | 6 (10.0) | 0.204 |
| Overall Cohort (n = 187) | PVS ≤ 3.1 (n = 127) | PVS > 3.1 (n = 60) | p-Value | |
|---|---|---|---|---|
| Neurological adverse events | ||||
| Transient ischemic attack, n (%) | 1 (0.5) | 0 (0.0) | 1 (1.7) | 0.321 |
| Postoperative stroke ≥ 72 h, n (%) | 7 (3.7) | 4 (3.1) | 3 (5.0) | 0.400 |
| Continuous Coma ≥ 24 h, n (%) | 4 (2.1) | 3 (2.4) | 1 (1.7) | 0.615 |
| Other neurological complications, n (%) | 15 (8.0) | 10 (7.9) | 5 (8.3) | 0.560 |
| Renal failure | ||||
| Acute Kidney Injury Stage III, n (%) | 16 (8.6) | 10 (7.9) | 6 (10.0) | 0.408 |
| Postoperative hemofiltration, n (%) | 13 (7.0) | 8 (6.3) | 5 (8.3) | 0.408 |
| Conduction disturbances | ||||
| New AV-Block III, n (%) | 10 (5.3) | 7 (5.5) | 3 (5.0) | 0.594 |
| New Atrial Fibrillation, n (%) | 36 (19.3) | 23 (18.1) | 13 (21.7) | 0.349 |
| Pulmonary adverse events | ||||
| Prolonged ventilation (>24 h), n (%) | 49 (26.2) | 27 (21.3) | 22 (36.7) | 0.021 |
| Pneumonia, n (%) | 20 (10.7) | 11 (8.7) | 9 (15.0) | 0.146 |
| Pulmonary embolism, n (%) | 1 (0.5) | 0 (0.0) | 1 (1.7) | 0.679 |
| Miscellaneous adverse events | ||||
| Acute peripheral ischemia, n (%) | 2 (1.1) | 1 (0.8) | 1 (1.7) | 0.540 |
| Complication of anticoagulation, n (%) | 5 (2.7) | 3 (2.4) | 2 (3.3) | 0.516 |
| Gastrointestinal complication, n (%) | 6 (3.2) | 5 (3.9) | 1 (1.7) | 0.373 |
| Perioperative myocardial infarction, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | n.s. |
| Cardiac tamponade, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | n.s. |
| Aortic dissection, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | n.s. |
| Multiorgan failure, n (%) | 9 (4.8) | 4 (3.1) | 5 (8.3) | 0.121 |
| Cardiac arrest, n (%) | 15 (8.0) | 9 (7.1) | 6 (10.0) | 0.337 |
| Reoperations | ||||
| Due to bleeding/tamponade, n (%) | 17 (9.1) | 7 (5.5) | 10 (16.7) | 0.016 |
| Due to valve dysfunction, n (%) | 5 (2.7) | 3 (2.4) | 2 (3.3) | 0.516 |
| Due to other cardiac reason, n (%) | 36 (19.3) | 22 (17.3) | 14 (23.3) | 0.218 |
| Due to other non-cardiac reason, n (%) | 25 (13.4) | 12 (9.4) | 13 (21.7) | 0.022 |
| Length of stay in days, median (±IQR) | 13.0 (13) | 13.0 (12) | 15.0 (28) | 0.063 |
| Hospital mortality n (%) | 19 (10.2) | 8 (6.3) | 11 (18.3) | 0.013 |
| 30-day all-cause mortality, n (%) | 8 (4.3) | 4 (3.1) | 4 (6.7) | 0.229 |
| Hospital readmission within 30 days, n (%) | 10 (5.3) | 4 (3.1) | 6 (10.0) | 0.059 |
| Multivariate Analysis | |||
|---|---|---|---|
| OR | 95% CI | p-Value | |
| Demographics | |||
| Age | 1.002 | 0.987–1.017 | 0.766 |
| Gender | 1.313 | 0.926–1.861 | 0.126 |
| Preoperative alkaline phosphatase | 1.001 | 0.995–1.008 | 0.658 |
| Logistic EuroSCORE | 1.010 | 0.992–1.028 | 0.268 |
| EuroSCORE II | 0.980 | 0.950–1.012 | 0.218 |
| Procedure type | 1.44 | 0.969–2.150 | 0.071 |
| PVS > 3.1 | 1.833 | 0.999–3.361 | 0.050 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaefer, A.-K.; Poschner, T.; Andreas, M.; Kocher, A.; Laufer, G.; Wiedemann, D.; Mach, M. Impact of Subclinical Congestion on Outcome of Patients Undergoing Mitral Valve Surgery. Biomedicines 2020, 8, 363. https://doi.org/10.3390/biomedicines8090363
Schaefer A-K, Poschner T, Andreas M, Kocher A, Laufer G, Wiedemann D, Mach M. Impact of Subclinical Congestion on Outcome of Patients Undergoing Mitral Valve Surgery. Biomedicines. 2020; 8(9):363. https://doi.org/10.3390/biomedicines8090363
Chicago/Turabian StyleSchaefer, Anne-Kristin, Thomas Poschner, Martin Andreas, Alfred Kocher, Günther Laufer, Dominik Wiedemann, and Markus Mach. 2020. "Impact of Subclinical Congestion on Outcome of Patients Undergoing Mitral Valve Surgery" Biomedicines 8, no. 9: 363. https://doi.org/10.3390/biomedicines8090363
APA StyleSchaefer, A.-K., Poschner, T., Andreas, M., Kocher, A., Laufer, G., Wiedemann, D., & Mach, M. (2020). Impact of Subclinical Congestion on Outcome of Patients Undergoing Mitral Valve Surgery. Biomedicines, 8(9), 363. https://doi.org/10.3390/biomedicines8090363






