Integrin-Targeting Peptides for the Design of Functional Cell-Responsive Biomaterials
Abstract
1. Introduction
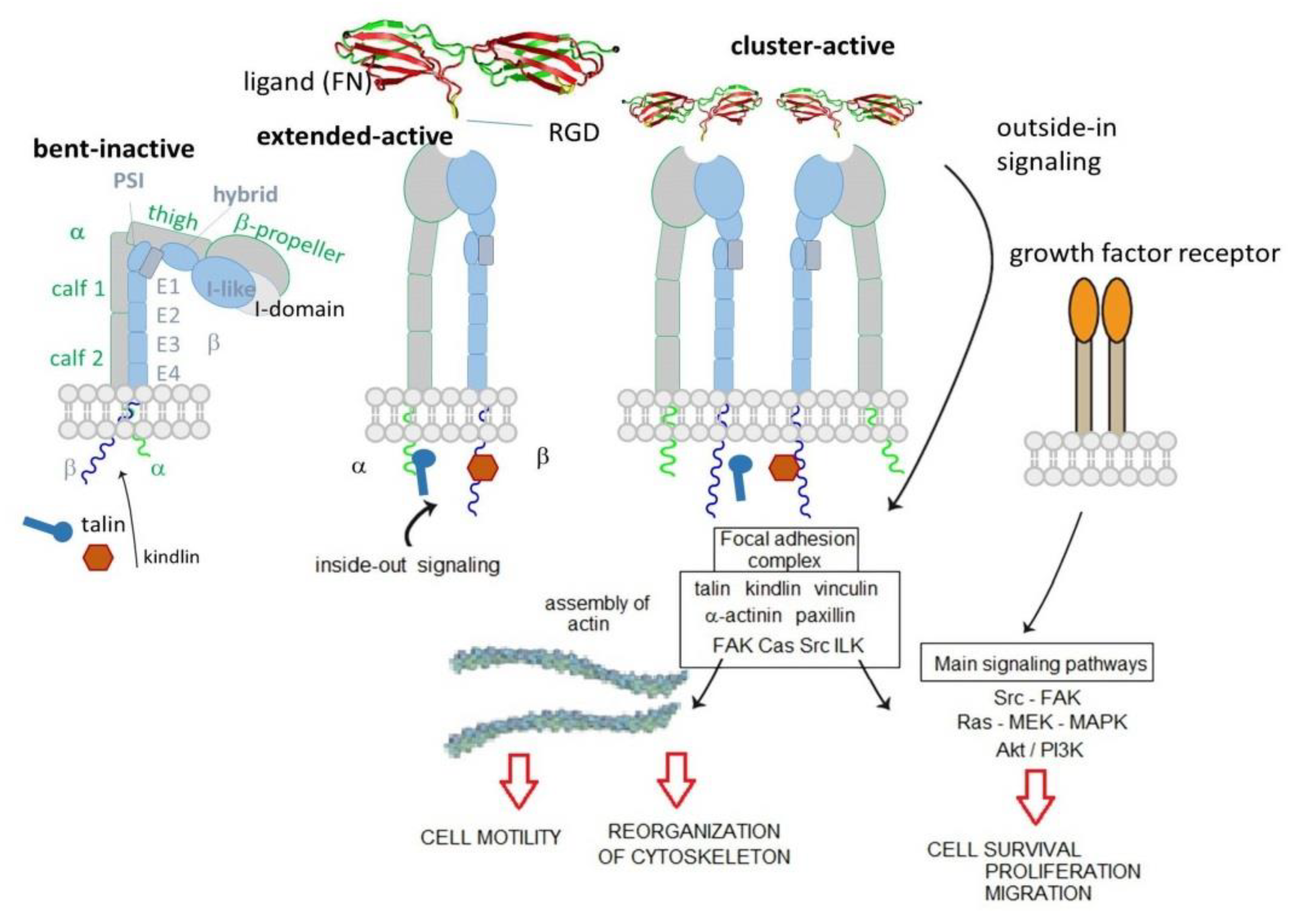
2. Integrins Structure and Functions
3. Integrin-Correlated Diseases
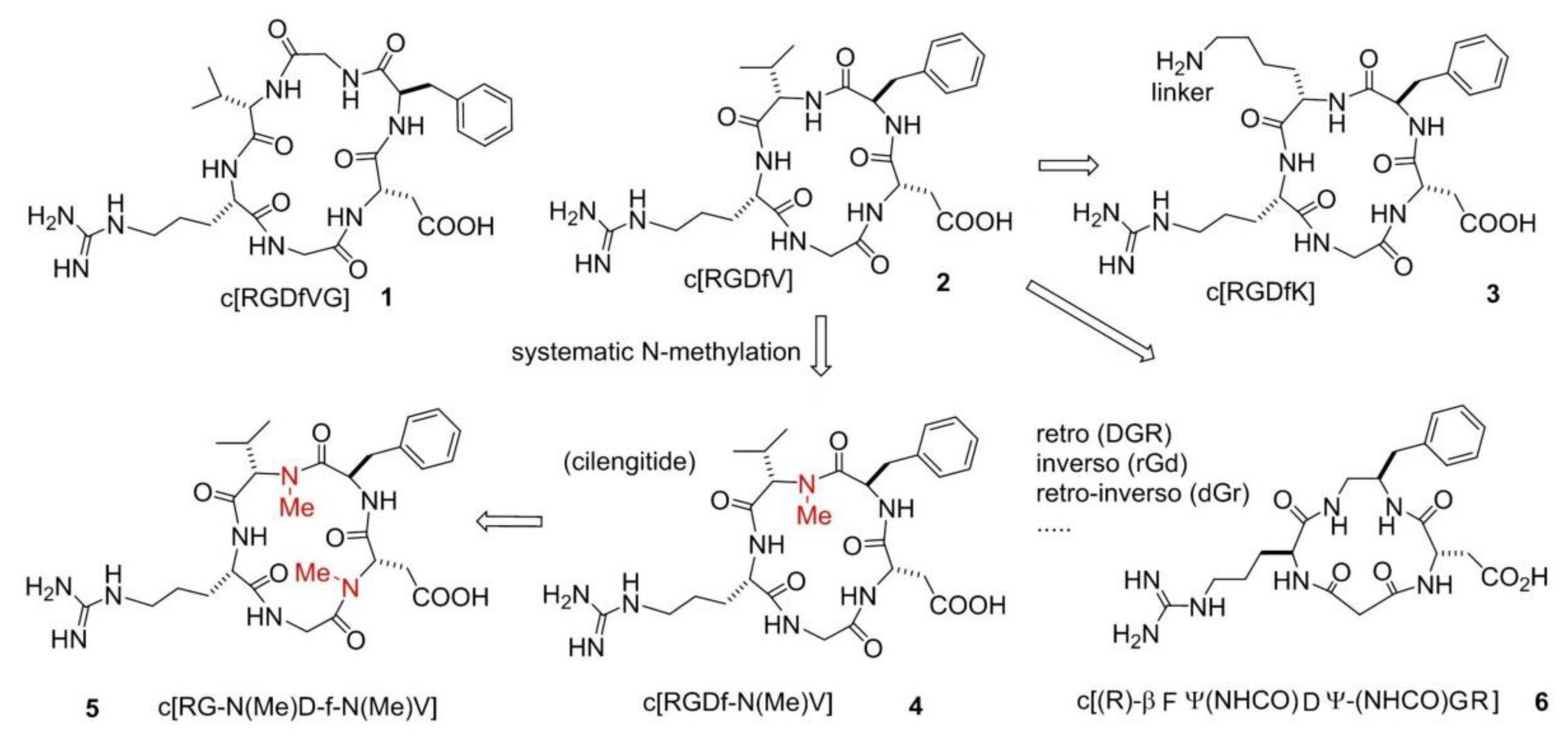
4. Peptide and Peptidomimetic Integrin Ligands
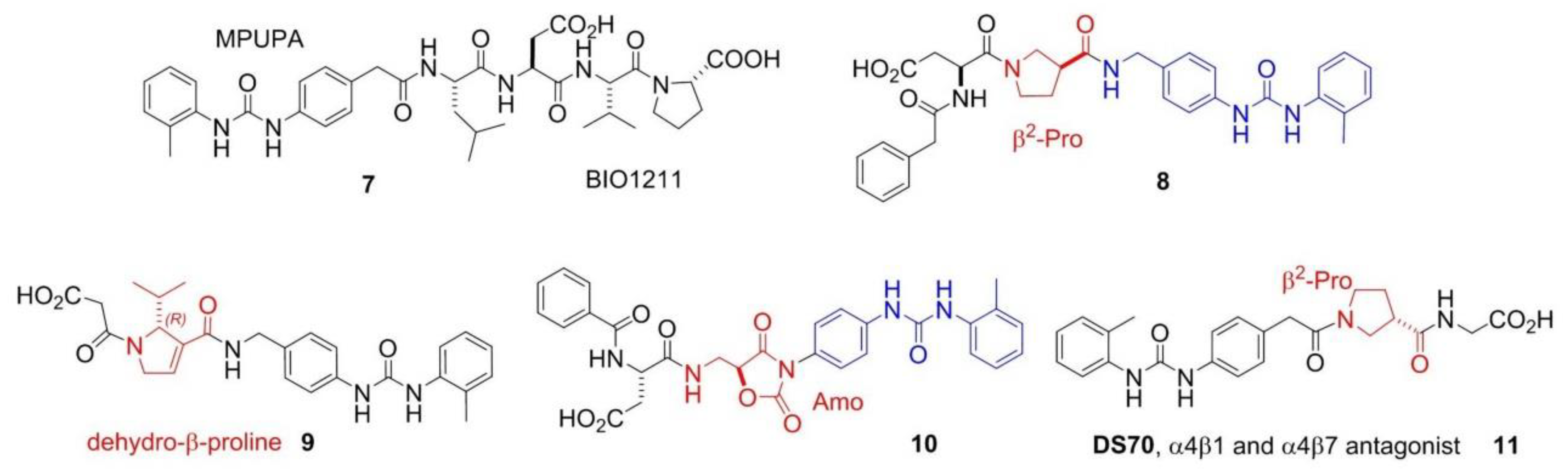
Addressing Integrins with Agonist Ligands
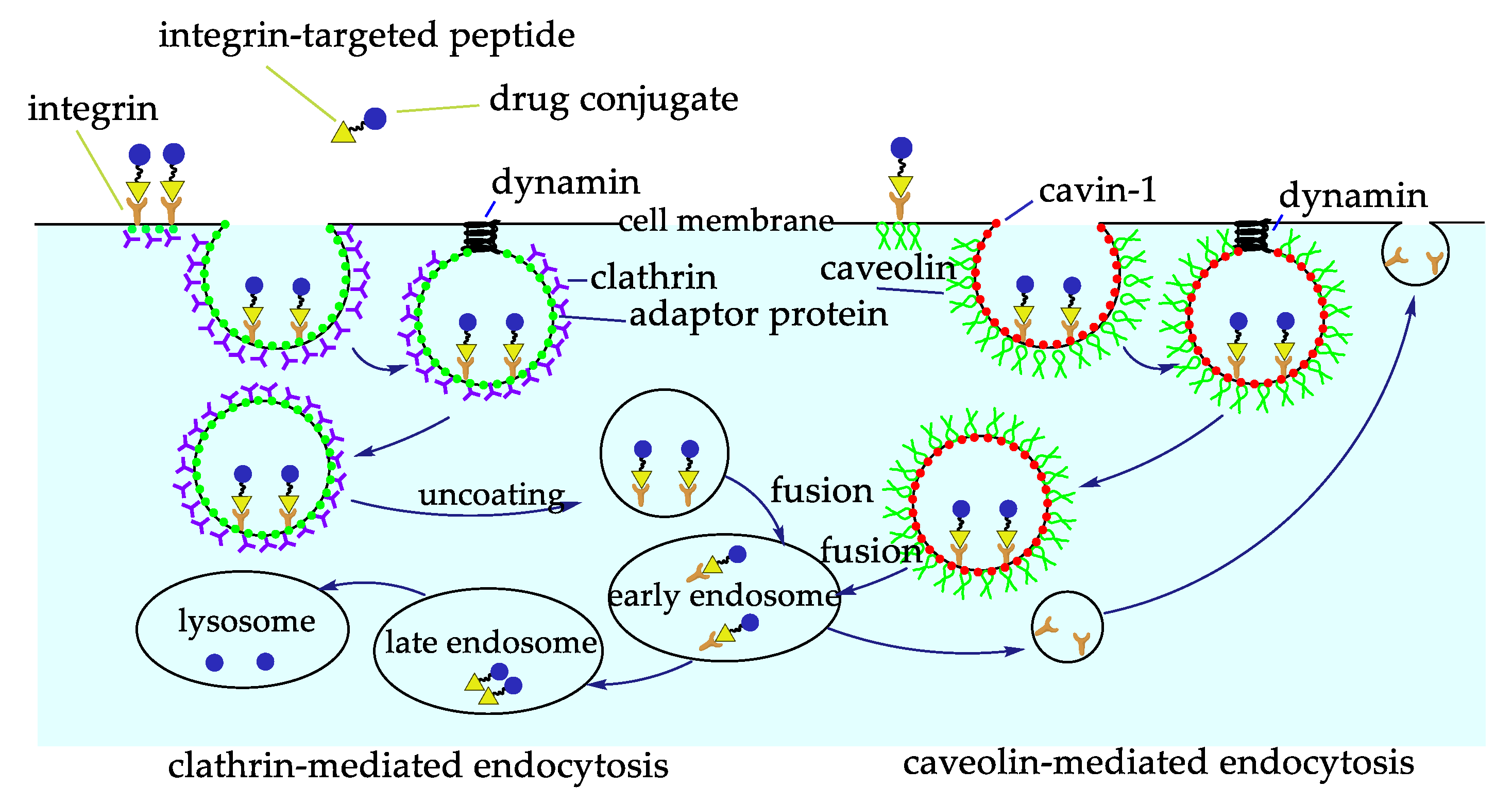
5. Integrin-Targeted Peptide Conjugates
6. Integrin-Targeted NPs
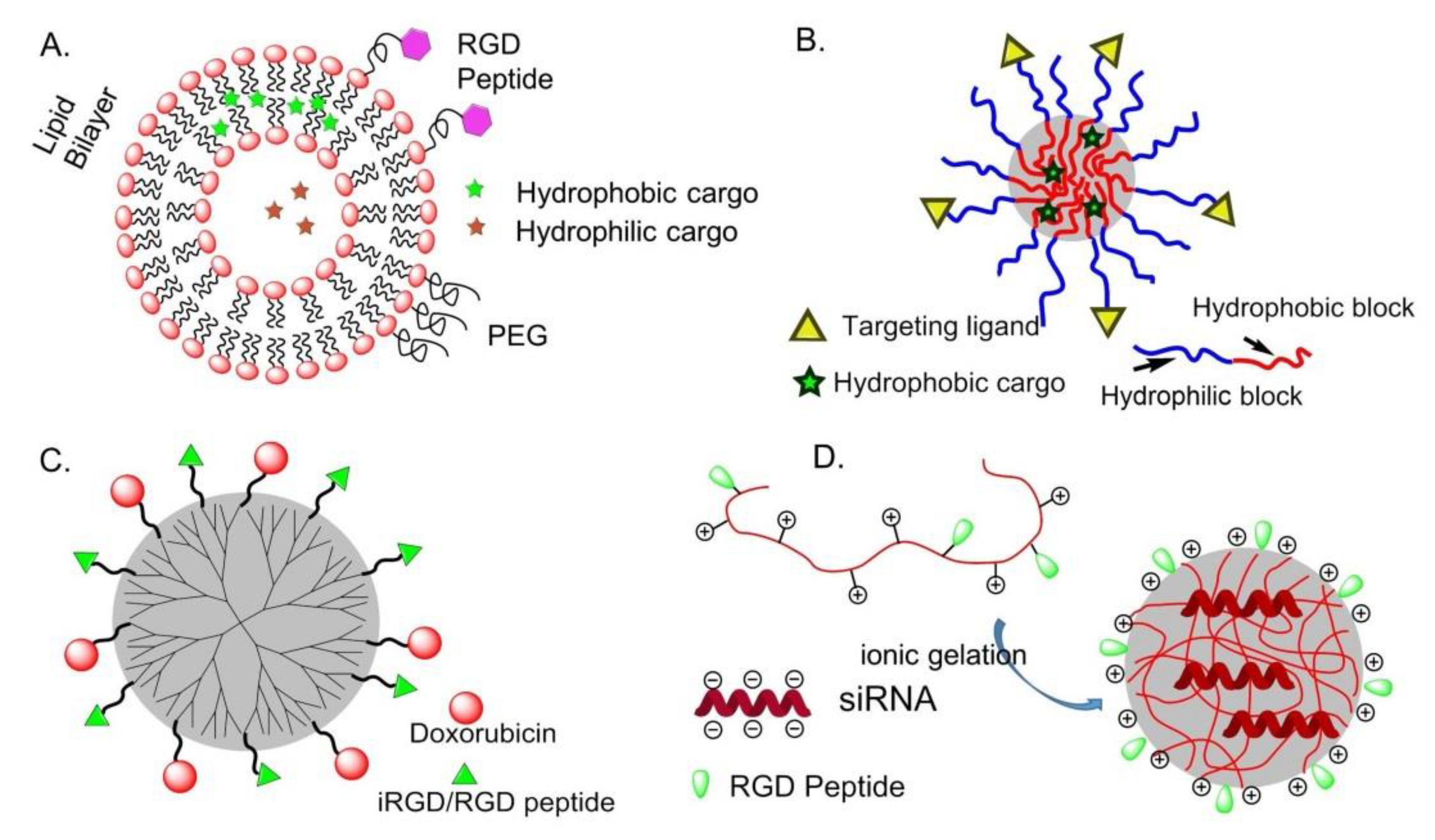
6.1. Integrin-Targeted Organic NPs
6.1.1. Liposomes
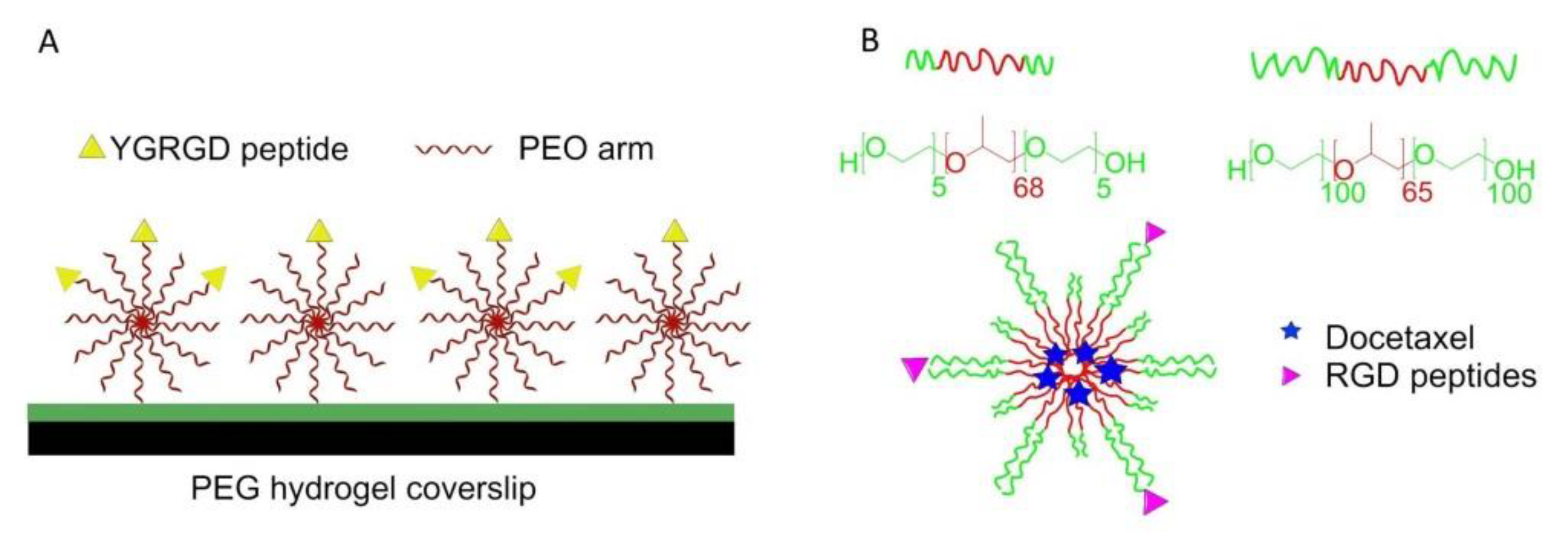
6.1.2. Polymeric Micelles (PM)
6.1.3. Dendrimers
6.1.4. Chitosan
6.1.5. Other Organic NPs
6.2. Integrin-Targeted Inorganic NPs and QDs
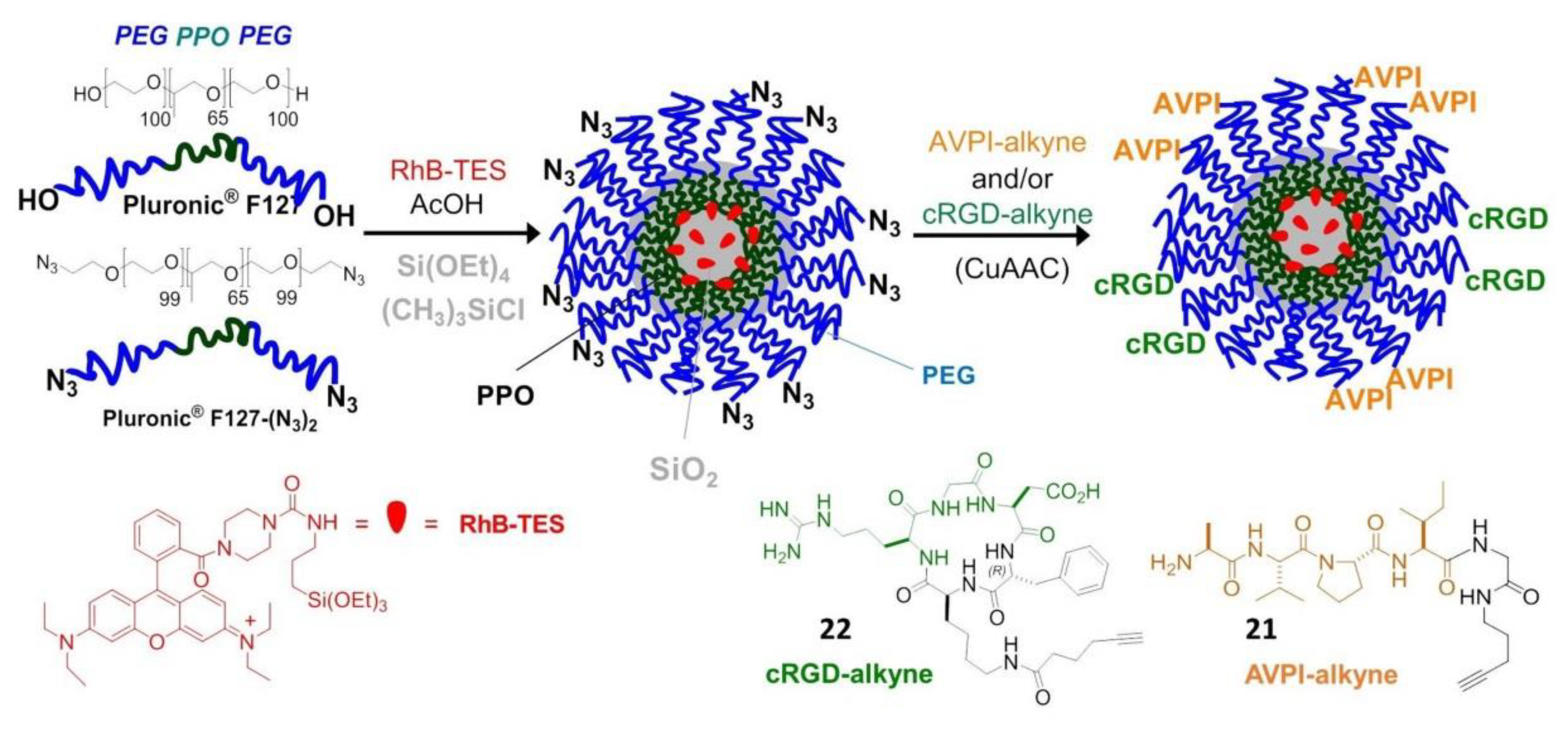
6.2.1. Silica NPs
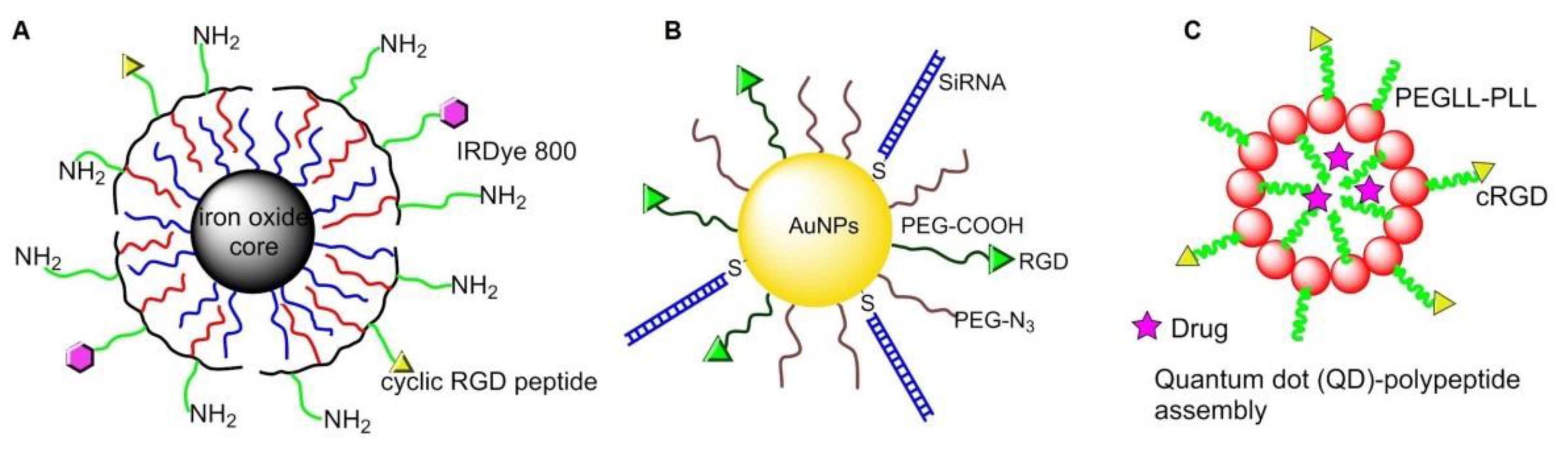
6.2.2. Magnetic NPs
6.2.3. Gold NPs
6.2.4. Quantum Dots (QDs)
7. Surfaces and Materials Functionalized with Peptidic Integrin Ligands
7.1. Self-Assembled Monolayers (SAMs)
7.2. Interfaces for Studying of Cell Adhesion, Spreading, and Differentiation
7.3. Application in Regenerative Medicine and Tissue Engineering
7.4. Fabrication Methods of Integrin Ligand Immobilized Nanostructured Surfaces
7.4.1. Blending Strategy for Surfaces Functionalization
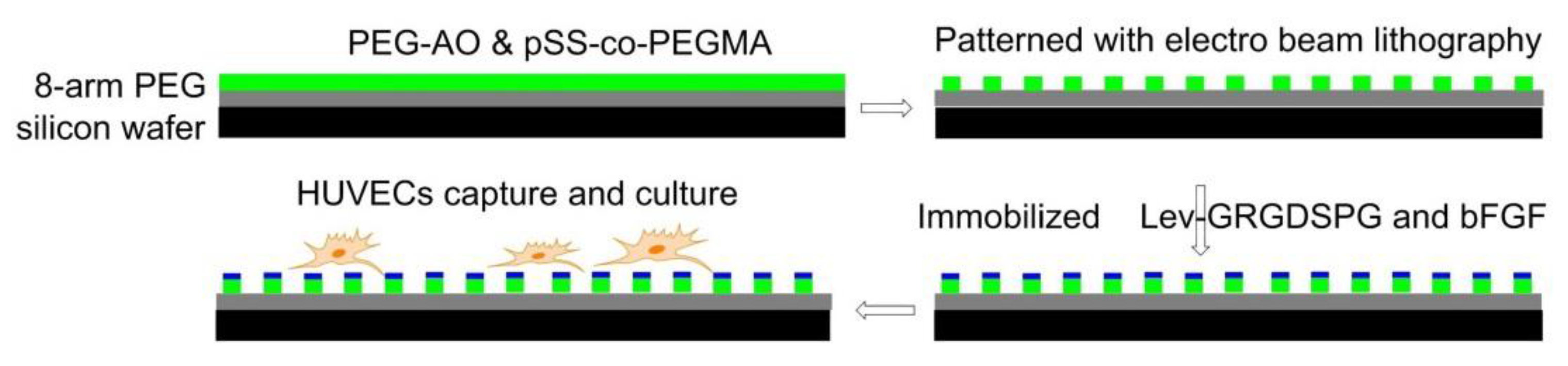
7.4.2. Electron Beam Fabricated Patterns
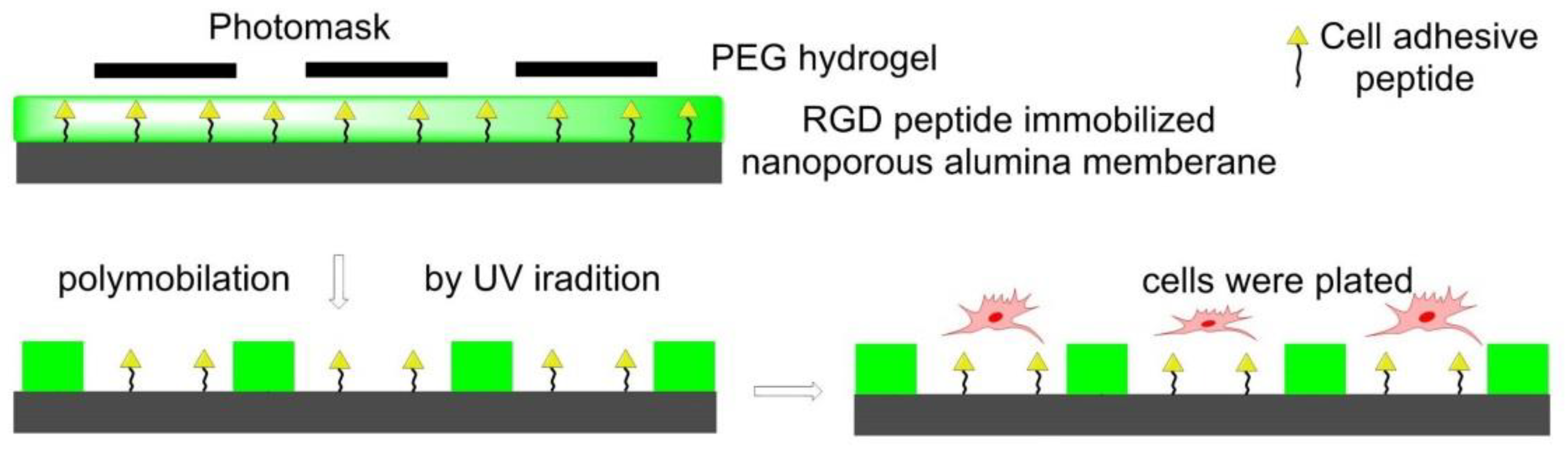
7.4.3. Photolithography Strategy for Surfaces Functionalization
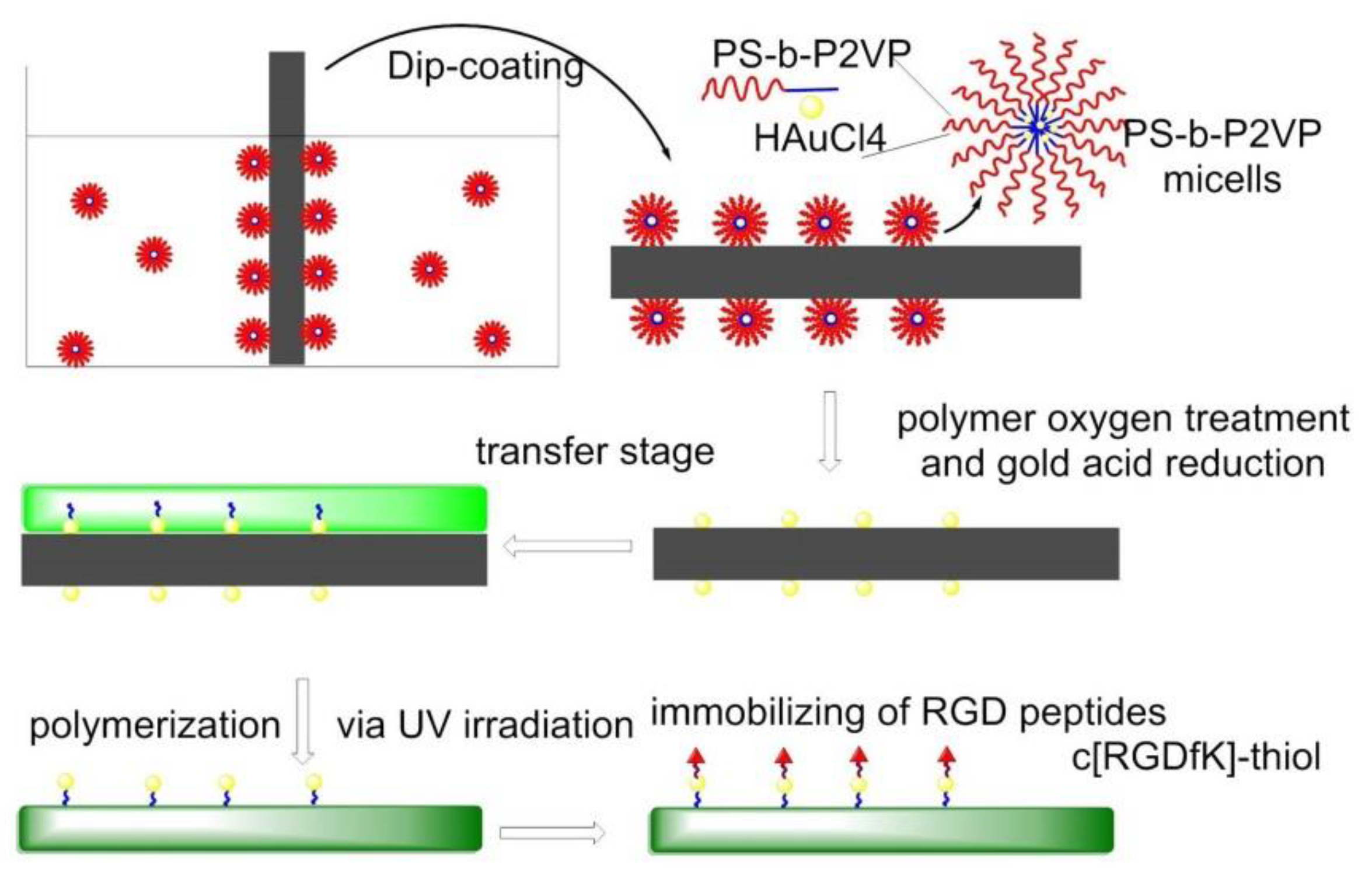
7.4.4. Nanolithography Strategy for Surfaces Functionalization
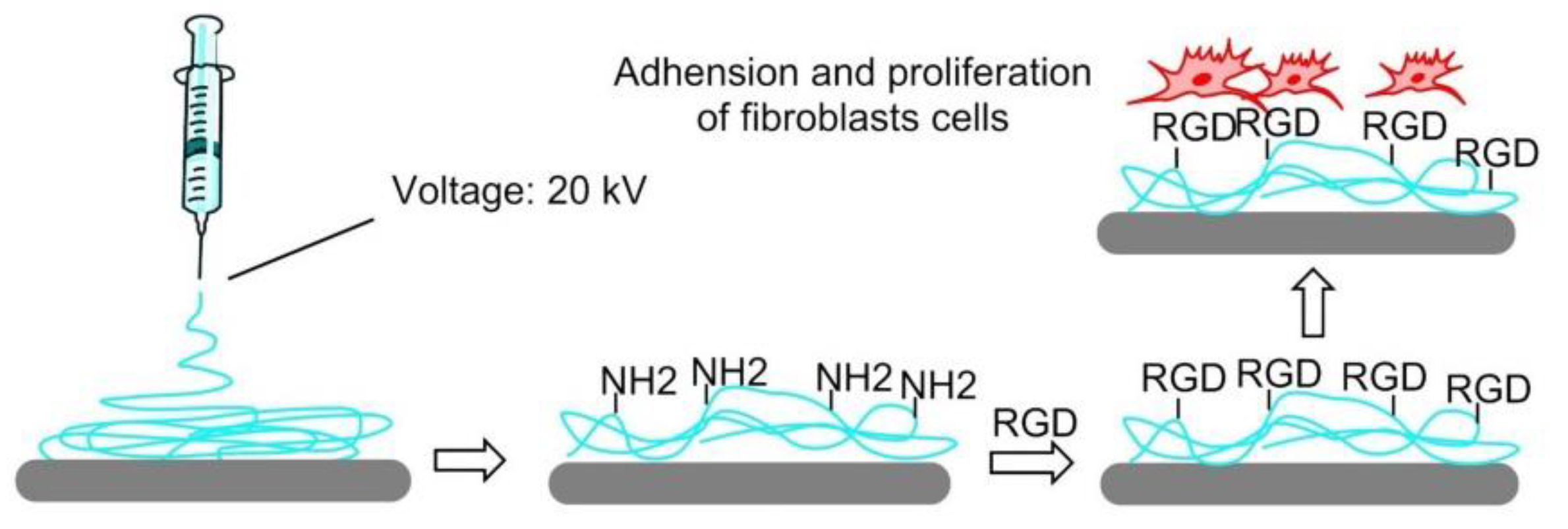
7.4.5. Electrospinning
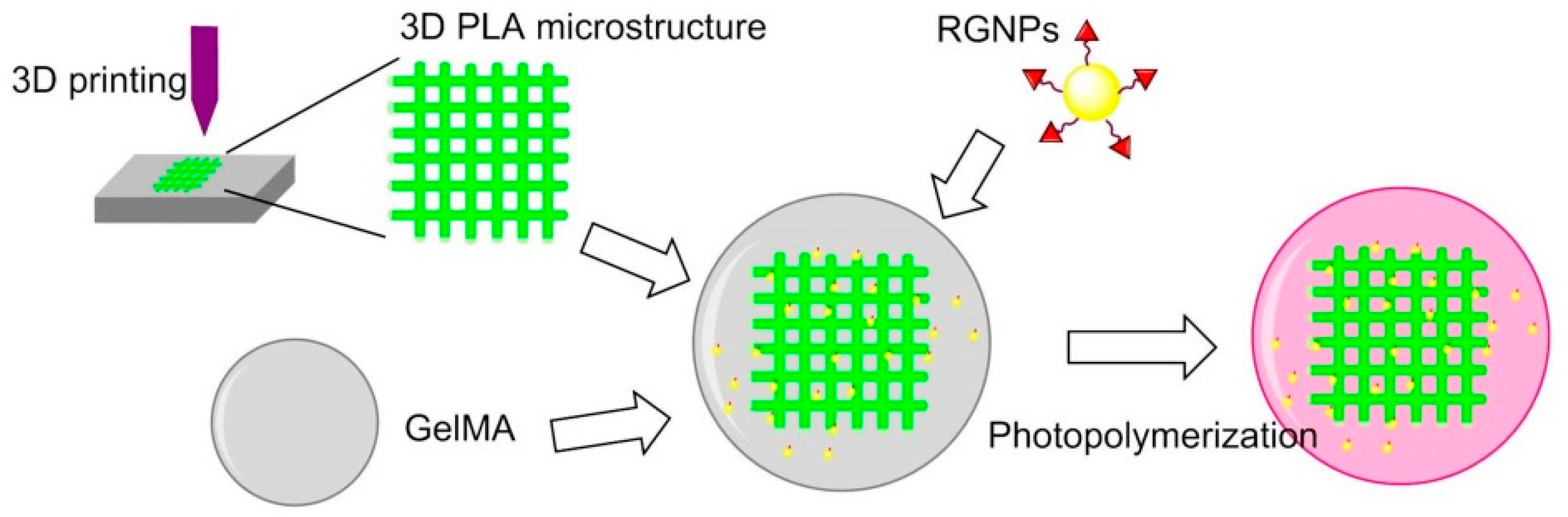
7.4.6. 3D Printing
7.5. Detection of Tumor Cells
7.6. Ligands Other than RGD
7.7. Multifunctional Integrin-Targeting Biocompatible Surfaces
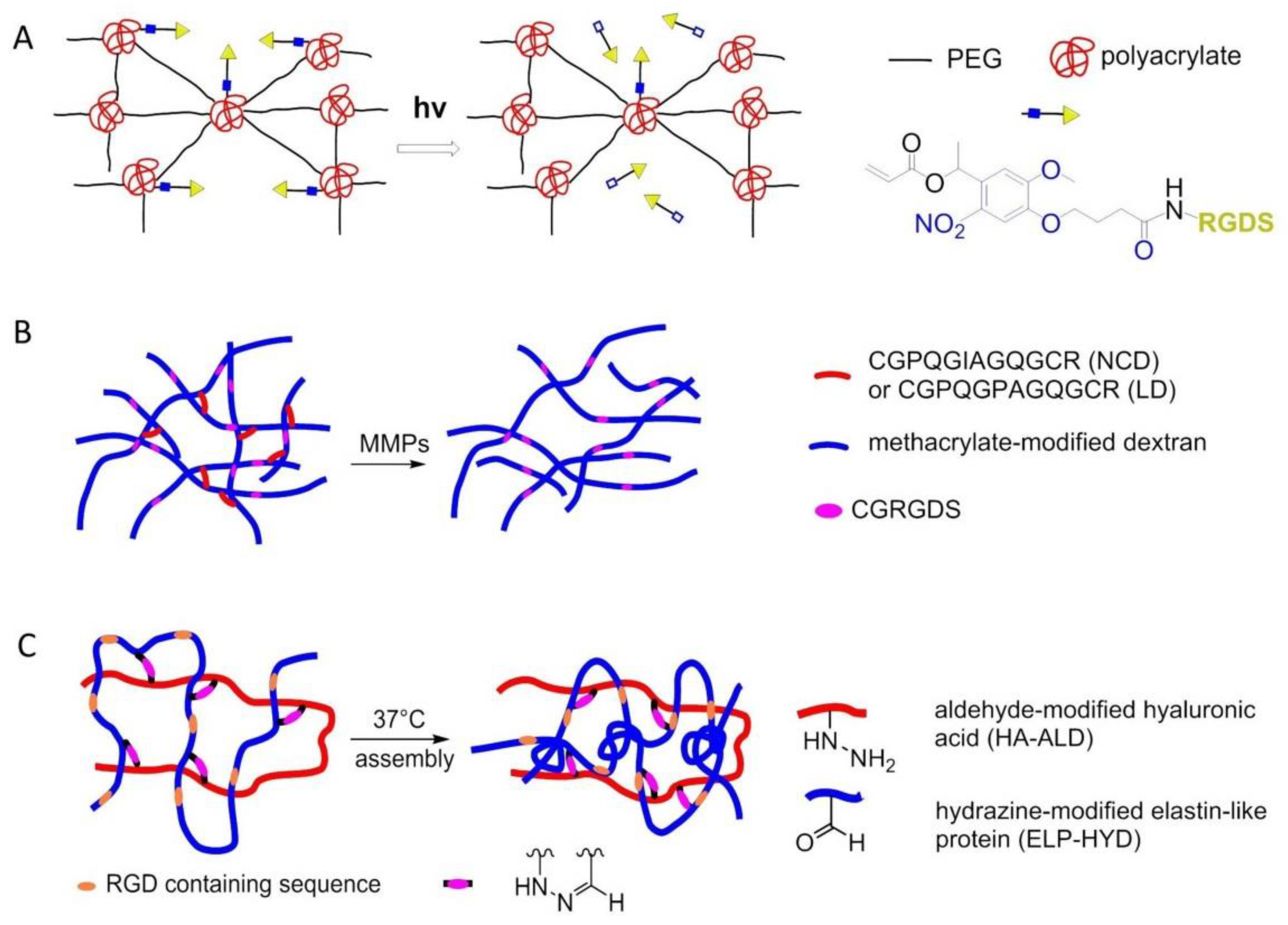
8. Nanostructured 2D or 3D Smart Interfaces for Dynamic Cell Adhesion
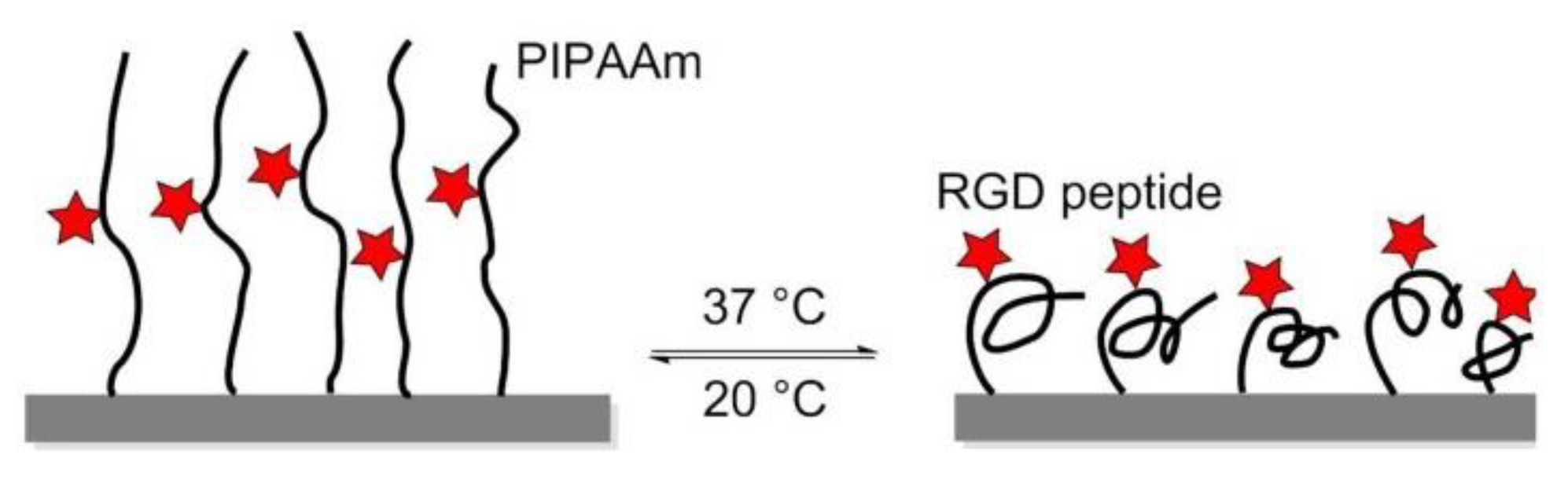
8.1. Thermoresponsive Polymers
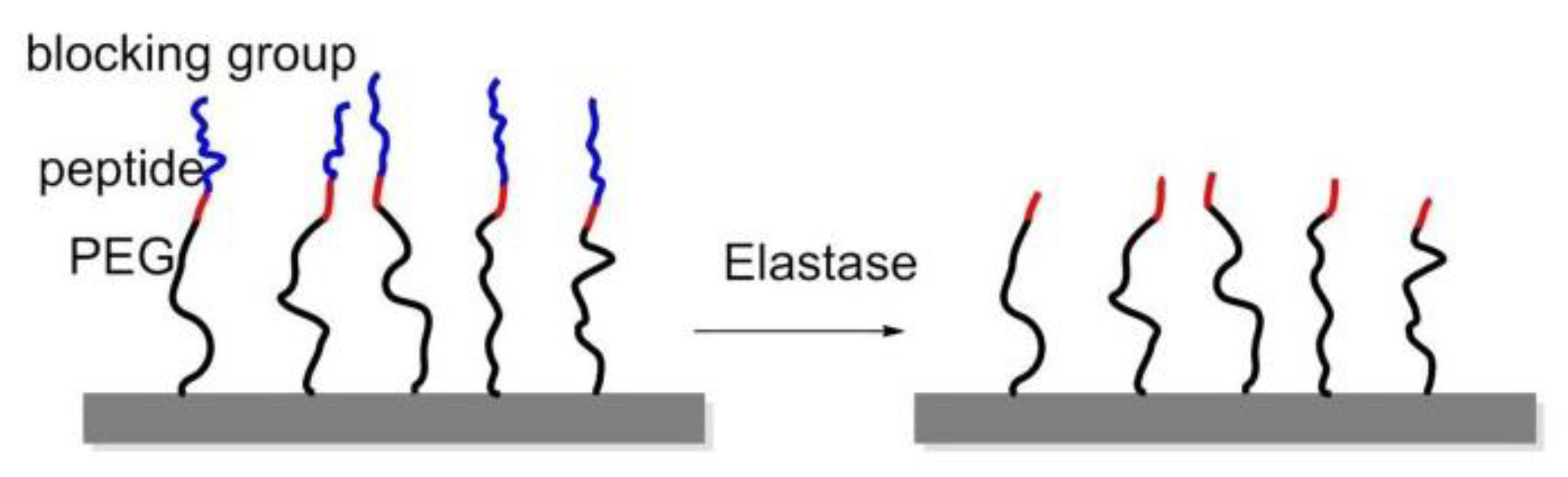
8.2. Enzyme-Triggered Polymers
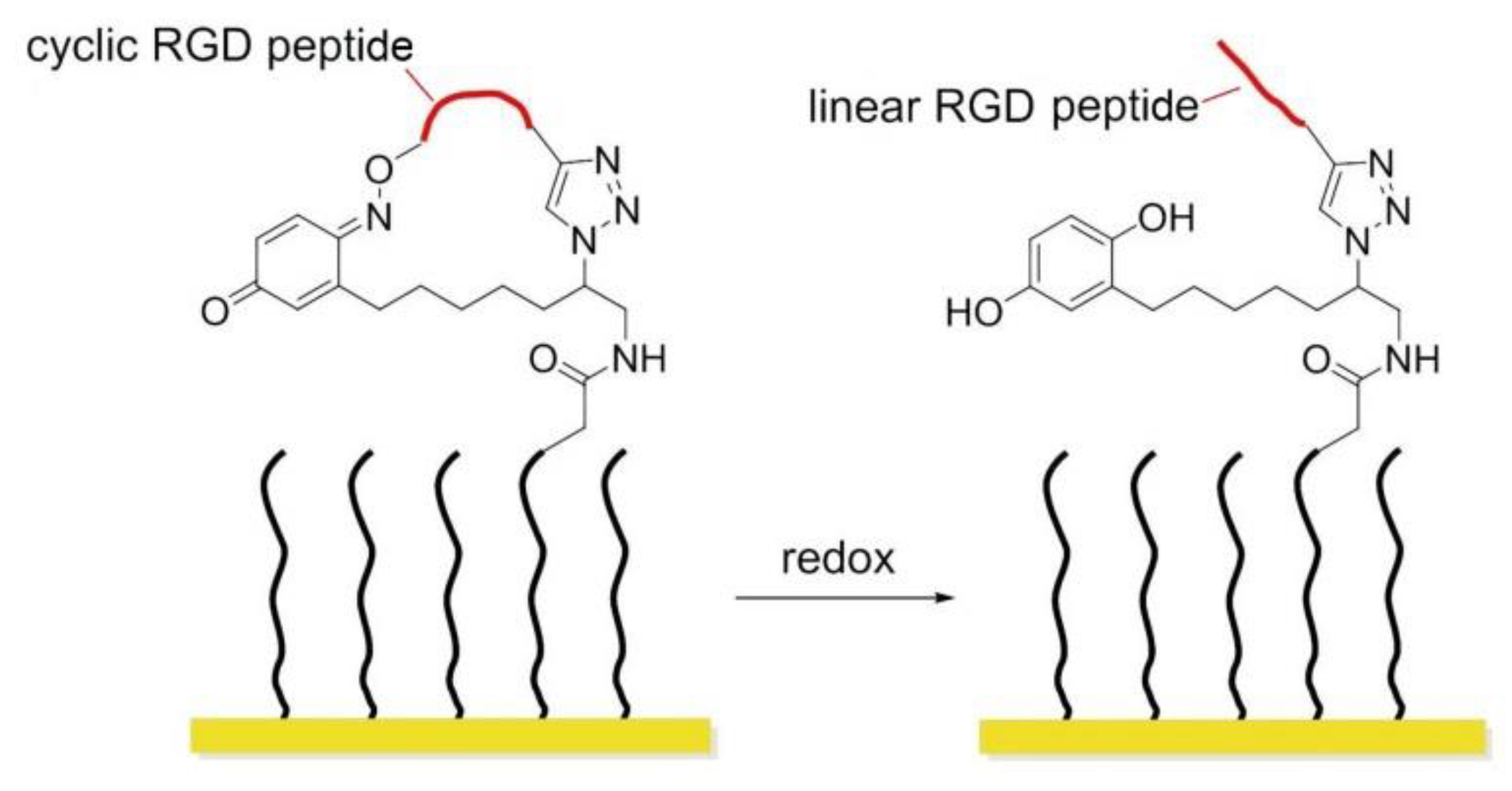
8.3. Redox-Switchable Polymers
8.4. Potential Responsive Polymers
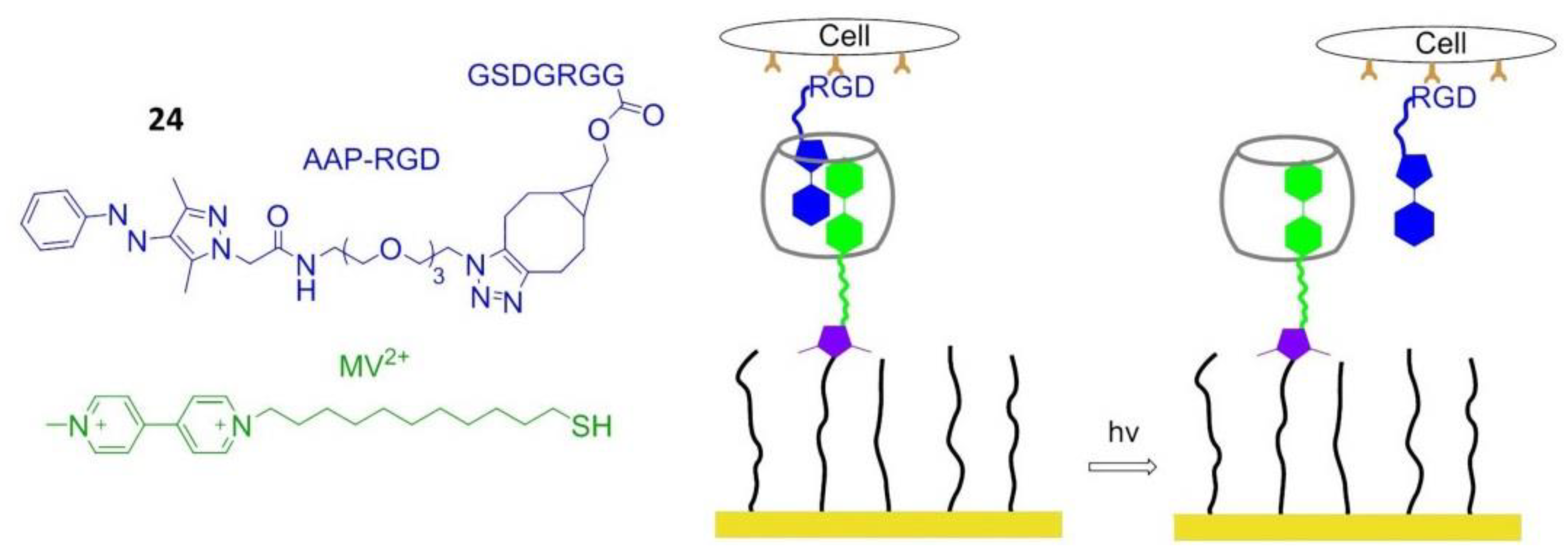
8.5. Photo Responsive Polymers
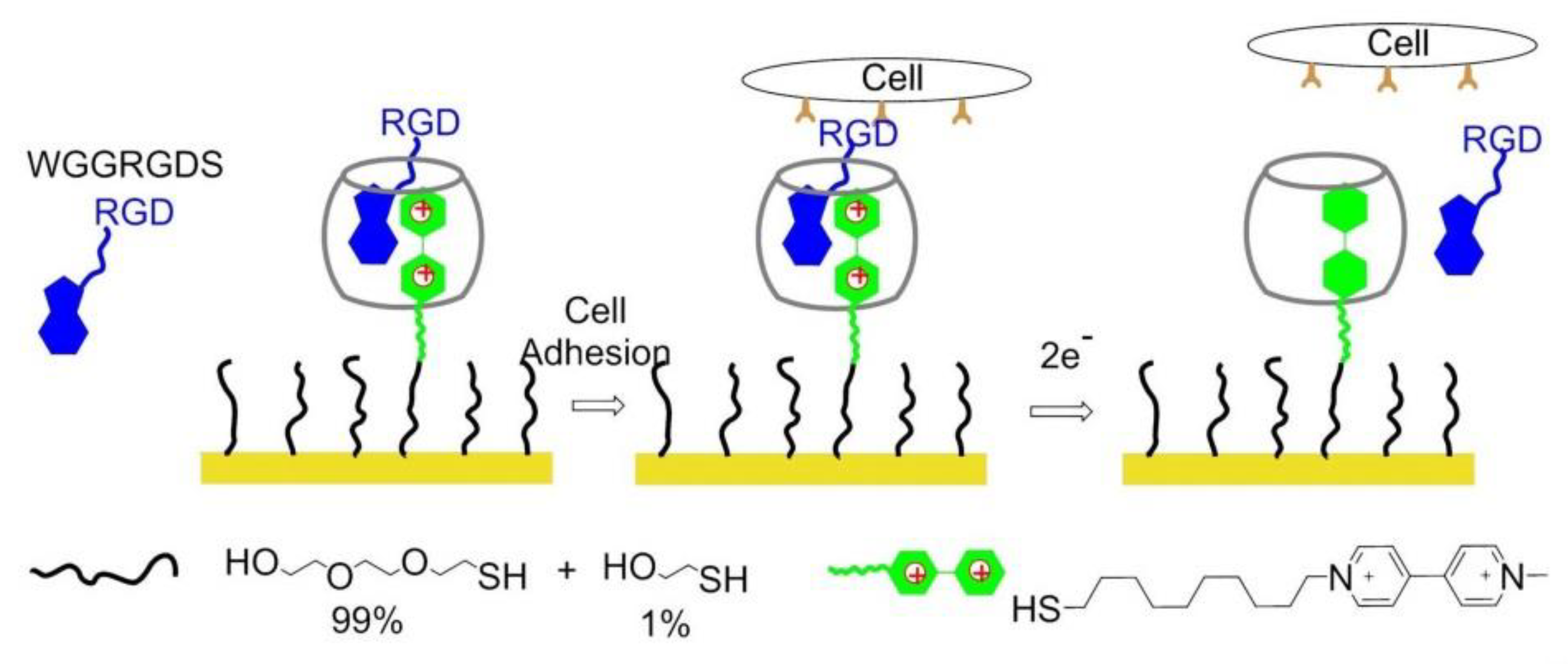
8.6. Electrochemically Controlled Polymers
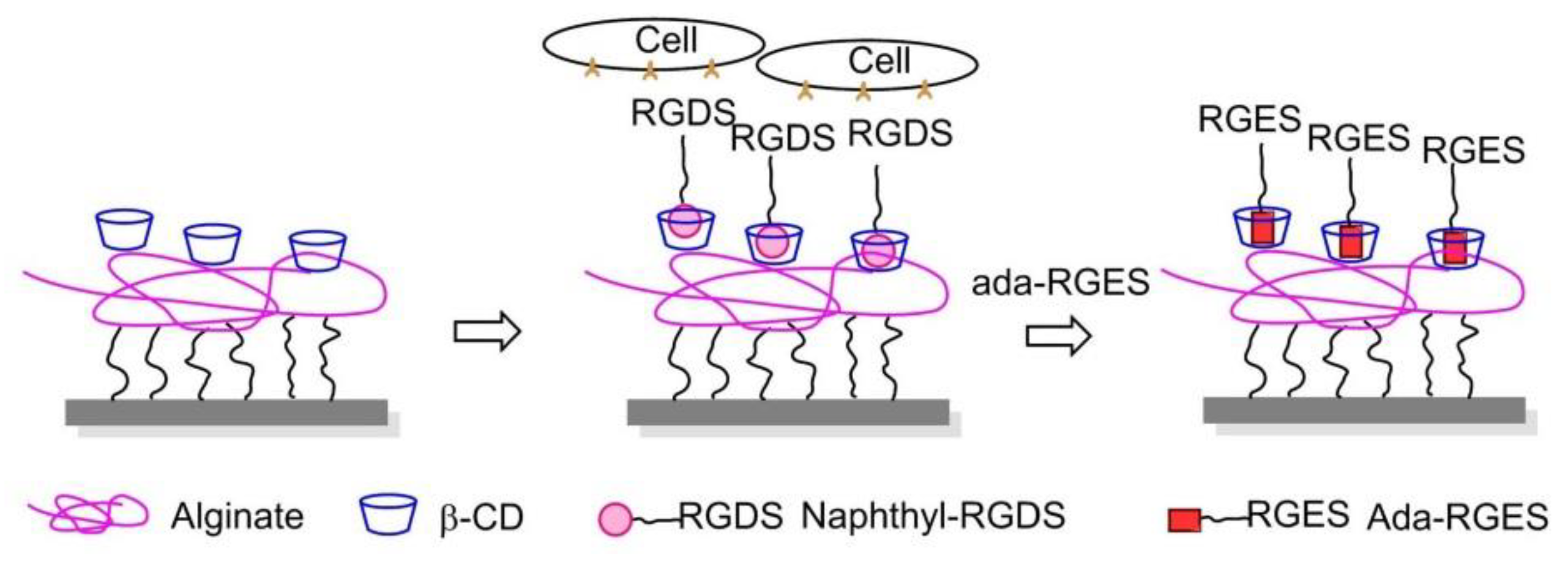
8.7. Dynamically Competitive Polymers
8.8. Dynamically Controlled Smart Interfaces for 3D Cell Culture
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baiula, M.; Spampinato, S.; Gentilucci, L.; Tolomelli, A. Novel ligands targeting α4β1 integrin: Therapeutic applications and perspectives. Front. Chem. 2019, 7, 489. [Google Scholar] [CrossRef]
- Danhier, F.; Le Breton, A.; Préat, V. RGD-based strategies to target ανβ3 integrin in cancer therapy and diagnosis. Mol. Pharm. 2012, 9, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- De Marco, R.; Tolomelli, A.; Juaristi, E.; Gentilucci, L. Integrin ligands with α/β-hybrid peptide structure: Design, bioactivity, and conformational aspects. Med. Res. Rev. 2016, 36, 389–424. [Google Scholar] [CrossRef]
- Curley, G.P.; Blum, H.; Humphries, M.J. Integrin antagonists. Cell. Mol. Life Sci. 1999, 56, 427–441. [Google Scholar] [CrossRef]
- Auzzas, L.; Zanardi, F.; Battistini, L.; Burreddu, P.; Carta, P.; Rassu, G.; Curti, C.; Casiraghi, G. Targeting ανβ3 integrin: Design and applications of mono- and multifunctional RGD-based peptides and semipeptides. Curr. Med. Chem. 2010, 17, 1255–1299. [Google Scholar] [CrossRef]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation and interaction. Cold Spring Harb. Perspect. Biol. 2011, 3, a004994. [Google Scholar] [CrossRef]
- Zent, R.; Pozzi, A. Cell-Extracellular Matrix Interaction in Cancer; Springer: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Li, J.; Springer, T.A. Integrin extension enables ultrasensitive regulation by cytoskeletal force. Proc. Natl. Acad. Sci. USA 2017, 114, 4685–4690. [Google Scholar] [CrossRef]
- Hillis, G.S.; MacLeod, A.M. lntegrins and disease. Clin. Sci. 1996, 91, 639–650. [Google Scholar] [CrossRef]
- Winograd-Katz, S.E.; Fassler, R.; Geiger, B.; Legate, K.R. The integrin adhesome: From genes and proteins to human disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 273–288. [Google Scholar] [CrossRef]
- Ni, H.; Freedman, J. Platelets in hemostasis and thrombosis: Role of integrins and their ligands. Transfus. Apher. Sci. 2003, 28, 257–264. [Google Scholar] [CrossRef]
- Topol, E.J.; Califf, R.M.; Weisman, H.F.; Ellis, S.G.; Tcheng, J.E.; Worley, S.; Ivanhoe, R.; George, B.S.; Fintel, D.; Weston, M.; et al. Randomised trial of coronary intervention with antibody against platelet IIb/IIIa iritegrin for reduction of clinical restenosis: Results at six months. Lancet 1994, 343, 881–886. [Google Scholar] [CrossRef]
- Chico, T.J.A.; Chamberlain, J.; Gunn, J.; Arnold, N.; Bullens, S.L.; Gadek, T.R.; Francis, S.E.; Bunting, S.; Horton, M.; Shepherd, L.; et al. Effect of selective or combined inhibition of integrins αIIbβ3 and ανβ3 on thrombosis and neointima after oversized porcine coronary angioplasty. Circulation 2001, 103, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Herter, J.; Zarbock, A. Integrin regulation during leukocytes recruitment. J. Immunol. 2013, 190, 4451–4457. [Google Scholar] [CrossRef]
- Von Andrian, U.H.; Engelhardt, B. Alpha(4) integrins as therapeutic targets in autoimmune disease. N. Engl. J. Med. 2003, 348, 68–72. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Osteoporosis and integrins. J. Bone Miner. Metab. 2000, 18, 344–349. [Google Scholar] [CrossRef]
- Friedlander, M.; Theesfeld, C.L.; Sugita, M.; Fruttiger, M.; Thomas, M.A.; Chang, S.; Cheresh, D.A. Involvement of integrins ανβ3 and ανβ5 in ocular neovascular diseases. Proc. Natl. Acad. Sci. USA 1996, 93, 9764–9769. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.E.; Choi, D.K.; Jang, J.Y.; Jung, J.J.; Kiyonari, H.; Shioi, G.; Chang, W.; Suda, T.; Mochizuki, N.; et al. Angiopoietin-1 guides directional angiogenesis through integrin ανβ5 signaling for recovery of ischemic retinopathy. Sci. Transl. Med. 2013, 5, 203ra127. [Google Scholar] [CrossRef]
- Avraamides, C.J.; Garmy-Susini, B.; Varner, J.A. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 2008, 8, 604–617. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Pierschbacher, M.D. Arg-Gly-Asp: A versatile cell recognition signal. Cell 1986, 44, 517–518. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Pierschbacher, M.D. New perspectives in cell adhesion: RGD and integrins. Science 1987, 238, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Haubner, R.; Gratias, R.; Diefenbach, B.; Goodman, S.L.; Jonczyk, A.; Kessler, H. Structural and functional aspects of RGD-containing cyclic pentapeptides as highly potent and selective integrin ανβ3 antagonists. J. Am. Chem. Soc. 1996, 118, 7461–7472. [Google Scholar] [CrossRef]
- Gentilucci, L.; De Marco, R.; Cerisoli, L. Chemical modifications designed to improve peptide stability: Incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr. Pharm. Des. 2010, 16, 3185–3203. [Google Scholar] [CrossRef] [PubMed]
- Gentilucci, L.; Tolomelli, A.; Squassabia, F. Peptides and peptidomimetics in medicine, surgery and biotechnology. Curr. Med. Chem. 2006, 13, 2449–2466. [Google Scholar] [CrossRef]
- Gentilucci, L.; Tosi, P.; Bauer, A.; De Marco, R. Modern tools for the chemical ligation and synthesis of modified peptides and proteins. Future Med. Chem. 2016, 8, 2287–2304. [Google Scholar] [CrossRef] [PubMed]
- Gentilucci, L.; Cardillo, G.; Spampinato, S.; Tolomelli, A.; Squassabia, F.; De Marco, R.; Bedini, A.; Baiula, M.; Belvisi, L.; Civera, M. Antiangiogenic effect of dual/selective α5β1/ανβ3 integrin antagonists designed on partially modified retro-inverso cyclotetrapeptide mimetics. J. Med. Chem. 2010, 53, 106–118. [Google Scholar] [CrossRef]
- Tolomelli, A.; Gentilucci, L.; Mosconi, E.; Viola, A.; Dattoli, S.D.; Baiula, M.; Spampinato, S.; Belvisi, L.; Civera, M. Development of isoxazoline-containing peptidomimetics as dual ανβ3 and αν5β1 integrin ligands. ChemMedChem 2011, 6, 2264–2272. [Google Scholar] [CrossRef]
- Tolomelli, A.; Baiula, M.; Belvisi, L.; Viola, A.; Gentilucci, L.; Troisi, S.; Dattoli, S.D.; Spampinato, S.; Civera, M.; Juaristi, E.; et al. Modulation of ανβ3- and α5β1-integrin-mediated adhesion by dehydro-β-amino acids containing peptidomimetics. Eur. J. Med. Chem. 2013, 66, 258–268. [Google Scholar] [CrossRef]
- De Marco, R.; Mazzotti, G.; Greco, A.; Gentilucci, L. Heterocyclic scaffolds in the design of peptidomimetic integrin ligands: Synthetic strategies, structural aspects, and biological activity. Curr. Top. Med. Chem. 2016, 16, 343–359. [Google Scholar] [CrossRef]
- Mitjans, F.; Meyer, T.; Fittschen, C.; Goodman, S.; Jonczyk, A.; Marshall, J.F.; Reyes, G.; Piulats, J. In Vivo therapy of malignant melanoma by means of antagonists of αν integrins. Int. J. Cancer 2000, 87, 716–723. [Google Scholar] [CrossRef]
- Lomonaco, S.L.; Finniss, S.; Xiang, C.; Lee, H.K.; Jiang, W.; Lemke, N.; Rempel, S.A.; Mikkelsen, T.; Brodie, C. Cilengitide induces autophagy-mediated cell death in glioma cells. Neurooncology 2011, 13, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Taga, T.; Suzuki, A.; Gonzalez-Gomez, I.; Gilles, F.H.; Stins, M.; Shimada, H.; Barsky, L.; Weinberg, K.I.; Laug, W.E. Alpha(ν)-integrin antagonist EMD 121974 induces apoptosis in brain tumor cells growing on vitronectin and tenascin. Int. J. Cancer 2002, 98, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Nabors, L.B.; Mikkelsen, T.; Rosenfeld, S.S.; Hochberg, F.; Akella, N.S.; Fisher, J.D.; Cloud, G.A.; Zhang, Y.; Carson, K.; Wittemer, S.M.; et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J. Clin. Oncol. 2007, 25, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, T.J.; Stewart, C.F.; Kocak, M.; Goldman, S.; Ellenbogen, R.G.; Phillips, P.; Lafond, D.; Poussaint, T.Y.; Kieran, M.W.; Boyett, J.M.; et al. Phase I clinical trial of cilengitide in children with refractory brain tumors: Pediatric brain tumor consortium study PBTC-012. J. Clin. Oncol. 2008, 26, 919–924. [Google Scholar] [CrossRef]
- Fink, K.; Mikkelsen, T.; Nabors, L.B.; Ravin, P.; Plotkin, S.R.; Schiff, D.; Hicking, C.; Picard, M.; Reardon, D.A. Long-term effects of cilengitide, a novel integrin inhibitor, in recurrent glioblastoma: A randomized phase IIa study. J. Clin. Oncol. 2010, 28, 15. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Kuhn, J.; Lamborn, K.R.; Lieberman, F.; Wen, P.Y.; Mehta, M.; Cloughesy, T.; Lassman, A.B.; Deangelis, L.M.; Chang, S.; et al. Cilengitide in patients with recurrent glioblastoma: The results of NABTC 03-02, a phase II trial with measures of treatment delivery. J. Neurooncol. 2012, 106, 147–153. [Google Scholar] [CrossRef]
- Massabeau, C.; Khalifa, J.; Filleron, T.; Modesto, A.; Bigay-Gamé, L.; Plat, G.; Dierickx, L.; Aziza, R.; Rouquette, I.; Gomez-Roca, C.; et al. Continuous infusion of cilengitide plus chemoradiotherapy for patients with stage III non–small-cell lung cancer: A phase I study. Clin. Lung Cancer 2018, 19, e277–e285. [Google Scholar] [CrossRef]
- Yang, G.X.; Hagmann, W.K. VLA-4 antagonists: Potent inhibitors of lymphocyte migration. Med. Res. Rev. 2003, 23, 369–392. [Google Scholar] [CrossRef]
- Lin, K.C.; Ateeq, H.S.; Hsiung, S.H.; Chong, L.T.; Zimmerman, C.N.; Castro, A.; Lee, W.C.; Hammond, C.E.; Kalkunte, S.; Chen, L.L.; et al. Selective, tight-binding inhibitors of integrin α4β1 that inhibit allergic airway responses. J. Med. Chem. 1999, 42, 920–934. [Google Scholar] [CrossRef]
- Dattoli, S.D.; De Marco, R.; Baiula, M.; Spampinato, S.; Greco, A.; Tolomelli, A.; Gentilucci, L. Synthesis and assay of retro-α4β1 integrin-targeting motifs. Eur. J. Med. Chem. 2014, 12, 225–232. [Google Scholar] [CrossRef]
- Tolomelli, A.; Baiula, M.; Viola, A.; Ferrazzano, L.; Gentilucci, L.; Dattoli, S.D.; Spampinato, S.; Juaristi, E.; Escudero, M. Dehydro-β-proline containing α4β1 integrin antagonists: Stereochemical recognition in ligand-receptor interplay. ACS Med. Chem. Lett. 2015, 6, 701–706. [Google Scholar] [CrossRef] [PubMed]
- De Marco, R.; Mazzotti, G.; Dattoli, S.D.; Baiula, M.; Spampinato, S.; Greco, A.; Gentilucci, L. 5-Aminomethyloxazolidine-2,4-dione hybrid α/β-dipeptide scaffolds as inductors of constrained conformations: Applications to the synthesis of integrin antagonists. Biopolymers 2015, 104, 636–649. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Tani, S.; De Marco, R.; Gentilucci, L. Synthesis and analysis of the conformational preferences of 5-aminomethyloxazolidine-2,4-dione scaffolds: First examples of β(2)-and β(2,2)-homo-freidinger lactam analogues. Chem. Eur. J. 2014, 20, 13390–13404. [Google Scholar] [CrossRef]
- Dattoli, S.D.; Baiula, M.; De Marco, R.; Bedini, A.; Anselmi, M.; Gentilucci, L.; Spampinato, S. DS-70, a novel and potent α4 integrin antagonist, is an effective treatment for experimental allergic conjunctivitis in guinea pigs. Br. J. Pharmacol. 2018, 175, 3891–3910. [Google Scholar] [CrossRef] [PubMed]
- Celik, E.; Faridi, M.H.; Kumar, V.; Deep, S.; Moy, V.T.; Gupta, V. Agonist leukadherin-1 increases CD11b/CD18-dependent adhesion via membrane tethers. Biophys. J. 2013, 105, 2517–2527. [Google Scholar] [CrossRef]
- Vanderslice, P.; Biediger, R.J.; Woodside, D.G.; Brown, W.S.; Khounlo, S.; Warier, N.D.; Gundlach, C.W.; Caivano, A.R.; Bornmann, W.G.; Maxwell, D.S.; et al. Small molecule agonist of very late antigen-4 (VLA-4) integrin induces progenitor cell adhesion. J. Biol. Chem. 2013, 288, 19414–19428. [Google Scholar] [CrossRef]
- Schwartz, M.A.; McRoberts, K.; Coyner, M.; Andarawewa, K.L.; Frierson, H.F.; Sanders, J.M.; Swenson, S.; Markland, F.; Conaway, M.R.; Theodorescu, D. Integrin agonists as adjuvants in chemotherapy for melanoma. Clin. Cancer Res. 2008, 14, 6193–6197. [Google Scholar] [CrossRef]
- Yang, W.; Carman, C.V.; Kim, M.; Salas, A.; Shimaoka, M.; Springer, T.A. A small molecule agonist of an integrin, αLβ2. J. Biol. Chem. 2006, 281, 37904–37912. [Google Scholar] [CrossRef]
- Galletti, P.; Soldati, R.; Pori, M.; Durso, M.; Tolomelli, A.; Gentilucci, L.; Dattoli, S.D.; Baiula, M.; Spampinato, S.; Giacomini, D. Targeting integrins ανβ3 and α5β1 with new β-lactam derivatives. Eur. J. Med. Chem. 2014, 83, 284–293. [Google Scholar] [CrossRef]
- Baiula, M.; Galletti, P.; Martelli, G.; Soldati, R.; Belvisi, L.; Civera, M.; Dattoli, S.D.; Spampinato, S.M.; Giacomini, D. New β-lactam derivatives modulate cell adhesion and signaling mediated by RGD-binding and leukocyte integrins. J. Med. Chem. 2016, 59, 9721–9742. [Google Scholar] [CrossRef]
- Martelli, G.; Baiula, M.; Caligiana, A.; Galletti, P.; Gentilucci, L.; Arta, R.; Spampinato, S.; Giacomini, D. Could dissecting the molecular framework of β-lactam integrin ligands enhance selectivity? J. Med. Chem. 2019, 62, 10156–10166. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P. Integrin-Mediated delivery of drugs and nucleic acids for anti-angiogenic cancer therapy: Current landscape and remaining challenges. Bioengineering 2018, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, R.E.; Norman, J.C.; Caswell, P.T. Integrin trafficking at a glance. J. Cell Sci. 2012, 125, 3695–3701. [Google Scholar] [CrossRef]
- Temming, K.; Schiffelers, R.M.; Molemad, G.; Kok, R.J. RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature. Drug Resist. Updat. 2005, 8, 381–402. [Google Scholar] [CrossRef] [PubMed]
- Arosio, D.; Casagrande, C.; Manzoni, L. Integrin-mediated drug delivery in cancer and cardiovascular diseases with peptide-functionalized nanoparticles. Curr. Med. Chem. 2012, 19, 3128–3151. [Google Scholar] [CrossRef] [PubMed]
- Pysz, I.; Jackson, P.J.M.; Thurston, D.E. Introduction to antibody-drug conjugates (ADCs). In Cytotoxic Payloads for Antibody-Drug Conjugates; Thurston, D.E., Jackson, P.J.M., Eds.; Royal Society of Chemistry: London, UK, 2019. [Google Scholar] [CrossRef]
- Dal Corso, A.; Pignataro, L.; Belvisi, L.; Gennari, C. Alpha(ν)beta(3) Integrin-targeted peptide/peptidomimetic-drug conjugates: In-Depth analysis of the linker technology. Curr. Top. Med. Chem. 2016, 16, 1–16. [Google Scholar] [CrossRef]
- Zhuang, C.; Guan, X.; Ma, H.; Cong, H.; Zhang, W.; Miao, Z. Small molecule-drug conjugates: A novel strategy for cancer-targeted treatment. Eur. J. Med. Chem. 2019, 163, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Olenyuk, B.Z.; Okamoto, C.T.; Hamm-Alvarez, S.F. Targeting receptor-mediated endocytotic pathways with nanoparticles: Rationale and advances. Adv. Drug Deliv. Rev. 2013, 65, 121–138. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.G.; Kim, N.; Hanes, J.; Ensign, L.M. Gylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Spicer, C.D.; Pashuck, E.T.; Stevens, M.M. Achieving controlled biomolecule-biomaterial conjugation. Chem. Rev. 2018, 118, 7702–7743. [Google Scholar] [CrossRef]
- Gabizon, A.; Papahadjopoulos, D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc. Natl. Acad. Sci. USA 1988, 85, 6949–6953. [Google Scholar] [CrossRef] [PubMed]
- Madni, A.; Sarfraz, M.; Rehman, M.; Ahmad, M.; Akhtar, N.; Ahmad, S.; Tahir, N.; Ijaz, S.; Al-Kassas, R.; Lobenberg, R. Liposomal drug delivery: A versatile platform for challenging clinical applications. J. Pharm. Pharm. Sci. 2014, 17, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Schiffeler, R.M.; Koning, G.A.; ten Hagen, T.L.M.; Fens, M.H.A.M.; Schraa, A.J.; Janssen, A.P.C.A.; Kok, R.J.; Molema, G.; Storm, G. Antitumor efficacy of tumor vasculature-targeted liposomal doxorubicin. J. Control. Release 2003, 91, 115–122. [Google Scholar] [CrossRef]
- Bianchini, F.; De Santis, A.; Portioli, E.; Krauss, I.R.; Battistini, L.; Curti, C.; Peppicelli, S.; Calorini, L.; D’Errico, G.; Zanardi, F.; et al. Integrin-Targeted AmpRGD sunitinib liposomes as integrated antiangiogenic tools. Nanomedicine 2019, 18, 135–145. [Google Scholar] [CrossRef]
- Meng, S.; Su, B.; Li, W.; Ding, Y.; Tang, L.; Zhou, W.; Song, Y.; Li, H.; Zhou, C. Enhanced antitumor effect of novel dual-targeted paclitaxel liposomes. Nantecnology 2010, 21, 1–7. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef]
- Nasongkla, N.; Shuai, X.; Ai, H.; Weinberg, B.D.; Pink, J.; Boothman, D.A.; Gao, J. cRGD-functionalized polymer micelles for targeted doxorubicin delivery. Angew. Chem. Int. Ed. 2004, 43, 6323–6327. [Google Scholar] [CrossRef]
- Nasongkla, N.; Bey, E.; Ren, J.; Ai, H.; Khemtong, C.; Guthi, J.S.; Chin, S.F.; Sherry, A.D.; Boothman, D.A.; Gao, J. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006, 6, 2427–2430. [Google Scholar] [CrossRef]
- Xiong, X.B.; Ma, Z.; Lai, L.; Lavasanifar, A. The therapeutic response to multifunctional polymeric nano conjugates in the targeted cellular and subcellular delivery of doxorubicin. Biomaterials 2010, 31, 757–768. [Google Scholar] [CrossRef]
- Danhier, F.; Vroman, B.; Lecouturier, N.; Crokart, N.; Pourcelle, V.; Freichels, H.; Jérôme, C.; Marchand-Brynaert, J.; Feron, O.; Préat, V. Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with Paclitaxel. J. Control. Release 2009, 140, 166–173. [Google Scholar] [CrossRef]
- Waite, C.L.; Roth, C.M. PAMAM-RGD conjugates enhance siRNA delivery through a multicellular spheroid model of malignant glioma. Bioconjugate Chem. 2009, 20, 1908–1916. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.F.; Liu, Y.; Liu, C.; Jiang, B.H.; Jiang, Y.Y. Tumor penetrability and anti-angiogenesis using iRGD-mediated delivery of doxorubicin-polymer conjugates. Biomaterials 2014, 35, 8735–8747. [Google Scholar] [CrossRef] [PubMed]
- Han, H.D.; Mangala, L.S.; Lee, J.W.; Shahzad, M.M.; Kim, H.S.; Shen, D.; Nam, E.J.; Mora, E.M.; Stone, R.L.; Lu, C.; et al. Targeted gene silencing using RGD-labeled chitosan nanoparticles. Clin. Cancer Res. 2010, 16, 3910–3922. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Rothweiler, F.; Anhorn, M.G.; Sauer, D.; Riemann, I.; Weiss, E.C.; Katsen-Globa, A.; Michaelis, M.; Cinatl Jr., J.; Schwartz, D.; et al. Enhanced drug targeting by attachment of an anti αν integrin antibody to doxorubicin loaded human serum albumin nanoparticles. Biomaterials 2010, 31, 2388–2398. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Carver, K.; Wu, L. Albumin-Based nanoconjugates for targeted delivery of therapeutic oligonucleotides. Biomaterials 2013, 34, 7939–7949. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nam, H.Y.; Kim, T.I.; Kim, P.H.; Ryu, J.; Yun, C.O.; Kim, S.W. Active targeting of RGD-conjugated bioreducible polymer for delivery of oncolytic adenovirus expressing shRNA against IL-8 mRNA. Biomaterials 2011, 32, 5158–5166. [Google Scholar] [CrossRef]
- Conde, J.; Dias, J.T.; Grazú, V.; Moros, M.; Baptista, P.V.; de la Fuente, J.M. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front. Chem. 2014, 2, 1–27. [Google Scholar] [CrossRef]
- Jia, T.; Choi, J.; Ciccione, J.; Henry, M.; Mehdi, A.; Martinez, J.; Eymin, B.; Subra, G.; Coll, J.L. Heteromultivalent targeting of integrin ανβ3 and neuropilin 1 promotes cell survival via the activation of the IGF-1/insulin receptors. Biomaterials 2018, 155, 64–79. [Google Scholar] [CrossRef]
- Juthani, R.; Madajewski, B.; Yoo, B.; Zhang, L.; Chen, P.M.; Chen, F.; Turker, M.Z.; Ma, K.; Overholtzer, M.; Longo, V.A.; et al. Ultrasmall core-shell silica nanoparticles for precision drug delivery in a high-grade malignant brain tumor model. Clin. Cancer Res. 2020, 26, 147–158. [Google Scholar] [CrossRef]
- De Marco, R.; Rampazzo, E.; Zhao, J.; Prodi, L.; Paolillo, M.; Picchetti, P.; Gallo, F.; Calonghi, N.; Gentilucci, L. Integrin-targeting dye-doped PEG-shell/silica-core nanoparticles mimicking the proapoptotic smac/DIABLO protein. Nanomaterials 2020, 10, 1211. [Google Scholar] [CrossRef]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Girard, O.M.; Hanahan, D.; Mattrey, R.F.; Ruoslahti, E. Tissue-Penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell 2009, 16, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Agemy, L.; Friedmann-Morvinski, D.; Kotamraju, V.R.; Roth, L.; Sugahara, K.N.; Girard, O.M.; Mattrey, R.F.; Verma, I.M.; Ruoslahti, E. Targeted nanoparticle enhanced proapoptotic peptide as potential therapy for glioblastoma. Proc. Natl. Acad. Sci. USA 2011, 108, 17450–17455. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xie, J.; Xu, H.; Behera, D.; Michalski, M.H.; Biswal, S.; Wang, A.; Chen, X. Triblock copolymer coated iron oxide nanoparticle conjugate for tumor integrin targeting. Biomaterials 2009, 30, 6912–6919. [Google Scholar] [CrossRef] [PubMed]
- Kluza, E.; van der Schaft, D.W.; Hautvast, P.A.; Mulder, W.J.; Mayo, K.H.; Griffioen, A.W.; Strijkers, G.J.; Nicolay, K. Synergistic targeting of ανβ3 integrin and galectin-1 with heteromultivalent paramagnetic liposomes for combined MR imaging and treatment of angiogenesis. Nano Lett. 2010, 10, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Tian, F.; Hernandez, Y.; Bao, C.; Cui, D.; Janssen, K.P.; Ibarra, M.R.; Baptista, P.V.; Stoeger, T.; de la Fuente, J.M. In vivo tumor targeting via nanoparticle-mediated therapeutic siRNA coupled to inflammatory response in lung cancer mouse models. Biomaterials 2013, 34, 7744–7753. [Google Scholar] [CrossRef]
- Li, K.G.; Zhang, Z.L.; Zheng, L.F.; Liu, H.; Wei, W.; Li, Z.Y.; He, Z.Y.; Larson, A.C.; Zhang, G.X. Arg-Gly-Asp-D-Phe-Lys peptide-modified PEGylated dendrimer-entrapped gold nanoparticles for targeted computed tomography imaging of breast carcinoma. Nanomedicine 2015, 10, 2185–2197. [Google Scholar] [CrossRef]
- Atmaja, B.; Lui, B.H.; Hu, Y.H.; Beck, S.E.; Frank, C.W.; Cochran, J.R. Targeting of cancer cells using quantum dot-polypeptide hybrid assemblies that function as molecular imaging agents and carrier systems. Adv. Funct. Mater. 2010, 20, 4091–4097. [Google Scholar] [CrossRef]
- Hu, K.Z.; Wang, H.L.; Tang, G.H.; Huang, T.T.; Tang, X.L.; Liang, X.; Yao, S.B.; Nie, D.H. In vivo cancer dual-targeting and dual-modality imaging with functionalized quantum dots. J. Nucl. Med. 2015, 56, 1278–1284. [Google Scholar] [CrossRef][Green Version]
- Hirano, Y.; Mooney, D.J. Peptide and protein presenting materials for tissue engineering. Adv. Mater. 2004, 16, 17–25. [Google Scholar] [CrossRef]
- Shekaran, A.; García, A.J. Extracellular matrix-mimetic adhesive biomaterials for bone repair. J. Biomed. Mater. Res. A 2011, 96, 261–272. [Google Scholar] [CrossRef]
- Meyers, S.R.; Grinstaff, M.W. Biocompatible and bioactive surface modifications for prolonged in vivo efficacy. Chem. Rev. 2012, 112, 1615–1632. [Google Scholar] [CrossRef] [PubMed]
- Elbert, D.L.; Hubbell, J.A. Surface treatments of polymers for biocompatibility. Annu. Rev. Mater. Sci. 1996, 26, 365–394. [Google Scholar] [CrossRef]
- Spicer, C.D.; Davis, B.G. Selective chemical protein modification. Nat. Commun. 2014, 5, 4740–4753. [Google Scholar] [CrossRef] [PubMed]
- De Marco, R.; Zhao, J.; Greco, A.; Ioannone, S.; Gentilucci, L. In-Peptide synthesis of imidazolidin-2-one scaffolds, equippable with proteinogenic or taggable/linkable side chains, general promoters of unusual secondary structures. J. Org. Chem. 2019, 84, 4992–5004. [Google Scholar] [CrossRef] [PubMed]
- Mendes, P.M. Cellular nanotechnology: Making biological interfaces smarter. Chem. Soc. Rev. 2013, 42, 9207–9218. [Google Scholar] [CrossRef]
- Houseman, B.T.; Mrksich, M. The microenvironment of immobilized Arg-Gly-Asp peptides is an important determinant of cell adhesion. Biomaterials 2001, 22, 943–955. [Google Scholar] [CrossRef]
- Shabbir, S.H.; Eisenberg, J.L.; Mrksich, M. An inhibitor of a cell adhesion receptor stimulates cell migration. Angew. Chem. Int. Ed. 2010, 49, 7706–7709. [Google Scholar] [CrossRef]
- Le Saux, G.; Magenau, A.; Gunaratnam, K.; Kilian, K.A.; Böcking, T.; Gooding, J.J.; Gaus, K. Spacing of integrin ligands influences signal transduction in endothelial cells. Biophys. J. 2011, 101, 764–773. [Google Scholar] [CrossRef]
- Cosenza, C.; Lettera, V.; Causa, F.; Scognamiglio, P.L.; Battista, E.; Netti, P.A. Cell mechanosensory recognizes ligand compliance at biomaterial interface. Biomaterials 2016, 76, 282–291. [Google Scholar] [CrossRef]
- Garcia, A.S.; Dellatore, S.M.; Messersmith, P.B.; Miller, W.M. Effects of supported lipid monolayer fluidity on the adhesion of hematopoietic progenitor cell lines to fibronectin-derived peptide ligands for α5β1 and α4β1 integrins. Langmuir 2009, 25, 2994–3002. [Google Scholar] [CrossRef]
- Marchi-Artzner, V.; Lorz, B.; Hellerer, U.; Kantlehner, M.; Kessler, H.; Sackmann, E. Selective adhesion of endothelial cells to artificial membranes with a synthetic RGD-lipopeptide. Chem. Eur. J. 2001, 7, 1095–1101. [Google Scholar] [CrossRef]
- Koçer, G.; Jonkheijm, P. Guiding hMSC adhesion and differentiation on supported lipid bilayers. Adv. Healthc. Mater. 2017, 6, 1600862. [Google Scholar] [CrossRef] [PubMed]
- Rechenmacher, F.; Neubauer, S.; Mas-Moruno, C.; Dorfner, P.M.; Polleux, J.; Guasch, J.; Conings, B.; Boyen, H.G.; Bochen, A.; Sobahi, T.R.; et al. A molecular toolkit for the functionalization of titanium-based biomaterials that selectively control integrin-mediated cell adhesion. Chem. Eur. J. 2013, 19, 9218–9223. [Google Scholar] [CrossRef] [PubMed]
- Fraioli, R.; Rechenmacher, F.; Neubauer, S.; Manero, J.M.; Gil, J.; Kessler, H.; Mas-Moruno, C. Mimicking bone extracellular matrix: Integrin-binding peptidomimetics enhance osteoblast-like cells adhesion, proliferation and differentiation on titanium. Colloids Surf. B Biointerfaces 2015, 128, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Rémy, M.; Bareille, R.; Rerat, V.; Bourget, C.; Marchand-Brynaert, J.; Bordenave, L. Polyethylene terephthalate membrane grafted with peptidomimetics: Endothelial cell compatibility and retention under shear stress. J. Biomater. Sci. Polym. Ed. 2013, 24, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Kantlehner, M.; Finsinger, D.; Meyer, J.; Schaffner, P.; Jonczyk, A.; Diefenbach, B.; Nies, B.; Kessler, H. Selective RGD mediated adhesion of osteoblasts at surfaces of implants. Angew. Chem. Int. Ed. 1999, 38, 560–562. [Google Scholar] [CrossRef]
- Schussler, O.; Coirault, C.; Louis-Tisserand, M.; Al-Chare, W.; Oliviero, P.; Menard, C.; Michelot, R.; Bochet, P.; Salomon, D.R.; Chachques, J.C.; et al. Use of Arginine-Glycine-Aspartic acid adhesion peptides coupled with a new collagen scaffold to engineer a myocardium-like tissue graft. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 240–249. [Google Scholar] [CrossRef]
- Kilian, K.A.; Mrksich, M. Directing stem cell fate by controlling the affinity and density of ligand-receptor interactions at the biomaterials interface. Angew. Chem. Int. Ed. 2012, 51, 4891–4895. [Google Scholar] [CrossRef]
- Karimi, F.; O’Connor, A.J.; Qiao, G.G.; Heath, D.E. Integrin clustering matters: A review of biomaterials functionalized with multivalent integrin-binding ligands to improve cell adhesion, migration, differentiation, angiogenesis, and biomedical device integration. Adv. Healthc. Mater. 2018, 7, 1701324. [Google Scholar] [CrossRef]
- Morgan, A.W.; Roskov, K.E.; Lin-Gibson, S.; Kaplan, D.L.; Becker, M.L.; Simon, C.G. Characterization and optimization of RGD-containing silk blends to support osteoblastic differentiation. Biomaterials 2008, 29, 2556–2563. [Google Scholar] [CrossRef]
- Yang, Y.J.; Kwon, Y.; Choi, B.H.; Jung, D.; Seo, J.H.; Lee, K.H.; Cha, H.J. Multifunctional adhesive silk fibroin with blending of RGD bioconjugated mussel adhesive protein. Biomacromolecules 2014, 15, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Janani, G.; Nandi, S.K.; Mandal, B.B. Functional hepatocyte clusters on bioactive blend silk matrices towards generating bioartificial liver constructs. Acta Biomater. 2018, 67, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, G.; Brown, G.; Lauffenburger, D.A.; Wells, A.; Griffith, L.G. Cell adhesion and motility depend on nanoscale RGD clustering. J. Cell Sci. 2000, 113, 1677–1686. Available online: https://jcs.biologists.org/content/113/10/1677.short (accessed on 25 August 2020). [PubMed]
- Lim, C.; Moon, J.; Sim, T.; Hoang, N.H.; Won, W.R.; Lee, E.S.; Youn, Y.S.; Choi, H.G.; Oh, K.; Oh, K.T. Cyclic RGD-conjugated Pluronic® blending system for active, targeted drug delivery. Int. J. Nanomed. 2018, 13, 4627–4639. [Google Scholar] [CrossRef]
- Rundqvist, J.; Mendoza, B.; Werbin, J.L.; Heinz, W.F.; Lemmon, C.; Romer, L.H.; Haviland, D.B.; Hoh, J.H. High fidelity functional patterns of an extracellular matrix protein by electron beam-based inactivation. J. Am. Chem. Soc. 2007, 129, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, C.M.; Kim, S.H.; Broyer, R.M.; Saxer, S.S.; Decker, C.G.; Maynard, H.D. Combination of integrin-binding peptide and growth factor promotes cell adhesion on electron-beam-fabricated patterns. J. Am. Chem. Soc. 2012, 134, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kwon, C.; Jeon, H. Genetically engineered phage induced selective H9c2 cardiomyocytes patterning in PDMS microgrooves. Materials 2017, 10, 973. [Google Scholar] [CrossRef]
- Bilem, I.; Chevallier, P.; Plawinski, L.; Sone, E.D.; Durrieu, M.C.; Laroche, G. Interplay of geometric cues and RGD/BMP-2 crosstalk in directing stem cell fate. ACS Biomater. Sci. Eng. 2017, 3, 2514–2523. [Google Scholar] [CrossRef]
- Bilem, I.; Plawinski, L.; Chevallier, P.; Ayela, C.; Sone, E.D.; Laroche, G.; Durrieu, M.C. The spatial patterning of RGD and BMP-2 mimetic peptides at the subcellular scale modulates human mesenchymal stem cells osteogenesis. J. Biomed. Mater. Res. A 2018, 106, 959–970. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, D.N.; Park, S.; Lee, Y.; Koh, W.G. Micropatterning of a nanoporous alumina membrane with poly(ethylene glycol) hydrogel to create cellular micropatterns on nanotopographic substrates. Acta Biomater. 2011, 7, 1281–1289. [Google Scholar] [CrossRef]
- Pallarola, D.; Bochen, A.; Boehm, H.; Rechenmacher, F.; Sobahi, T.R.; Spatz, J.P.; Kessler, H. Interface immobilization chemistry of cRGD-based peptides regulates integrin mediated cell adhesion. Adv. Funct. Mater. 2014, 24, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Medda, R.; Helth, A.; Herre, P.; Pohl, D.; Rellinghaus, B.; Perschmann, N.; Neubauer, S.; Kessler, H.; Oswald, S.; Eckert, J.; et al. Investigation of early cell-surface interactions of human mesenchymal stem cells on nanopatterned β-type titanium-niobium alloy surfaces. Interface Focus 2014, 4, 20130046. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, C.; Ye, K.; He, Y.; Li, Z.; Ding, J. Effect of RGD nanospacing on differentiation of stem cells. Biomaterials 2013, 34, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.A.; Zouani, O.F.; Glinel, K.; Jonas, A.M.; Durrieu, M.C. Bioactive chemical nanopatterns impact human mesenchymal stem cell fate. Nano Lett. 2013, 13, 3923–3929. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Park, T.G. Biomimicking extracellular matrix: Cell adhesive RGD peptide modified electrospun poly (D, L-lactic-co-glycolic acid) nanofiber mesh. Tissue Eng. 2006, 12, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.H.; Famili, A.; Lee, Y.M.; Jenkins, P.M.; Freed, C.R.; Park, D. Biomimetic poly(serinol hexamethylene urea) for promotion of neurite outgrowth and guidance. J. Biomater. Sci. Polym. Ed. 2014, 25, 354–369. [Google Scholar] [CrossRef]
- Madhavan, K.; Frid, M.G.; Hunter, K.; Shandas, R.; Stenmark, K.R.; Park, D. Development of an electrospun biomimetic polyurea scaffold suitable for vascular grafting. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2018, 106, 278–290. [Google Scholar] [CrossRef]
- Jeong, S.I.; Burns, N.A.; Bonino, C.A.; Kwon, I.K.; Khan, S.A.; Alsberg, E. Improved cell infiltration of highly porous D nanofibrous scaffolds formed by combined fiber-fiber charge repulsions and ultra-sonication. J. Mater. Chem. B 2014, 2, 8116–8122. [Google Scholar] [CrossRef]
- Antonova, L.V.; Seifalian, A.M.; Kutikhin, A.G.; Sevostyanova, V.V.; Matveeva, V.G.; Velikanova, E.A.; Mironov, A.V.; Shabaev, A.R.; Glushkova, T.V.; Senokosova, E.A.; et al. Conjugation with RGD peptides and incorporation of vascular endothelial growth factor are equally efficient for biofunctionalization of tissue-engineered vascular grafts. Int. J. Mol. Sci. 2016, 17, 1920. [Google Scholar] [CrossRef]
- Wang, J.L.; Yang, M.Y.; Zhu, Y.; Wang, L.; Tomsia, A.P.; Mao, C.B. Phage nanofibers induce vascularized osteogenesis in D printed bone scaffolds. Adv. Mater. 2014, 26, 4961–4966. [Google Scholar] [CrossRef]
- Heo, D.N.; Castro, N.J.; Lee, S.J.; Noh, H.; Zhu, W.; Zhang, L.G. Enhanced bone tissue regeneration using a D printed microstructure incorporated with hybrid nano hydrogel. Nanoscale 2017, 9, 5055–5062. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.; Busari, H.; Seims, K.B.; Schwarzenberg, P.; Dailey, H.L.; Chow, L.W. D printing with peptide-polymer conjugates for single-step fabrication of spatially functionalized scaffolds. Biomater. Sci. 2019, 7, 4237–4247. [Google Scholar] [CrossRef] [PubMed]
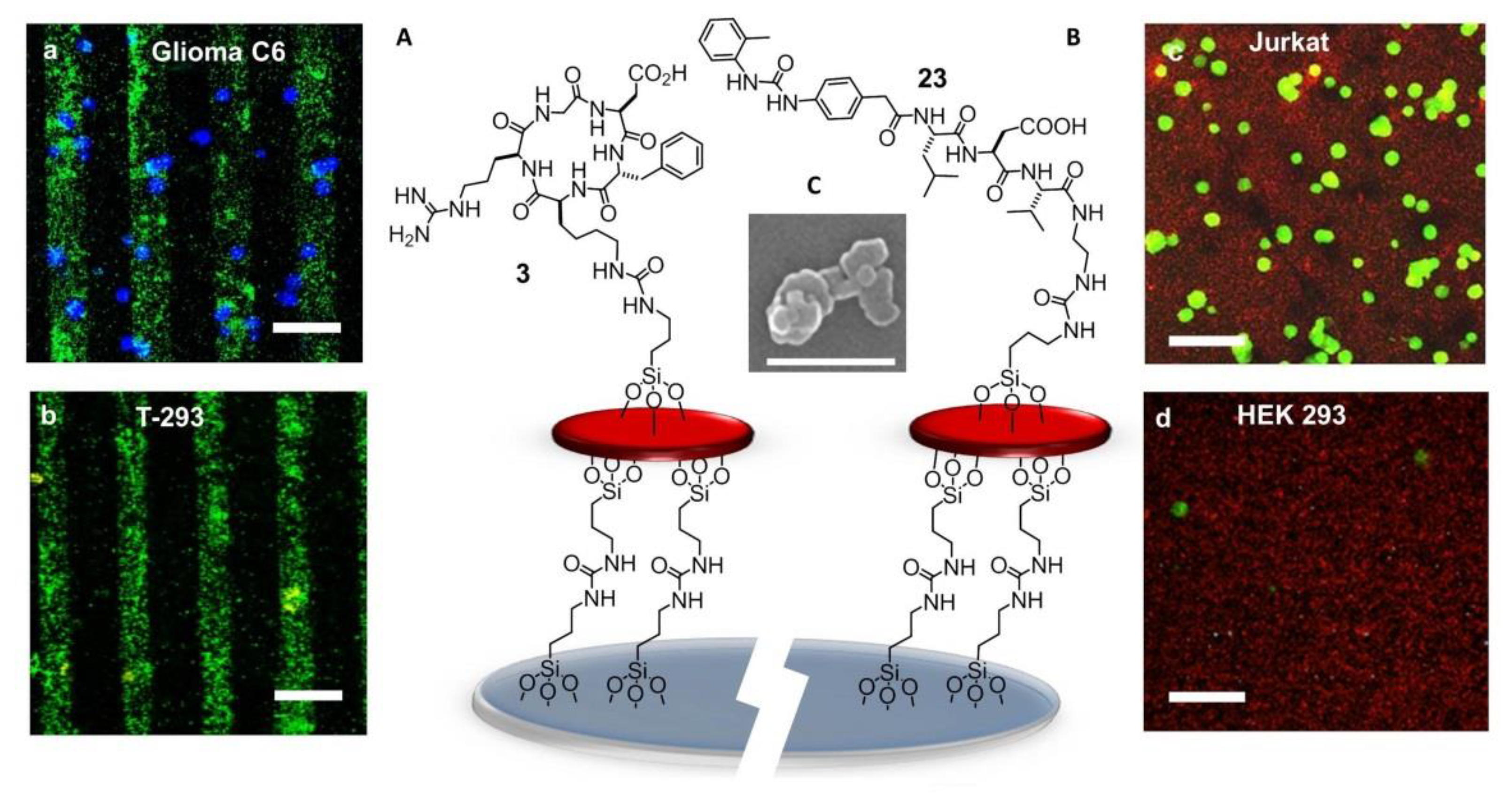
- Greco, A.; Maggini, L.; De Cola, L.; De Marco, R.; Gentilucci, L. Diagnostic implementation of fast and selective integrin-mediated adhesion of cancer cells on functionalized zeolite L monolayers. Bioconjugate Chem. 2015, 26, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- De Marco, R.; Greco, A.; Calonghi, N.; Dattoli, S.D.; Baiula, M.; Spampinato, S.; Picchetti, P.; De Cola, L.; Anselmi, M.; Cipriani, F.; et al. Selective detection of α4β1 integrin (VLA-4)-expressing cells using peptide-functionalized nanostructured materials mimicking endothelial surfaces adjacent to inflammatory sites. Pept. Sci. 2018, 110, e23081. [Google Scholar] [CrossRef]
- Fraioli, R.; Tsimbouri, P.M.; Fisher, L.E.; Nobbs, A.H.; Su, B.; Neubauer, S.; Rechenmacher, F.; Kessler, H.; Ginebra, M.P.; Dalby, M.J.; et al. Towards the cell-instructive bactericidal substrate: Exploring the combination of nanotopographical features and integrin selective synthetic ligands. Sci. Rep. 2017, 7, 16363. [Google Scholar] [CrossRef]
- Martelli, G.; Bloise, N.; Merlettini, A.; Bruni, G.; Visai, L.; Focarete, M.L.; Giacomini, D. Combining biologically active β-lactams integrin agonists with poly(L-lactic acid) nanofibers: Enhancement of human mesenchymal stem cell adhesion. Biomacromolecules 2020, 21, 1157–1170. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Kaufman, D.S.; Shen, W. A synthetic substrate to support early mesodermal differentiation of human embryonic stem cells. Biomaterials 2011, 32, 8058–8066. [Google Scholar] [CrossRef][Green Version]
- Mas-Moruno, C.; Fraioli, R.; Albericio, F.; Manero, J.M.; Gil, F.J. Novel peptide-based platform for the dual presentation of biologically active peptide motifs on biomaterials. ACS Appl. Mater. Interfaces 2014, 6, 6525–6536. [Google Scholar] [CrossRef]
- Zhao, N.; Battig, M.R.; Xu, M.; Wang, X.; Xiong, N.; Wang, Y. Development of a dual-functional hydrogel using RGD and anti-VEGF aptamer. Macromol. Biosci. 2017, 17, 1700201. [Google Scholar] [CrossRef]
- Karimi, F.; Thombare, V.J.; Hutton, C.A.; O’Connor, A.J.; Qiao, G.G.; Heath, D.E. Beyond RGD; nanoclusters of syndecan- and integrin-binding ligands synergistically enhance cell/material interactions. Biomaterials 2018, 187, 81–92. [Google Scholar] [CrossRef]
- Koçer, G.; Jonkheijm, P. About chemical strategies to fabricate cell-instructive biointerfaces with static and dynamic complexity. Adv. Healthc. Mater. 2018, 7, 1701192. [Google Scholar] [CrossRef] [PubMed]
- Ebara, M.; Yamato, M.; Aoyagi, T.; Kikuchi, A.; Sakai, K.; Okano, T. A novel approach to observing synergy effects of PHSRN on integrin-RGD binding using intelligent surfaces. Adv. Mater. 2008, 20, 3034–3038. [Google Scholar] [CrossRef]
- Kobayashi, J.; Yamato, M.; Okano, T. On-Off affinity binding modulation on thermoresponsive polymer-grafted surfaces for capture and release of proteins and cells. J. Biomater. Sci. Polym. Ed. 2017, 28, 939–957. [Google Scholar] [CrossRef] [PubMed]
- Simnick, A.J.; Valencia, C.A.; Liu, R.; Chilkoti, A. Morphing low-affinity ligands into high-avidity nanoparticles by thermally triggered self-assembly of a genetically encoded polymer. ACS Nano 2010, 4, 2217–2227. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.N.; Sahoo, J.K.; McNamara, L.E.; Burgess, K.V.; Yang, J.L.; Alakpa, E.V.; Anderson, H.J.; Hay, J.; Turner, L.A.; Yarwood, S.J.; et al. Dynamic surfaces for the study of mesenchymal stem cell growth through adhesion regulation. ACS Nano 2016, 10, 6667–6679. [Google Scholar] [CrossRef]
- Lamb, B.M.; Yousaf, M.N. Redox-Switchable surface for controlling peptide structure. J. Am. Chem. Soc. 2011, 133, 8870–8873. [Google Scholar] [CrossRef]
- Luo, W.; Yousaf, M.N. Tissue morphing control on dynamic gradient surfaces. J. Am. Chem. Soc. 2011, 133, 10780–10783. [Google Scholar] [CrossRef]
- Ng, C.C.A.; Magenau, A.; Ngalim, S.H.; Ciampi, S.; Chockalingham, M.; Harper, J.B.; Gaus, K.; Gooding, J.J. Using an electrical potential to reversibly switch surfaces between two states for dynamically controlling cell adhesion. Angew. Chem. Int. Ed. 2012, 51, 7706–7710. [Google Scholar] [CrossRef]
- Li, J.; Lei, Y.F.; Sun, C.L.; Zheng, W.F.; Jiang, X.Y.; Zhang, H.L. Rationally designed peptide interface for potential modulated cell adhesion and migration. Adv. Mater. Interfaces 2015, 2, 1500335. [Google Scholar] [CrossRef]
- Salierno, M.J.; García, A.J.; del Campo, A. Photo-Activatable surfaces for cell migration assays. Adv. Funct. Mater. 2013, 23, 5974–5980. [Google Scholar] [CrossRef]
- Wiemann, M.; Niebuhr, R.; Juan, A.; Cavatorta, E.; Ravoo, B.J.; Jonkheijm, P. Photo-Responsive bioactive surfaces based on cucurbit[8]uril-mediated host–guest interactions of arylazopyrazoles. Chem. Eur. J. 2018, 24, 813–817. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Brinkmann, J.; Huskens, J.; Krabbenborg, S.; de Boer, J.; Jonkheijm, P. A supramolecular system for the electrochemically controlled release of cells. Angew. Chem. Int. Ed. 2012, 51, 12233–12237. [Google Scholar] [CrossRef] [PubMed]
- Boekhoven, J.; Rubert Pérez, C.M.; Sur, S.; Worthy, A.; Stupp, S.I. Dynamic display of bioactivity through host-guest chemistry. Angew. Chem. Int. Ed. 2013, 52, 12077–12080. [Google Scholar] [CrossRef] [PubMed]
- Kloxin, A.M.; Kasko, A.M.; Salinas, C.N.; Anseth, K.S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 2009, 324, 59–63. [Google Scholar] [CrossRef]
- Trappmann, B.; Baker, B.M.; Polacheck, W.J.; Choi, C.K.; Burdick, J.A.; Chen, C.S. Matrix degradability controls multicellularity of D cell migration. Nat. Commun. 2017, 8, 371. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, D.; Paul, A.; Cai, L.; Enejder, A.; Yang, F.; Heilshorn, S.C. Covalently adaptable elastin-like protein-hyaluronic acid (ELP-HA) hybrid hydrogels with secondary thermoresponsive crosslinking for injectable stem cell delivery. Adv. Funct. Mater. 2017, 27, 1605609. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Santino, F.; Giacomini, D.; Gentilucci, L. Integrin-Targeting Peptides for the Design of Functional Cell-Responsive Biomaterials. Biomedicines 2020, 8, 307. https://doi.org/10.3390/biomedicines8090307
Zhao J, Santino F, Giacomini D, Gentilucci L. Integrin-Targeting Peptides for the Design of Functional Cell-Responsive Biomaterials. Biomedicines. 2020; 8(9):307. https://doi.org/10.3390/biomedicines8090307
Chicago/Turabian StyleZhao, Junwei, Federica Santino, Daria Giacomini, and Luca Gentilucci. 2020. "Integrin-Targeting Peptides for the Design of Functional Cell-Responsive Biomaterials" Biomedicines 8, no. 9: 307. https://doi.org/10.3390/biomedicines8090307
APA StyleZhao, J., Santino, F., Giacomini, D., & Gentilucci, L. (2020). Integrin-Targeting Peptides for the Design of Functional Cell-Responsive Biomaterials. Biomedicines, 8(9), 307. https://doi.org/10.3390/biomedicines8090307





