The Effectiveness of Multi-Session FMT Treatment in Active Ulcerative Colitis Patients: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Characteristics of FMT Recipients
2.3. Characteristics of Fecal Donors
2.4. Fecal Sample Preparation and FMT Intervention
2.5. Metagenomic Analysis and Data Processing
2.5.1. Sample Collection and DNA Extraction
2.5.2. 16. S rRNA Gene Amplification and Sequencing
2.6. Biochemical Assessment
2.7. Statistical Analysis
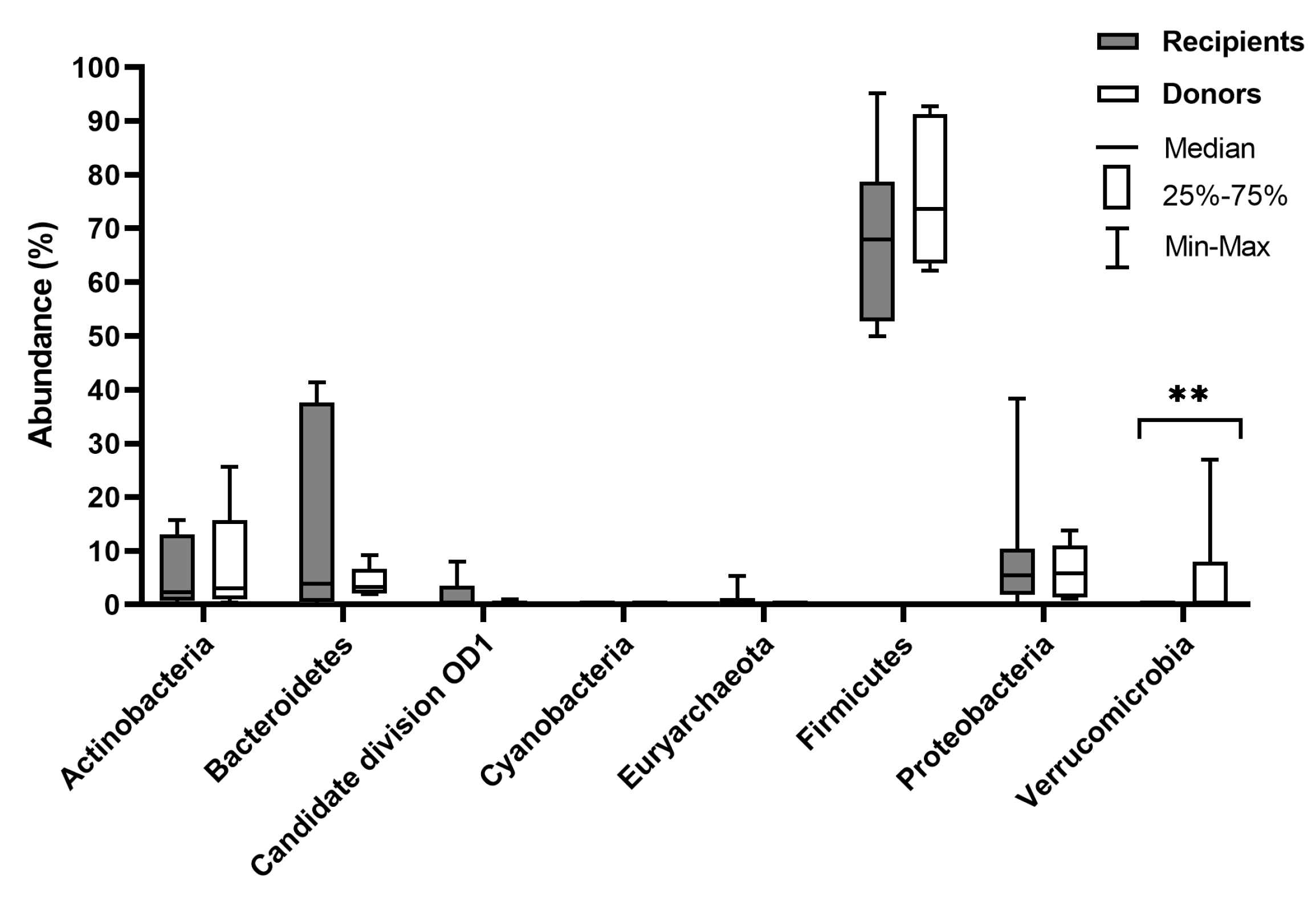
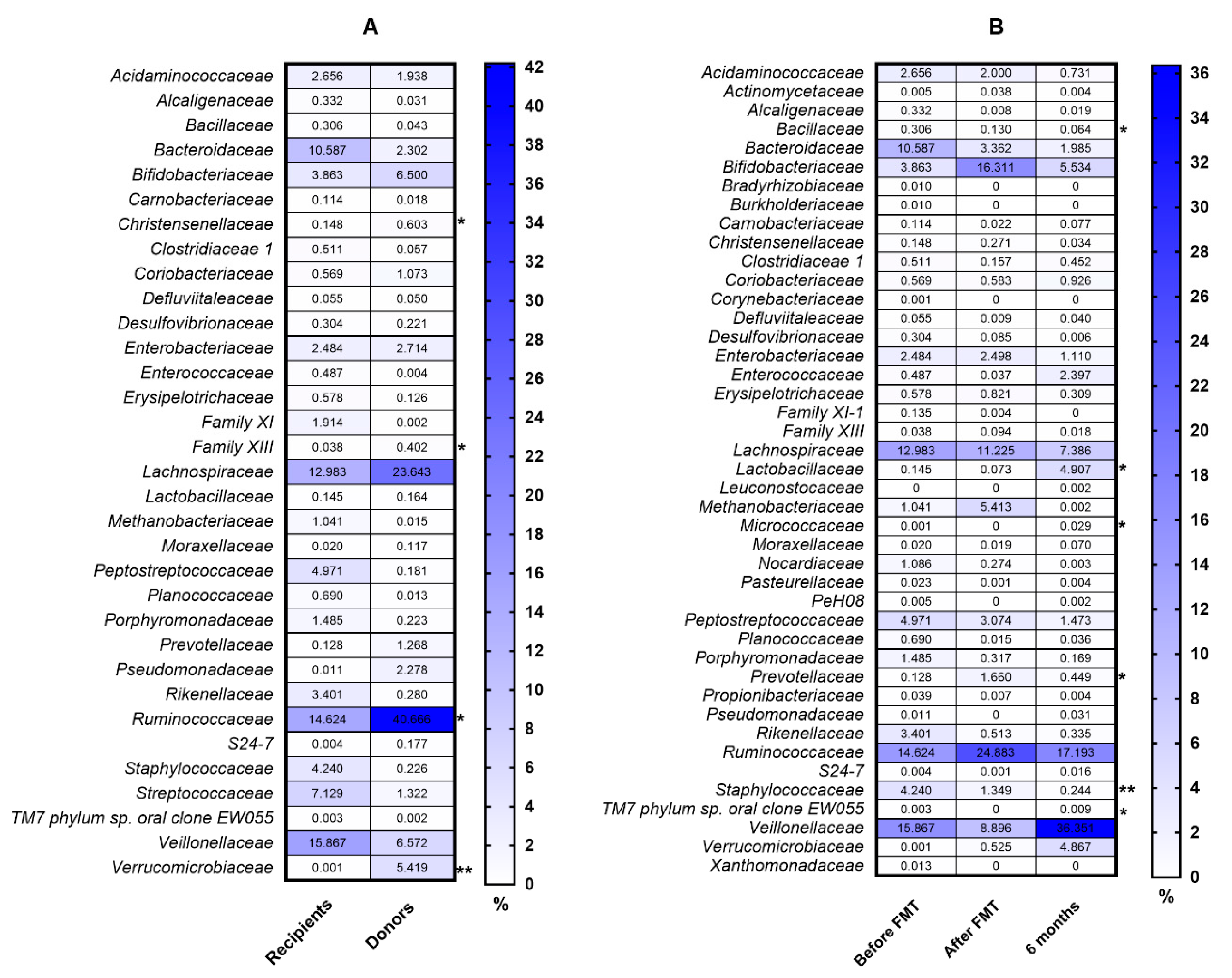
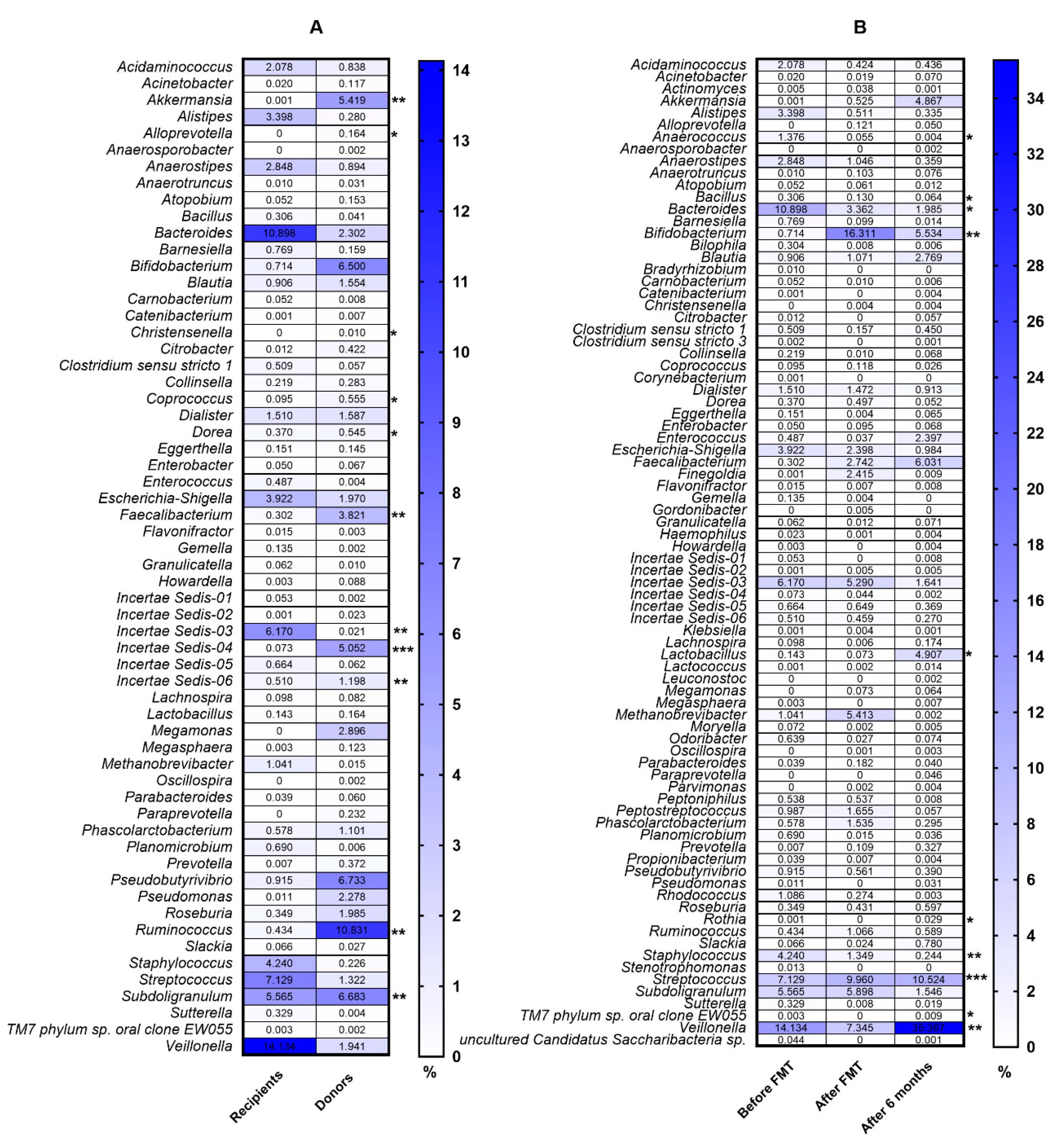
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FMT | Fecal Microbiota Transplantation |
| IBD | Inflammatory Bowel Disease |
| UC | Ulcerative Colitis |
| CD | Crohn’s Disease |
| BMI | Body Mass Index |
| MAM | Mucosal-Associated Microbiota |
| CALPR | Calprotectin |
| SCFAs | Short-Chain Fatty Acids |
| CRP | C Reactive Protein |
| ESR | Erythrocyte Sedimentation Rate |
| IDA | Iron Deficiency Anemia |
| ACD | Anemia of Chronic Disease |
References
- Zhang, Y.-Z.; Li, Y.-Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. WJG 2014, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Knox, N.C.; Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The gut microbiome as a target for IBD treatment: Are we there yet? Curr. Treat. Options Gastroenterol. 2019, 17, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, G.; Bellavia, M.; Palumbo, V.D.; Gioviale, M.C.; Damiani, P.; Lo Monte, A.I. From gut microflora imbalance to mycobacteria infection: Is there a relationship with chronic intestinal inflammatory diseases? Ann. Ital. Di Chir. 2011, 82, 3613–3668. [Google Scholar]
- Arseneau, K.O.; Cominelli, F. Leukocytapheresis in ulcerative colitis: A possible alternative to biological therapy? Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2009, 41, 551. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.; Bevins, C.L.; Ghosh, D.; Ganz, T. The multifaceted Paneth cell. Cellular and molecular life sciences. CMLS 2002, 59, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-H.; Zhu, C.-X.; Quan, Y.-S.; Yang, Z.-Y.; Wu, S.; Luo, W.-W.; Tan, B.; Wang, X.-Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018, 24, 5. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Xu, B.; Wang, X.; Zhang, Y.; Wang, H.; Kong, X.; Zhu, H.; Wu, K. The biodiversity and composition of the dominant fecal microbiota in patients with inflammatory bowel disease. Diagn. Microbiol. Infect. Dis. 2013, 75, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Imhann, F.; Vila, A.V.; Bonder, M.J.; Fu, J.; Gevers, D.; Visschedijk, M.C.; Spekhorst, L.M.; Alberts, R.; Franke, L.; Van Dullemen, H.M. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut 2018, 67, 108–119. [Google Scholar] [CrossRef]
- Santoru, M.L.; Piras, C.; Murgia, A.; Palmas, V.; Camboni, T.; Liggi, S.; Ibba, I.; Lai, M.A.; Orrù, S.; Blois, S. Cross sectional evaluation of the gut-microbiome metabolome axis in an Italian cohort of IBD patients. Sci. Rep. 2017, 7, 11–14. [Google Scholar] [CrossRef]
- Michail, S.; Durbin, M.; Turner, D.; Griffiths, A.M.; Mack, D.R.; Hyams, J.; Leleiko, N.; Kenche, H.; Stolfi, A.; Wine, E. Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm. Bowel Dis. 2012, 18, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, X.; Ghozlane, A.; Hu, H.; Li, X.; Xiao, Y.; Li, D.; Yu, G.; Zhang, T. Characteristics of faecal microbiota in paediatric Crohn’s disease and their dynamic changes during infliximab therapy. J. Crohn’s Colitis 2018, 12, 337–346. [Google Scholar] [CrossRef]
- Liguori, G.; Lamas, B.; Richard, M.L.; Brandi, G.; Da Costa, G.; Hoffmann, T.W.; Di Simone, M.P.; Calabrese, C.; Poggioli, G.; Langella, P. Fungal dysbiosis in mucosa-associated microbiota of Crohn’s disease patients. J. Crohn’s Colitis 2016, 10, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.O.; Gluck, M. Fecal microbiota transplantation: An update on clinical practice. Clin. Endosc. 2019, 52, 137. [Google Scholar] [CrossRef]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; De Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, M.N.; Widlak, M.; Bhala, N.A.; Moore, D.; Price, M.; Sharma, N.; Iqbal, T. Systematic review with meta-analysis: The efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment. Pharmacol. Ther. 2017, 46, 479–493. [Google Scholar] [CrossRef]
- Kellingray, L.; Le Gall, G.; Defernez, M.; Beales, I.L.; Franslem-Elumogo, N.; Narbad, A. Microbial taxonomic and metabolic alterations during faecal microbiota transplantation to treat Clostridium difficile infection. J. Infect. 2018, 77, 107–118. [Google Scholar] [CrossRef]
- Kelly, C.R.; Khoruts, A.; Staley, C.; Sadowsky, M.J.; Abd, M.; Alani, M.; Bakow, B.; Curran, P.; McKenney, J.; Tisch, A. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: A randomized trial. Ann. Intern. Med. 2016, 165, 609–616. [Google Scholar] [CrossRef]
- Cui, B.; Xu, F.; Zhang, F. Methodology, not concept of fecal microbiota transplantation, affects clinical findings. Gastroenterology 2016, 150, 285–286. [Google Scholar] [CrossRef]
- Zhang, F.; Cui, B.; He, X.; Nie, Y.; Wu, K.; Fan, D.; Group, F.-s.S. Microbiota transplantation: Concept, methodology and strategy for its modernization. Protein Cell 2018, 9, 462–473. [Google Scholar] [CrossRef]
- Borody, T.J.; Paramsothy, S.; Agrawal, G. Fecal microbiota transplantation: Indications, methods, evidence, and future directions. Curr. Gastroenterol. Rep. 2013, 15, 337. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Blanchaert, C.; Strubbe, B.; Peeters, H. Fecal microbiota transplantation in ulcerative colitis. Acta Gastro Enterol. Belg. 2019, 82, 519–528. [Google Scholar]
- Cui, B.; Li, P.; Xu, L.; Peng, Z.; Xiang, J.; He, Z.; Zhang, T.; Ji, G.; Nie, Y.; Wu, K. Step-up fecal microbiota transplantation (FMT) strategy. Gut Microbes 2016, 7, 323–328. [Google Scholar] [CrossRef]
- Long, C.; Yu, Y.; Cui, B.; Jagessar, S.A.R.; Zhang, J.; Ji, G.; Huang, G.; Zhang, F. A novel quick transendoscopic enteral tubing in mid-gut: Technique and training with video. BMC Gastroenterol. 2018, 18, 37. [Google Scholar] [CrossRef]
- Ni, X.; Fan, S.; Zhang, Y.; Wang, Z.; Ding, L.; Li, Y.; Li, J. Coordinated hospital-home fecal microbiota transplantation via percutaneous endoscopic cecostomy for recurrent steroid-dependent ulcerative colitis. Gut Liver 2016, 10, 975. [Google Scholar] [CrossRef]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015, 149, 102–109. [Google Scholar] [CrossRef]
- Rossen, N.G.; MacDonald, J.K.; De Vries, E.M.; D’Haens, G.R.; De Vos, W.M.; Zoetendal, E.G.; Ponsioen, C.Y. Fecal microbiota transplantation as novel therapy in gastroenterology: A systematic review. World J. Gastroenterol. WJG 2015, 21, 5359. [Google Scholar] [CrossRef]
- Kassam, Z.; Lee, C.H.; Yuan, Y.; Hunt, R.H. Fecal Microbiota Transplantation forClostridium difficileInfection: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2013, 108, 500–508. [Google Scholar] [CrossRef]
- Goyal, A.; Yeh, A.; Bush, B.R.; Firek, B.A.; Siebold, L.M.; Rogers, M.B.; Kufen, A.D.; Morowitz, M.J. Safety, clinical response, and microbiome findings following fecal microbiota transplant in children with inflammatory bowel disease. Inflamm. Bowel Dis. 2018, 24, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Nielsen, S.; Kamm, M.A.; Deshpande, N.P.; Faith, J.J.; Clemente, J.C.; Paramsothy, R.; Walsh, A.J.; Van Den Bogaerde, J.; Samuel, D. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology 2019, 156, 1440–1454. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Steiner, T.; Petrof, E.O.; Smieja, M.; Roscoe, D.; Nematallah, A.; Weese, J.S.; Collins, S.; Moayyedi, P.; Crowther, M. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: A randomized clinical trial. JAMA 2016, 315, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Mancabelli, L.; Milani, C.; Lugli, G.A.; Turroni, F.; Cocconi, D.; Van Sinderen, D.; Ventura, M. Identification of universal gut microbial biomarkers of common human intestinal diseases by meta-analysis. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; Shanahan, F.; Guarner, F.; De Vos, W.M. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm. Bowel Dis. 2013, 19, 481–488. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef]
- Kummen, M.; Holm, K.; Anmarkrud, J.A.; Nygård, S.; Vesterhus, M.; Høivik, M.L.; Trøseid, M.; Marschall, H.-U.; Schrumpf, E.; Moum, B. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut 2017, 66, 611–619. [Google Scholar] [CrossRef]
- Kump, P.; Wurm, P.; Gröchenig, H.; Wenzl, H.; Petritsch, W.; Halwachs, B.; Wagner, M.; Stadlbauer, V.; Eherer, A.; Hoffmann, K. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Aliment. Pharmacol. Ther. 2018, 47, 67–77. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Vigsnæs, L.K.; Brynskov, J.; Steenholdt, C.; Wilcks, A.; Licht, T.R. Gram-negative bacteria account for main differences between faecal microbiota from patients with ulcerative colitis and healthy controls. Benef. Microbes 2012, 3, 287–297. [Google Scholar] [CrossRef]
- Hirano, A.; Umeno, J.; Okamoto, Y.; Shibata, H.; Ogura, Y.; Moriyama, T.; Torisu, T.; Fujioka, S.; Fuyuno, Y.; Kawarabayasi, Y. Comparison of the microbial community structure between inflamed and non-inflamed sites in patients with ulcerative colitis. J. Gastroenterol. Hepatol. 2018, 33, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Leoni, G.; Quiros, M.; Wu, H.; Desai, C.; Nishio, H.; Jones, R.M.; Nusrat, A.; Neish, A.S. The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat. Microbiol. 2016, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Uchiyama, K.; Takagi, T. A next-generation beneficial microbe: Akkermansia muciniphila. J. Clin. Biochem. Nutr. 2018, 63, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Kamm, M.A.; Kaakoush, N.O.; Walsh, A.J.; Van Den Bogaerde, J.; Samuel, D.; Leong, R.W.; Connor, S.; Ng, W.; Paramsothy, R. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet 2017, 389, 1218–1228. [Google Scholar] [CrossRef]
- Walujkar, S.A.; Kumbhare, S.V.; Marathe, N.P.; Patangia, D.V.; Lawate, P.S.; Bharadwaj, R.S.; Shouche, Y.S. Molecular profiling of mucosal tissue associated microbiota in patients manifesting acute exacerbations and remission stage of ulcerative colitis. World J. Microbiol. Biotechnol. 2018, 34, 76. [Google Scholar] [CrossRef]
- Kiernan, M.G.; Coffey, J.C.; McDermott, K.; Cotter, P.D.; Cabrera-Rubio, R.; Kiely, P.A.; Dunne, C.P. The human mesenteric lymph node microbiome differentiates between Crohn’s disease and ulcerative colitis. J. Crohn’s Colitis 2019, 13, 58–66. [Google Scholar] [CrossRef]
- Chen, H.T.; Huang, H.L.; Xu, H.M.; Luo, Q.L.; He, J.; Li, Y.Q.; Zhou, Y.L.; Nie, Y.Q.; Zhou, Y.J. Fecal microbiota transplantation ameliorates active ulcerative colitis. Exp. Ther. Med. 2020, 19, 2650–2660. [Google Scholar] [CrossRef]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef]
- Fuentes, S.; Rossen, N.G.; Van Der Spek, M.J.; Hartman, J.H.; Huuskonen, L.; Korpela, K.; Salojärvi, J.; Aalvink, S.; De Vos, W.M.; D’Haens, G.R. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. Isme J. 2017, 11, 1877–1889. [Google Scholar] [CrossRef]
- Dutta, S.K.; Girotra, M.; Garg, S.; Dutta, A.; Von Rosenvinge, E.C.; Maddox, C.; Song, Y.; Bartlett, J.G.; Vinayek, R.; Fricke, W.F. Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin. Gastroenterol. Hepatol. 2014, 12, 1572–1576. [Google Scholar] [CrossRef]
- Scanlan, P.D.; Shanahan, F.; O’Mahony, C.; Marchesi, J.R. Culture-independent analyses of temporal variation of the dominant fecal microbiota and targeted bacterial subgroups in Crohn’s disease. J. Clin. Microbiol. 2006, 44, 3980–3988. [Google Scholar] [CrossRef] [PubMed]
- Kabeerdoss, J.; Jayakanthan, P.; Pugazhendhi, S.; Ramakrishna, B.S. Alterations of mucosal microbiota in the colon of patients with inflammatory bowel disease revealed by real time polymerase chain reaction amplification of 16S ribosomal ribonucleic acid. Indian J. Med Res. 2015, 142, 23. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; Amand, A.L.S.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed]
- Andoh, A.; Imaeda, H.; Aomatsu, T.; Inatomi, O.; Bamba, S.; Sasaki, M.; Saito, Y.; Tsujikawa, T.; Fujiyama, Y. Comparison of the fecal microbiota profiles between ulcerative colitis and Crohn’s disease using terminal restriction fragment length polymorphism analysis. J. Gastroenterol. 2011, 46, 479–486. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Factories 2017, 16, 79. [Google Scholar] [CrossRef]
- Venegas, D.P.; Marjorie, K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Reddavide, R.; Rotolo, O.; Gabriella, C.M.; Stasi, E.; Notarnicola, M.; Miraglia, C.; Nouvenne, A.; Meschi, T.; Luigi De’ Angelis, G.; Di Mario, F.; et al. The role of diet in the prevention and treatment of Inflammatory Bowel Diseases. Acta Bio-Med. Atenei Parm. 2018, 89, 60. [Google Scholar] [CrossRef]
- Png, C.W.; Lindén, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.A.; Florin, T.H. Mucolytic Bacteria With Increased Prevalence in IBD Mucosa AugmentIn VitroUtilization of Mucin by Other Bacteria. Am. J. Gastroenterol. 2010, 105, 2420–2428. [Google Scholar] [CrossRef]
- Nishida, A.; Imaeda, H.; Ohno, M.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Efficacy and safety of single fecal microbiota transplantation for Japanese patients with mild to moderately active ulcerative colitis. J. Gastroenterol. 2017, 52, 476–482. [Google Scholar] [CrossRef]
- Hourigan, S.; Chen, L.; Grigoryan, Z.; Laroche, G.; Weidner, M.; Sears, C.L.; Oliva-Hemker, M. Microbiome changes associated with sustained eradication of Clostridium difficile after single faecal microbiota transplantation in children with and without inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015, 42, 741–752. [Google Scholar] [CrossRef]
- Sabino, J.; Vieira-Silva, S.; Machiels, K.; Joossens, M.; Falony, G.; Ballet, V.; Ferrante, M.; Van Assche, G.; Van Der Merwe, S.; Vermeire, S. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut 2016, 65, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Kanai, T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015, 37, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, A.; Ansari, S.; Hershberg, R.M.; Mckay, D.M. Colonic bacterial superantigens evoke an inflammatory response and exaggerate disease in mice recovering from colitis. Gastroenterology 2003, 125, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Vesterlund, S.; Karp, M.; Salminen, S.; Ouwehand, A.C. Staphylococcus aureus adheres to human intestinal mucus but can be displaced by certain lactic acid bacteria. Microbiology 2006, 152, 1819–1826. [Google Scholar] [CrossRef]
- Salem, F.; Kindt, N.; Marchesi, J.R.; Netter, P.; Lopez, A.; Kokten, T.; Danese, S.; Jouzeau, J.-Y.; Peyrin-Biroulet, L.; Moulin, D. Gut microbiome in chronic rheumatic and inflammatory bowel diseases: Similarities and differences. United Eur. Gastroenterol. J. 2019, 7, 1008–1032. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020. [Google Scholar] [CrossRef]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef]
- Zhang, T.; Cui, B.; Li, P.; He, Z.; Long, C.; Wei, L.; Peng, Z.; Ji, G.; Zhang, F. Short-term surveillance of cytokines and C-reactive protein cannot predict efficacy of fecal microbiota transplantation for ulcerative colitis. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Uygun, A.; Ozturk, K.; Demirci, H.; Oger, C.; Avci, I.Y.; Turker, T.; Gulsen, M. Fecal microbiota transplantation is a rescue treatment modality for refractory ulcerative colitis. Medicine 2017, 96. [Google Scholar] [CrossRef]
- Cold, F.; Browne, P.D.; Günther, S.; Halkjaer, S.; Petersen, A.; Al-Gibouri, Z.; Hansen, L.; Christensen, A. Multidonor FMT capsules improve symptoms and decrease fecal calprotectin in ulcerative colitis patients while treated–an open-label pilot study. Scand. J. Gastroenterol. 2019, 54, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Zackular, J.P.; Chazin, W.J.; Skaar, E.P. Nutritional immunity: S100 proteins at the host-pathogen interface. J. Biol. Chem. 2015, 290, 18991–18998. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.A.; Skaar, E.P. The impact of dietary transition metals on host-bacterial interactions. Cell Host Microbe 2018, 23, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Damo, S.M.; Kehl-Fie, T.E.; Sugitani, N.; Holt, M.E.; Rathi, S.; Murphy, W.J.; Zhang, Y.; Betz, C.; Hench, L.; Fritz, G. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc. Natl. Acad. Sci. USA 2013, 110, 3841–3846. [Google Scholar] [CrossRef] [PubMed]
- Das, N.K.; Schwartz, A.J.; Barthel, G.; Inohara, N.; Liu, Q.; Sankar, A.; Hill, D.R.; Ma, X.; Lamberg, O.; Schnizlein, M.K. Microbial metabolite signaling is required for systemic iron homeostasis. Cell Metab. 2020, 31, 115–130.e116. [Google Scholar] [CrossRef]


| Analyzed Variable | N = 10 |
|---|---|
| Age (years) | 47.5 ± 18.16 |
| Disease duration (years) | 5.9 (3−10) |
| BMI (kg/m2) | 22 ± 2.75 |
| Male sex | 5 |
| Disease extent | |
| -Proctitis | 0 |
| -Left-sided colitis | 4 |
| -Extensive colitis | 4 |
| -Pancolitis | 2 |
| Medical treatment * | |
| -Oral 5-ASA | 10 |
| -Oral steroids | 4 |
| -Immunomodulators | 4 |
| Nonsmoker | 6 |
| Analyzed Parameter | n | Before FMT | After FMT | After 6 Months | p-Value |
|---|---|---|---|---|---|
| WBC (103/L) | 10 | 22.40 (20–24) | 7.15 (5.2–9.4) | 7.2 (6.2–9.4) | 0.904 b |
| RBC (10⁶/L) | 10 | 3.97 ± 0.62 | 4.11 ± 0.63 | 4.438 ± 0.46 | 0.029 a |
| Before vs. After 0.675 | |||||
| Before vs. After 6mo 0.027 | |||||
| After vs. After 6mo 0.141 | |||||
| HGB (g/dL) | 10 | 11.61 ± 2.38 | 12.18 ± 1.88 | 12.63 ± 1.92 | 0.099 a |
| HCT (%) | HCT | 36 (2.7–39) | 36.65 (33.6–39.5) | 40.35 (35.5–42.7) | 0.049 b |
| Before vs. After 0.281 | |||||
| Before vs. After 6mo 0.057 | |||||
| After vs. After 6mo 1.000 | |||||
| MCV (fL) | 10 | 86.75 (84–88) | 86.35 (84.9–88) | 85.35 (84.9–87) | 0.905 b |
| RDW-CV (fL) | 10 | 12.55 (12.3–13.7) | 13.7 (12.1–16) | 13.85 (12.8–14.5) | 0.283 b |
| PLT (103/L) | 10 | 313.4 ± 128.17 | 344.7 ± 118.83 | 299.1 ± 104.82 | 0.059 a |
| Iron (µg/dL) | 10 | 48.1 ± 24.6 | 51.7 ± 22.92 | 71.1 ± 39.75 | 0.075 a |
| TIBC (µg/dL) | 10 | 243.2 ± 72.81 | 278.6 ± 36.81 | 316.5 ± 45.01 | 0.004 a |
| Before vs. After 0.171 | |||||
| Before vs. After 6mo 0.003 | |||||
| After vs. After 6mo 0.135 | |||||
| CRP (mg/L) | 10 | 9.5 (7.7–82) | 5.2 (3.2–5.7) | 3.4 (2.4–7.4) | 0.0004 b |
| Before vs. After 0.011 | |||||
| Before vs. After 6mo 0.0004 | |||||
| After vs. After 6mo 1.000 | |||||
| Ferritin (ng/mL) | 10 | 33 (23–50) | 35.5 (19–42) | 37 (24–44) | 0.388 b |
| TP (g/dL) | 10 | 6.7 ± 0.45 | 7.18 ± 0.51 | 7.325 ± 0.52 | 0.001 a |
| Before vs. After 0.013 | |||||
| Before vs. After 6mo 0.002 | |||||
| After vs. After 6mo 0.604 | |||||
| ALB (g/dL) | 10 | 3.72 ± 0.65 | 4.06 ± 0.28 | 4.196 ± 0.33 | 0.127 a |
| Calprotectin (µg/g) | 10 | 1500 (969–1590) | 1095 (1000–1280) | 510 (91–800) | 0.002 b |
| Before vs. After 0.221 | |||||
| Before vs. After 6mo 0.001 | |||||
| After vs. After 6mo 0.221 | |||||
| Disease activity (Truelove and Witts Severity Index) | 10 | 3 (3–3) | 2 (2–2) | 1 (1–2) | 0.0001 b |
| Before vs. After 0.016 | |||||
| Before vs. After 6mo 0.0003 | |||||
| After vs. After 6mo 0.791 |
| Phylum | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Selected Bacteria | Before FMT | After FMT | After 6 Months | ||||||
| CRP | Ferritin | CALPR * | CRP | Ferritin | CALPR * | CRP | Ferritin | CALPR * | |
| Firmicutes | 0.405 (0.297) | 0.947 (0.024) | 0.575 (−0.202) | 0.382 (0.311) | 0.556 (−0.212) | 0.275 (−0.383) | 0.511 (−0.236) | 0.345 (−0.334) | 0.128 (−0.515) |
| Family | |||||||||
| Lactobacillaceae | 0.851 (0.068) | 0.035 (−0.668) | 0.427 (−0.283) | 0.228 (−0.419) | 0.570 (0.205) | 0.903 (0.045) | 0.379 (0.313) | 0.852 (−0.068) | 0.672 (0.153) |
| Staphylococcaceae | 0.336 (0.340) | 0.662 (0.159) | 0.001 (0.861) | 0.353 (0.329) | 0.059 (0.612) | 0.097 (0.553) | 0.211 (0.433) | 0.293 (0.370) | 0.131 (0.511) |
| Genus | |||||||||
| Anaerococcus | 0.793 (−0.096) | 0.756 (0.113) | 0.97 (−0.014) | 0.227 (−0.42) | 0.141 (−0.5) | 0.918 (−0.037) | 0.416 (0.29) | 0.415 (0.291) | 0.416 (0.29) |
| Bacillus | 0.135 (0.506) | 0.424 (0.285) | 0.003 (0.829) | 0.021 (0.713) | 0.947 (−0.024) | 0.973 (0.012) | 0.173 (0.467) | 0.102 (0.547) | 0.65 (0.164) |
| Bacteroides | 0.511 (−0.236) | 0.854 (−0.067) | 0.461 (−0.264) | 0.802 (−0.091) | 0.108 (−0.539) | 0.106 (−0.541) | 0.679 (−0.15) | 0.615 (0.182) | 0.199 (0.444) |
| Bifidobacterium | 0.651 (0.164) | 0.364 (0.322) | 0.599 (0.19) | 0.464 (−0.262) | 0.31 (0.358) | 0.213 (0.432) | 0.365 (0.321) | 0.028 (0.687) | 0.855 (0.067) |
| Lactobacillus | 0.851 (0.068) | 0.035 (−0.668) | 0.428 (−0.283) | 0.228 (−0.419) | 0.57 (−0.205) | 0.903 (0.045) | 0.379 (0.313) | 0.853 (−0.068) | 0.672 (0.153) |
| Staphylococcus | 0.336 (0.34) | 0.662 (0.159) | 0.001 (0.862) | 0.353 (0.329) | 0.06 (0.612) | 0.097 (0.553) | 0.211 (0.433) | 0.293 (0.37) | 0.131 (0.511) |
| Veillonella | 0.551 (0.215) | 0.046 (−0.64) | 0.878 (0.056) | 0.865 (−0.062) | 0.906 (0.043) | 0.598 (−0.191) | 0.385 (−0.309) | 0.097 (−0.553) | 0.385 (−0.309) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mańkowska-Wierzbicka, D.; Stelmach-Mardas, M.; Gabryel, M.; Tomczak, H.; Skrzypczak-Zielińska, M.; Zakerska-Banaszak, O.; Sowińska, A.; Mahadea, D.; Baturo, A.; Wolko, Ł.; et al. The Effectiveness of Multi-Session FMT Treatment in Active Ulcerative Colitis Patients: A Pilot Study. Biomedicines 2020, 8, 268. https://doi.org/10.3390/biomedicines8080268
Mańkowska-Wierzbicka D, Stelmach-Mardas M, Gabryel M, Tomczak H, Skrzypczak-Zielińska M, Zakerska-Banaszak O, Sowińska A, Mahadea D, Baturo A, Wolko Ł, et al. The Effectiveness of Multi-Session FMT Treatment in Active Ulcerative Colitis Patients: A Pilot Study. Biomedicines. 2020; 8(8):268. https://doi.org/10.3390/biomedicines8080268
Chicago/Turabian StyleMańkowska-Wierzbicka, Dorota, Marta Stelmach-Mardas, Marcin Gabryel, Hanna Tomczak, Marzena Skrzypczak-Zielińska, Oliwia Zakerska-Banaszak, Anna Sowińska, Dagmara Mahadea, Alina Baturo, Łukasz Wolko, and et al. 2020. "The Effectiveness of Multi-Session FMT Treatment in Active Ulcerative Colitis Patients: A Pilot Study" Biomedicines 8, no. 8: 268. https://doi.org/10.3390/biomedicines8080268
APA StyleMańkowska-Wierzbicka, D., Stelmach-Mardas, M., Gabryel, M., Tomczak, H., Skrzypczak-Zielińska, M., Zakerska-Banaszak, O., Sowińska, A., Mahadea, D., Baturo, A., Wolko, Ł., Słomski, R., & Dobrowolska, A. (2020). The Effectiveness of Multi-Session FMT Treatment in Active Ulcerative Colitis Patients: A Pilot Study. Biomedicines, 8(8), 268. https://doi.org/10.3390/biomedicines8080268





