EDTA Chelation Therapy in the Treatment of Neurodegenerative Diseases: An Update
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Design
2.3. Chelation Test
2.4. Chelation Therapy
2.5. Toxic-Metal Analysis
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Percentage of Patients Affected by Each Toxic-Metal Burden vs. Total Poisoned Population
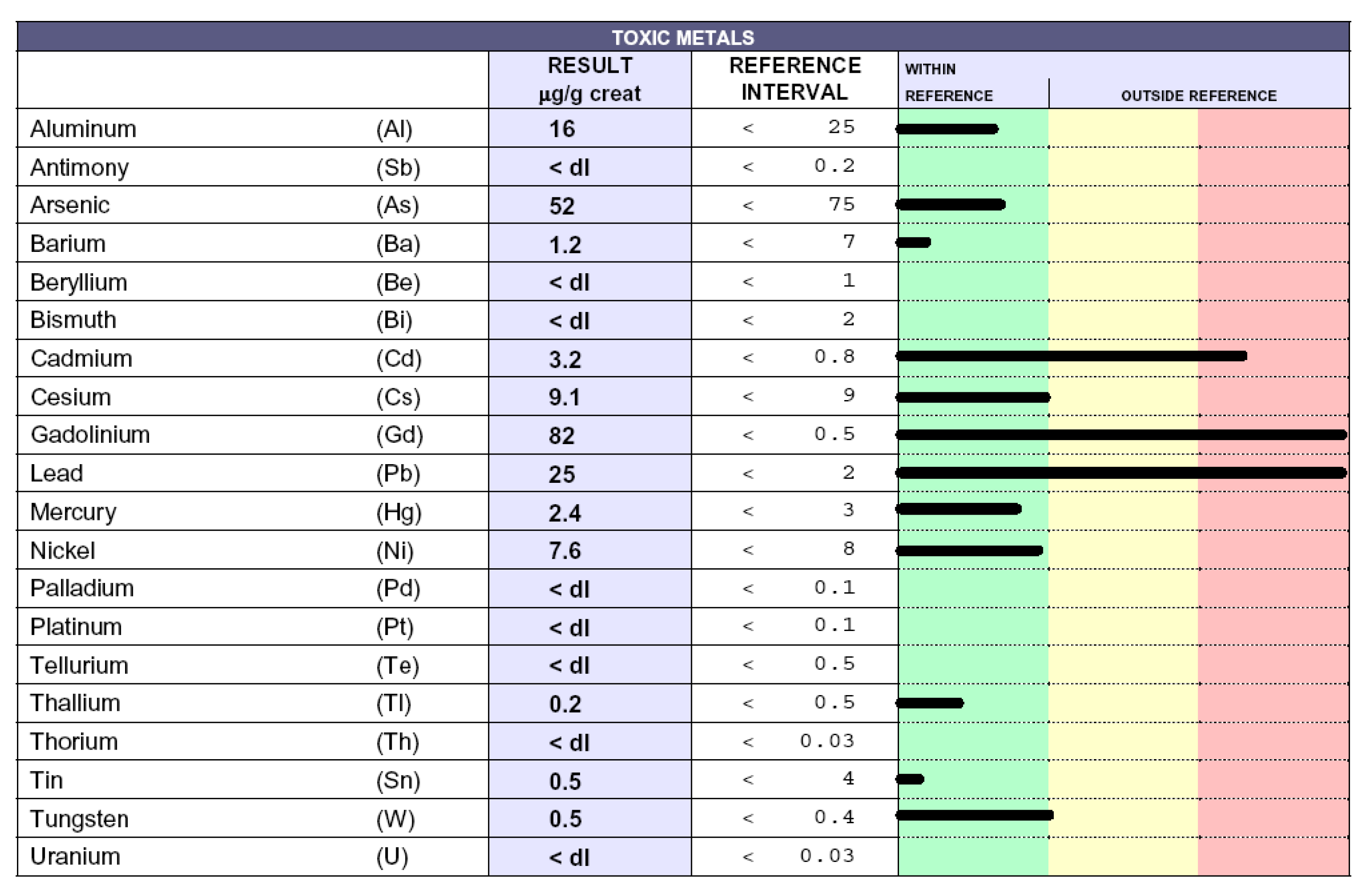
3.3. Toxic-Metal Burdens in Patient Urine Samples Following Chelation Test
3.4. Reduction of Poisoning Following Chelation Therapy
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ND | Neurodegenerative diseases |
| Non-ND | Non neurodegenerative diseases |
| HC | Healthy controls |
| EDTA | calcium disodium ethylenediaminetetraacetic acid |
| AD | Alzheimer’s disease |
| ALS | Amyotrophic lateral sclerosis |
| MS | Multiple sclerosis |
| PD | Parkinson’s disease |
| ICP | MS inductively coupled plasma mass spectrometry |
| Al | aluminum |
| Sb | antimony |
| As | arsenicum |
| Ba | barium |
| Be | beryllium |
| Bi | bismuth |
| Cd | cadmium |
| Cs | cesium |
| Gd | gadolinium |
| Pb | lead |
| Hg | mercury |
| Ni | nickel |
| Pd | palladium |
| Pt | platinum |
| Te | tellurium |
| Tl | thallium |
| Th | thorium |
| Sn | tin |
| W | tungsten |
| U | uranium |
References
- Fulgenzi, A.; Ferrero, M.E. EDTA Chelation Therapy for the Treatment of Neurotoxicity. Int. J. Mol. Sci. 2019, 20, 1019. [Google Scholar]
- Park, J.-S.; Davis, R.L.; Sue, C.M. Mitochondrial Dysfunction in Parkinson’s Disease: New Mechanistic Insights and Therapeutic Perspectives. Curr. Neurol. Neurosci. Rep. 2018, 18, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.; Lipton, S.A. Nitric Oxide-Dependent Protein Post-Translational Modifications Impair Mitochondrial Function and Metabolism to Contribute to Neurodegenerative Diseases. Antioxid. Redox Signal. 2020, 32, 817–833. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; Baroni, M.; Senin, U.; Boccardi, V. Brain Aging and Late-Onset Alzheimer’s Disease: A Matter of Increased Amyloid or Reduced Energy? J. Alzheimer’s Dis. 2018, 64, S397–S404. [Google Scholar] [CrossRef] [PubMed]
- Meldolesi, J. Neurotrophin receptors in the pathogenesis, diagnosis and therapy of neurodegenerative diseases. Pharmacol. Res. 2017, 121, 129–137. [Google Scholar] [CrossRef]
- Spielman, L.J.; Gibson, D.L.; Klegeris, A. Unhealthy gut, unhealthy brain: The role of the intestinal microbiota in neurodegenerative diseases. Neurochem. Int. 2018, 120, 149–163. [Google Scholar] [CrossRef]
- Mazon, J.N.; de Mello, A.H.; Ferreira, G.K.; Rezin, G.T. The impact of obesity on neurodegenerative diseases. Life Sci. 2017, 182, 22–28. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef] [Green Version]
- Ferrero, M.E. Rationale for the Successful Management of EDTA Chelation Therapy in Human Burden by Toxic Metals. Biomed Res. Int. 2016. [Google Scholar] [CrossRef] [Green Version]
- Lamas, G.A.; Navas-Acien, A.; Mark, D.B.; Lee, K.L. Heavy Metals, Cardiovascular Disease, and the Unexpected Benefits of Chelation Therapy. J. Am. Coll. Cardiol. 2016, 67, 2411–2418. [Google Scholar] [CrossRef] [Green Version]
- Peguero, J.G.; Arenas, I.; Lamas, G.A. Chelation therapy and cardiovascular disease: Connecting scientific silos to benefit cardiac patients. Trends Cardiovasc. Med. 2014, 24, 232–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjorklund, G.; Stejskal, V.; Urbina, M.A.; Dadar, M.; Chirumbolo, S.; Mutter, J. Metals and Parkinson’s Disease: Mechanisms and Biochemical Processes. Curr. Med. Chem. 2018, 25, 2198–2214. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Tinkov, A.A.; Dadar, M.; Rahman, M.M.; Chirumbolo, S.; Skalny, A.V.; Skalnaya, M.G.; Haley, B.E.; Ajsuvakova, O.P.; Aaseth, J. Insights into the Potential Role of Mercury in Alzheimer’s Disease. J. Mol. Neurosci. 2019, 67, 511–533. [Google Scholar] [CrossRef] [PubMed]
- Aliomrani, M.; Sahraian, M.A.; Shirkhanloo, H.; Sharifzadeh, M.; Khoshayand, M.R.; Ghahremani, M.H. Correlation between heavy metal exposure and GSTM1 polymorphism in Iranian multiple sclerosis patients. Neurol. Sci. 2017, 38, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.D.; Escolar, E.; Lamas, G.A. Chelation therapy after the Trial to Assess Chelation Therapy: Results of a unique trial. Curr. Opin. Cardiol. 2014, 29, 481. [Google Scholar] [CrossRef] [PubMed]
- Fulgenzi, A.; Vietti, D.; Maria Elena, F. Chronic toxic-metal poisoning and neurodegenerative diseases. Int. J. Curr. Res. 2017, 9, 57899–57999. [Google Scholar]
- Jayaraj, R.L.; Rodriguez, E.A.; Wang, Y.; Block, M.L. Outdoor Ambient Air Pollution and Neurodegenerative Diseases: The Neuroinflammation Hypothesis. Curr. Environ. Health Rep. 2017, 4, 166–179. [Google Scholar] [CrossRef]
- Ferrante, M.; Conti, G.O. Environment and Neurodegenerative Diseases: An Update on miRNA Role. MicroRNA 2017, 6, 157–165. [Google Scholar] [CrossRef]
- Cicero, C.E.; Mostile, G.; Vasta, R.; Rapisarda, V.; Signorelli, S.S.; Ferrante, M.; Zappia, M.; Nicoletti, A. Metals and neurodegenerative diseases. A systematic review. Environ. Res. 2017, 159, 82–94. [Google Scholar] [CrossRef]
- Farina, M.; Avila, D.S.; Da Rocha, J.B.T.; Aschner, M. Metals, oxidative stress and neurodegeneration: A focus on iron, manganese and mercury. Neurochem. Int. 2013, 62, 575–594. [Google Scholar] [CrossRef] [Green Version]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simão, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Nuran Ercal, B.S.P.; Hande Gurer-Orhan, B.S.P.; Nukhet Aykin-Burns, B.S.P. Toxic Metals and Oxidative Stress Part I: Mechanisms Involved in Metal induced Oxidative Damage. Curr. Top. Med. Chem. 2005. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Mirhosseini, N.; Eslahi, M.; Bahmani, F.; Shokrpour, M.; Chamani, M.; Asemi, Z. The effects of magnesium-zinc-calcium-vitamin D co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. BMC Pregnancy Childbirth 2019, 19, 107. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Peng, Y.; Li, J.; Holmgren, A.; Lu, J. Modulation of thiol-dependent redox system by metal ions via thioredoxin and glutaredoxin systems. Metallomics 2018, 10, 218–228. [Google Scholar] [CrossRef]
- Meleleo, D.; Notarachille, G.; Mangini, V.; Arnesano, F. Concentration-dependent effects of mercury and lead on Aβ42: Possible implications for Alzheimer’s disease. Eur. Biophys. J. 2019, 48, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Forcella, M.; Lau, P.; Oldani, M.; Melchioretto, P.; Bogni, A.; Gribaldo, L.; Fusi, P.; Urani, C. Neuronal specific and non-specific responses to cadmium possibly involved in neurodegeneration: A toxicogenomics study in a human neuronal cell model. Neurotoxicology 2020, 76, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Miah, M.; Culbreth, M.; Aschner, M. Autophagy in neurodegenerative diseases and metal neurotoxicity. Neurochem. Res. 2016, 41, 409–422. [Google Scholar] [CrossRef]
- Maiuolo, J.; Macrì, R.; Bava, I.; Gliozzi, M.; Musolino, V.; Nucera, S.; Carresi, C.; Scicchitano, M.; Bosco, F.; Scarano, F.; et al. Myelin disturbances produced by sub-toxic concentration of heavy metals: The role of oligodendrocyte dysfunction. Int. J. Mol. Sci. 2019, 20, 4554. [Google Scholar] [CrossRef] [Green Version]
- Fulgenzi, A.; De Giuseppe, R.; Bamonti, F.; Vietti, D.; Ferrero, M.E. Efficacy of chelation therapy to remove aluminium intoxication. J. Inorg. Biochem. 2015, 152, 214–218. [Google Scholar] [CrossRef]
- Exley, C. The toxicity of aluminium in humans. Morphologie 2016, 100, 51–55. [Google Scholar] [CrossRef]
- Crespo-Lopez, M.E. Role for apolipoprotein E in neurodegeneration and mercury intoxication. Front. Biosci. 2018, 10, 819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, R.; Ramond, A.; O’Keeffe, L.M.; Shahzad, S.; Kunutsor, S.K.; Muka, T.; Gregson, J.; Willeit, P.; Warnakula, S.; Khan, H.; et al. Environmental toxic metal contaminants and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2018, 362, k3310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigra, A.E.; Ruiz-Hernandez, A.; Redon, J.; Navas-Acien, A.; Tellez-Plaza, M. Environmental Metals and Cardiovascular Disease in Adults: A Systematic Review Beyond Lead and Cadmium. Curr. Environ. Health Rep. 2016, 3, 416–433. [Google Scholar] [CrossRef] [PubMed]
- Fulgenzi, A.; Zito, F.; Marchelli, D.; Colombo, F.; Ferrero, M.E. New Insights into EDTA In Vitro Effects on Endothelial Cells and on In Vivo Labeled EDTA Biodistribution. J. Heavy Met. Toxic. Dis. 2016, 1, 7. [Google Scholar]
- Sabolić, I. Common mechanisms in nephropathy induced by toxic metals. Nephron-Physiol. 2006, 10, p107–p114. [Google Scholar] [CrossRef] [PubMed]
- Foglieni, C.; Fulgenzi, A.; Ticozzi, P.; Pellegatta, F.; Sciorati, C.; Belloni, D.; Ferrero, E.; Ferrero, M.E. Protective effect of EDTA preadministration on renal ischemia. BMC Nephrol. 2006, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fulgenzi, A.; Giuseppe, R.D.; Bamonti, F.; Ferrero, M.E. Improvement of oxidative and metabolic parameters by cellfood administration in patients affected by neurodegenerative diseases on chelation treatment. Biomed Res. Int. 2014, 2014, 281510. [Google Scholar] [CrossRef]
- Dellanoce, C.; Fulgenzi, A.; Ferrero, M.E. Glutathione Redox Status in Neurodegenerative Diseases. Austin J. Clin. Neurol. 2019, 6, 6. [Google Scholar]

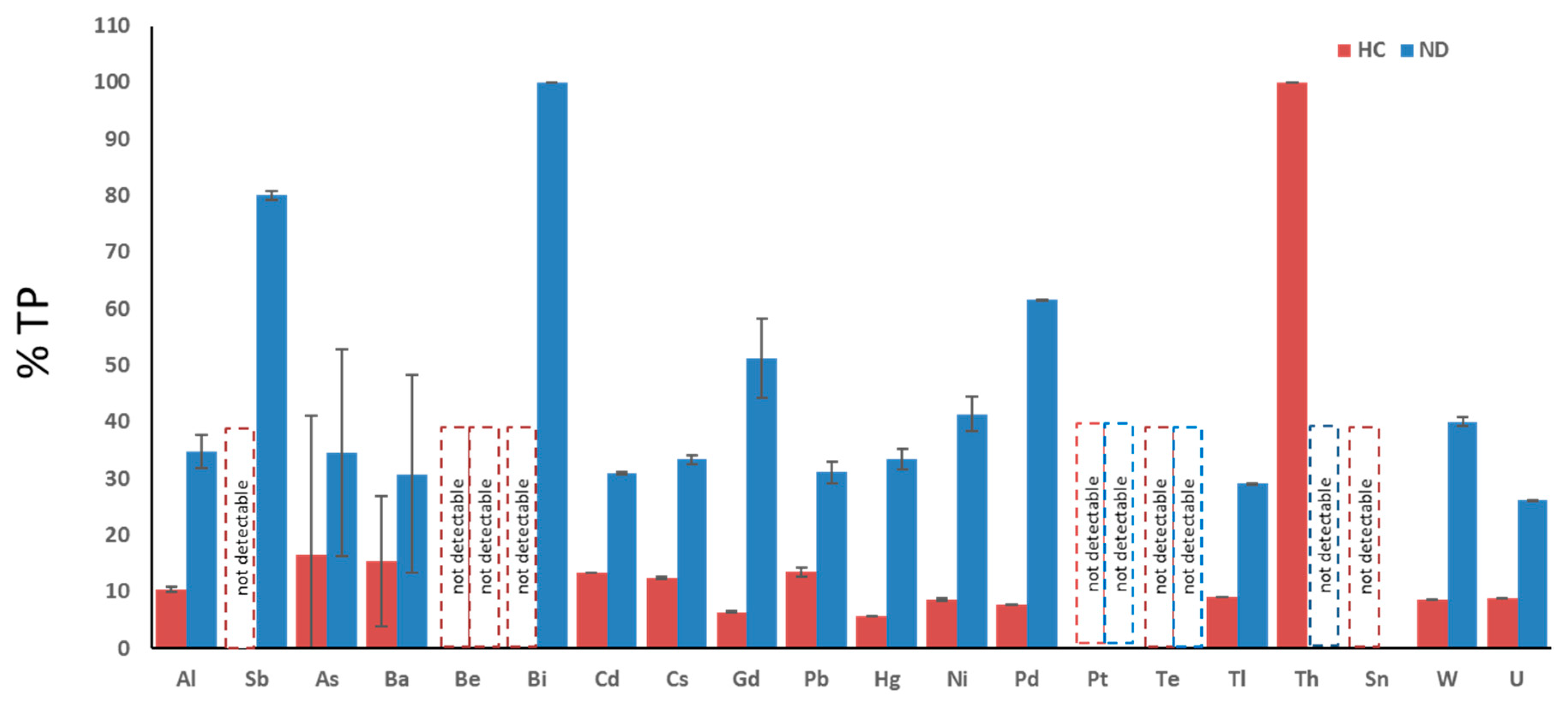

| Cutoff | N° Pz > Cutoff (A) | % TP | Mean (>Cutoff) | SEM | N° Pz > Cutoff ND | % Vs A | Mean (>Cutoff) | SEM | N° Pz > Cutoff ALS | % Vs A | Mean (>Cutoff) | SEM | N° Pz > Cutoff non ND | % Vs A | Mean (>Cutoff) | SEM | N° Pz > Cutoff HC | % Vs A | Mean (>Cutoff) | SEM | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aluminum | 25.00 | 135 | 36.4 | 41.93 | 1.74 | 47 | 35 | 46.70 | 2.90 * | 14 | 10 | 56.86 | 2.21 | 65 | 48 | 47.67 | 2.11 ° | 14 | 10 | 32.43 | 0.53 *,° |
| Antimony | 0.30 | 5 | 1.6 | 1.25 | 0.27 | 4 | 80 | 1.53 | 0.73 | 1 | 20 | 0.07 | nd | 1 | 20 | 1.80 | nd | 0 | nd | nd | nd |
| Arsenic | 108.00 | 55 | 15.3 | 252.07 | 13.85 | 19 | 35 | 269.47 | 18.34 | 1 | 2 | 77.95 | nd | 25 | 45 | 317.27 | 21.35 | 9 | 16 | 288.89 | 24.73 |
| Barium | 7.00 | 13 | 3.7 | 76.43 | 7.42 | 4 | 31 | 52.83 | 17.49 | 2 | 15 | 3.05 | 23.43 | 6 | 46 | 43.78 | 48.45 | 2 | 15 | 40.50 | 11.53 |
| Beryllium | 1.00 | 0 | nd | nd | nd | nd | nd | nd | nd | 0 | 0 | 0.00 | nd | 0 | nd | nd | nd | 0 | nd | nd | nd |
| Bismuth | 10.00 | 1 | 0.3 | 11.00 | 2.87 | 1 | 100 | 11.00 | nd | 1 | 100 | 0.00 | nd | 0 | nd | 1.20 | nd | 0 | nd | nd | nd |
| Cadmium | 0.80 | 346 | 92.9 | 3.49 | 0.16 | 107 | 31 | 3.96 | 0.21 * | 24 | 7 | 3.08 | 0.16 | 179 | 52 | 4.30 | 0.14 | 46 | 13 | 2.52 | 0.08 * |
| Cesium | 9.00 | 177 | 47.5 | 14.63 | 0.53 | 59 | 33 | 16.00 | 0.82 * | 16 | 9 | 8.78 | 0.64 | 86 | 49 | 16.78 | 0.53 | 22 | 12 | 12.88 | 0.25 * |
| Gadolinium | 0.30 | 172 | 45.9 | 31.19 | 5.41 | 88 | 51 | 41.55 | 6.96 | 7 | 4 | 58.83 | 0.81 | 65 | 38 | 8.48 | 4.34 | 11 | 6 | 2.62 | 0.18 |
| Lead | 2.00 | 370 | 99.7 | 26.76 | 1.56 | 115 | 31 | 28.00 | 1.91 * | 24 | 6 | 22.92 | 1.75 | 192 | 52 | 38.79 | 1.53 ° | 50 | 14 | 20.16 | 0.77 *,° |
| Mercury | 3.00 | 18 | 5.0 | 7.58 | 0.66 | 6 | 33 | 7.17 | 1.78 | 3 | 17 | 1.55 | 0.12 | 11 | 61 | 7.53 | 2.57 | 1 | 6 | 4.60 | nd |
| Nickel | 10.00 | 58 | 16.1 | 16.84 | 0.99 | 24 | 41 | 21.50 | 3.07 * | 7 | 12 | 8.80 | 0.96 | 29 | 50 | 21.93 | 0.58 | 5 | 9 | 12.00 | 0.16 * |
| Palladium | 0.30 | 13 | 3.4 | 0.48 | 0.04 | 8 | 62 | 0.48 | 0.05 | 1 | 8 | nd | nd | 5 | 38 | 0.65 | 0.03 | 1 | 8 | 0.40 | nd |
| Platinum | 1.00 | 2 | 0.5 | 10.65 | 9.35 | nd | nd | nd | nd | 0 | 0 | nd | nd | 2 | nd | nd | 9.35 | 0 | nd | nd | nd |
| Tellurium | 0.80 | 0 | 0.0 | nd | nd | nd | nd | nd | nd | 0 | 0 | nd | nd | 0 | nd | nd | nd | 0 | nd | nd | nd |
| Thallium | 0.50 | 55 | 14.8 | 1.20 | 0.06 | 16 | 29 | 1.33 | 0.12 | 8 | 15 | nd | 0.03 | 31 | 56 | 1.13 | 0.12 | 5 | 9 | 0.72 | 0.02 |
| Thorium | 0.03 | 1 | 0.3 | 0.08 | 0.04 | nd | nd | nd | nd | 0 | 0 | nd | nd | 0 | nd | nd | nd | 1 | 100 | 0.08 | nd |
| Tin | 9.00 | 4 | 1.1 | 24.00 | 1.47 | nd | nd | nd | nd | 0 | 0 | nd | nd | 3 | 75 | 48.00 | 2.29 | 0 | nd | nd | nd |
| Tungsten | 0.40 | 35 | 9.5 | 1.18 | 0.18 | 14 | 40 | 2.06 | 0.82 | 7 | 20 | nd | 1.14 | 19 | 54 | 2.88 | 0.04 | 3 | 9 | 0.60 | 0.03 |
| Uranium | 0.03 | 23 | 6.3 | 0.39 | 0.24 | 6 | 26 | 0.25 | 0.076 | 1 | 4 | nd | nd | 15 | 65 | 0.31 | 0.299 | 2 | 9 | 0.09 | 0.004 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fulgenzi, A.; Vietti, D.; Ferrero, M.E. EDTA Chelation Therapy in the Treatment of Neurodegenerative Diseases: An Update. Biomedicines 2020, 8, 269. https://doi.org/10.3390/biomedicines8080269
Fulgenzi A, Vietti D, Ferrero ME. EDTA Chelation Therapy in the Treatment of Neurodegenerative Diseases: An Update. Biomedicines. 2020; 8(8):269. https://doi.org/10.3390/biomedicines8080269
Chicago/Turabian StyleFulgenzi, Alessandro, Daniele Vietti, and Maria Elena Ferrero. 2020. "EDTA Chelation Therapy in the Treatment of Neurodegenerative Diseases: An Update" Biomedicines 8, no. 8: 269. https://doi.org/10.3390/biomedicines8080269
APA StyleFulgenzi, A., Vietti, D., & Ferrero, M. E. (2020). EDTA Chelation Therapy in the Treatment of Neurodegenerative Diseases: An Update. Biomedicines, 8(8), 269. https://doi.org/10.3390/biomedicines8080269





