The New Proactive Approach and Precision Medicine in Crohn’s Disease
Abstract
1. Introduction
2. Natural History
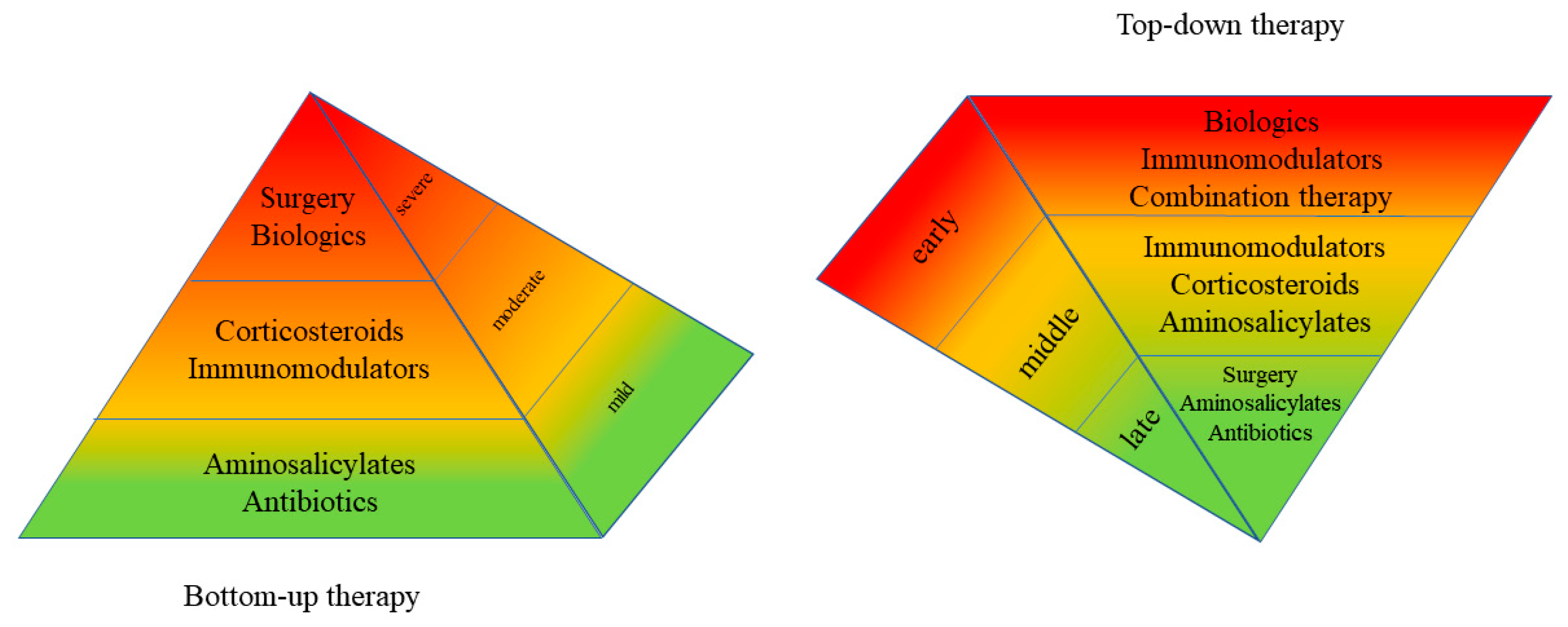
3. Evidence for Top-Down Remission Induction in IBD
4. Proactive Optimization of CD Therapy with Tight Control
5. Objective Measurements of CD Activity
5.1. Fecal Calprotectin
5.2. CRP
5.3. Hsp60
5.4. Multifactorial Disease Activity Indices
5.5. Small-Bowel Cross-Sectional Assessment
6. Multidisciplinary Care
7. A Proactive Algorithmic Approach
8. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Abautret-Daly, Á.; Dempsey, E.; Parra-Blanco, A.; Medina, C.; Harkin, A. Gut–brain actions underlying comorbid anxiety and depression associated with inflammatory bowel disease. Acta Neuropsychiatr. 2017, 30, 275–296. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Weimers, P.; Munkholm, P. The Natural History of IBD: Lessons Learned. Curr. Treat. Options Gastroenterol. 2018, 16, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Frolkis, A.D.; Dykeman, J.; Negrón, M.E.; Debruyn, J.; Jette, N.; Fiest, K.M.; Frolkis, T.; Barkema, H.W.; Rioux, K.P.; Panaccione, R.; et al. Risk of Surgery for Inflammatory Bowel Diseases Has Decreased Over Time: A Systematic Review and Meta-analysis of Population-Based Studies. Gastroenterology 2013, 145, 996–1006. [Google Scholar] [CrossRef]
- De Cruz, P.; A Kamm, M.; Hamilton, A.L.; Ritchie, K.J.; O Krejany, E.; Gorelik, A.; Liew, D.; Prideaux, L.; Lawrance, I.C.; Andrews, J.M.; et al. Crohn’s disease management after intestinal resection: A randomised trial. Lancet 2015, 385, 1406–1417. [Google Scholar] [CrossRef]
- Travis, S.P.L.; Stange, E.F.; Lémann, M.; Öresland, T.; Chowers, Y.; Forbes, A. European evidence based consensus on the diagnosis and management of Crohn’s disease: Current management. Gut 2006, 55 (Suppl. 1), i16–i35. [Google Scholar] [CrossRef]
- Hommes, D.W.; Oldenburg, B.; A Van Bodegraven, A.; A Van Hogezand, R.; De Jong, D.J.; Romberg-Camps, M.J.L.; Van Der Woude, J.; Dijkstra, G. Guidelines for treatment with infliximab for Crohn’s disease. Neth. J. Med. 2006, 64, 219–229. [Google Scholar]
- Oldenburg, B.; Hommes, D. Biological therapies in inflammatory bowel disease: Top-down or bottom-up? Curr. Opin. Gastroenterol. 2007, 23, 395–399. [Google Scholar] [CrossRef]
- Bouguen, G.; Levesque, B.G.; Feagan, B.G.; Kavanaugh, A.; Peyrin-Biroulet, L.; Colombel, J.; Hanauer, S.B.; Sandborn, W.J. Treat to Target: A Proposed New Paradigm for the Management of Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2015, 13, 1042–1050.e2. [Google Scholar] [CrossRef]
- Louis, E.; Collard, A.; Oger, A.F.; DeGroote, E.; El Yafi, F.A.N.; Belaiche, J. Behaviour of Crohn’s disease according to the Vienna classification: Changing pattern over the course of the disease. Gut 2001, 49, 777–782. [Google Scholar] [CrossRef]
- Cosnes, J.; Cattan, S.; Blain, A.; Beaugerie, L.; Carbonnel, F.; Parc, R.; Gendre, J.-P. Long-Term Evolution of Disease Behavior of Crohn’s Disease. Inflamm. Bowel Dis. 2002, 8, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Thia, K.T.; Sandborn, W.J.; Harmsen, W.S.; Zinsmeister, A.R.; Loftus, E.V., Jr. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010, 139, 1147–1155. [Google Scholar] [CrossRef]
- Pariente, B.; Mary, J.-Y.; Colombel, J.-F.; Cosnes, J. Development of the Crohn’s disease digestive damage score, the Lemann score. Inflamm. Bowel. Dis. 2013, 7, 1415–1422. [Google Scholar] [CrossRef][Green Version]
- Fiorino, G.; Bonifacio, C.; Allocca, M.; Repici, A.; Balzarini, L.; Malesci, A.; Peyrin-Biroulet, L.; Danese, S. Bowel Damage as Assessed by the Lemann Index is Reversible on Anti-TNF Therapy for Crohn’s Disease. J. Crohns Colitis 2015, 9, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Frøslie, K.F.; Jelsness-Jørgensen, L.-P.; Moum, B.; Vatn, M.H. Mucosal Healing in Inflammatory Bowel Disease: Results From a Norwegian Population-Based Cohort. Gastroenterology 2007, 133, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Björkesten, C.-G.A.; Nieminen, U.; Sipponen, T.; Turunen, U.; Arkkila, P.; Färkkilä, M. Mucosal healing at 3 months predicts long-term endoscopic remission in anti-TNF-treated luminal Crohn’s disease. Scand. J. Gastroenterol. 2013, 48, 543–551. [Google Scholar] [CrossRef]
- Colombel, J.-F.; Rutgeerts, P.; Sandborn, W.J.; Yang, M.; Camez, A.; Pollack, P.F.; Thakkar, R.B.; Robinson, A.M.; Chen, N.; Mulani, P.M.; et al. Adalimumab Induces Deep Remission in Patients With Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2014, 12, 414–422.e5. [Google Scholar] [CrossRef]
- Shah, S.C.; Colombel, J.; Sands, B.E.; Narula, N. Systematic review with meta-analysis: Mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment. Pharmacol. Ther. 2015, 43, 317–333. [Google Scholar] [CrossRef]
- Côté-Daigneault, J.; Bouin, M.; Lahaie, R.; Colombel, J.-F.; Poitras, P. Biologics in inflammatory bowel disease: What are the data? United Eur. Gastroenterol. J. 2015, 3, 419–428. [Google Scholar] [CrossRef]
- D’Haens, G.; Baert, F.; Van Assche, G.; Caenepeel, P.; Vergauwe, P.; Tuynman, H.; De Vos, M.; Van Deventer, S.; Stitt, L.; Donner, A.; et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: An open randomised trial. Lancet 2008, 371, 660–667. [Google Scholar] [CrossRef]
- Colombel, J.-F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; D’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, Azathioprine, or Combination Therapy for Crohn’s Disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Bressler, B.; Levesque, B.G.; Zou, G.; Stitt, L.W.; Greenberg, G.R.; Panaccione, R.; Bitton, A.; Pare, P.; Vermeire, S.; et al. Early combined immunosuppression for the management of Crohn’s disease (REACT): A cluster randomised controlled trial. Lancet 2015, 386, 1825–1834. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Feagan, B.G.; Cohen, R.D.; Salzberg, B.A.; Diamond, R.H.; Chen, D.M.; Pritchard, M.L.; Sandborn, W.J. Serious Infections and Mortality in Association With Therapies for Crohn’s Disease: TREAT Registry. Clin. Gastroenterol. Hepatol. 2006, 4, 621–630. [Google Scholar] [CrossRef]
- Hanauer, S.; Feagan, B.G.; Lichtenstein, G.R.; Mayer, L.F.; Schreiber, S.; Colombel, J.F.; Rachmilewitz, D.; Wolf, D.C.; Olson, A.; Bao, W.; et al. Maintenance infliximab for Crohn’s disease: The ACCENT I randomised trial. Lancet 2002, 359, 1541–1549. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Oussalah, A.; Williet, N.; Pillot, C.; Bresler, L.; Bigard, M.-A. Impact of azathioprine and tumour necrosis factor antagonists on the need for surgery in newly diagnosed Crohn’s disease. Gut 2011, 60, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Panaccione, R.; Sandborn, W.J.; D’Haens, G.R.; Schreiber, S.; Rutgeerts, P.J.; Jr, E.V.L.; Lomax, K.G.; Yu, A.P.; Wu, E.Q.; et al. Effects of Adalimumab Therapy on Incidence of Hospitalization and Surgery in Crohn’s Disease: Results From the CHARM Study. Gastroenterology 2008, 135, 1493–1499. [Google Scholar] [CrossRef]
- Dubinsky, M.; Mei, L.; Friedman, M.; Hakonarson, H.; Haritunians, T.; Kim, C.; Glessner, J.; Targan, S.R.; McGovern, D.; Taylor, K.D.; et al. 76 Genome Wide Association (GWA) Predictors of Anti-TNFα Therapeutic Responsiveness in Pediatric Inflammatory Bowel Disease (IBD). Gastroenterology 2009, 136, 1357–1366. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Loftus, E.V., Jr.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; E Sands, B. ACG Clinical Guideline: Management of Crohnʼs Disease in Adults. Am. J. Gastroenterol. 2018, 113, 481–517. [Google Scholar] [CrossRef]
- Kopylov, U.; Al-Taweel, T.; Yaghoobi, M.; Nauche, B.; Bitton, A.; Lakatos, P.L.; Ben-Horin, S.; Afif, W.; Seidman, E. Adalimumab monotherapy versus combination therapy with immunomodulators in patients with Crohn’s disease: A systematic review and meta-analysis. J. Crohns Colitis 2014, 8, 1632–1641. [Google Scholar] [CrossRef]
- Colombel, J.-F.; Panaccione, R.; Bossuyt, P.; Lukáš, M.; Baert, F.; Vanasek, T.; Danalioğlu, A.; Novacek, G.; Armuzzi, A.; Hebuterne, X.; et al. Effect of tight control management on Crohn’s disease (CALM): A multicentre, randomised, controlled phase 3 trial. Lancet 2017, 390, 2779–2789. [Google Scholar] [CrossRef]
- Casteele, N.V.; Ferrante, M.; Van Assche, G.; Ballet, V.; Compernolle, G.; Van Steen, K.; Simoens, S.; Rutgeerts, P.; Gils, A.; Vermeire, S. Trough Concentrations of Infliximab Guide Dosing for Patients With Inflammatory Bowel Disease. Gastroenterology 2015, 148, 1320–1329.e3. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Hanauer, S.B. Assessing Response and Loss of Response to Biological Therapies in IBD. Am. J. Gastroenterol. 2011, 106, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Ordas, I.; Eckmann, L.; Talamini, M. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Zittan, E.; Kabakchiev, B.; Milgrom, R.; Nguyen, G.C.; Croitoru, K.; Steinhart, A.; Silverberg, M.S. Higher Adalimumab Drug Levels are Associated with Mucosal Healing in Patients with Crohn’s Disease. J. Crohns Colitis 2016, 10, 510–515. [Google Scholar] [CrossRef]
- D’Haens, G.R.; Ferrante, M.; Vermeire, S.; Baert, F.; Noman, M.; Moortgat, L.; Geens, P.; Iwens, D.; Aerden, I.; Van Assche, G.; et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm. Bowel Dis. 2012, 18, 2218–2224. [Google Scholar] [CrossRef]
- Jones, J.; Loftus, E.V., Jr.; Panaccione, R.; Chen, L.; Peterson, S.; McConnell, J.; Baudhuin, L.; Hanson, K.; Feagan, B.G.; Harmsen, S.W.; et al. Relationships Between Disease Activity and Serum and Fecal Biomarkers in Patients With Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2008, 6, 1218–1224. [Google Scholar] [CrossRef]
- Langhorst, J.; Elsenbruch, S.; Koelzer, J.; Rueffer, A.; Michalsen, A.; Dobos, G.J. Noninvasive Markers in the Assessment of Intestinal Inflammation in Inflammatory Bowel Diseases: Performance of Fecal Lactoferrin, Calprotectin, and PMN-Elastase, CRP, and Clinical Indices. Am. J. Gastroenterol. 2008, 103, 162–169. [Google Scholar] [CrossRef]
- Chen, J.-M.; Liu, T.; Gao, S.; Tong, X.-D.; Deng, F.-H.; Nie, B. Efficacy of noninvasive evaluations in monitoring inflammatory bowel disease activity: A prospective study in China. World J. Gastroenterol. 2017, 23, 8235–8247. [Google Scholar] [CrossRef]
- Mosli, M.H.; Zou, G.; Garg, S.K.; Feagan, S.G.; Macdonald, J.K.; Chande, N.; Sandborn, W.J.; Feagan, B.G. C-Reactive Protein, Fecal Calprotectin, and Stool Lactoferrin for Detection of Endoscopic Activity in Symptomatic Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2015, 110, 802–819. [Google Scholar] [CrossRef]
- Sipponen, T.; Savilahti, E.; Kolho, K.L.; Nuutinen, H.; Turunen, U.; Färkkilä, M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: Correlation with Crohn’s disease activity index and endoscopic findings. Inflamm. Bowel Dis. 2008, 14, 40–46. [Google Scholar] [CrossRef]
- Masoodi, I.; Kochhar, R.; Dutta, U.; Vaishnavi, C.; Prasad, K.K.; Vaiphei, K.; Kaur, S.; Singh, K. Fecal lactoferrin, myeloperoxidase and serum C-reactive are effective biomarkers in the assessment of disease activity and severity in patients with idiopathic ulcerative colitis. J. Gastroenterol. Hepatol. 2009, 24, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Schoepfer, A.M.; Beglinger, C.; Straumann, A.; Trummler, M.; Vavricka, S.R.; E Bruegger, L.; Seibold, F. Fecal Calprotectin Correlates More Closely With the Simple Endoscopic Score for Crohnʼs Disease (SES-CD) than CRP, Blood Leukocytes, and the CDAI. Am. J. Gastroenterol. 2010, 105, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Kurokawa, M.S.; Yoshikawa, H.; Nara, K.; Takada, E.; Masuda, C.; Tsukikawa, S.; Ozaki, S.; Matsuda, T.; Suzuki, N. Involvement of Th1 cells and heat shock protein 60 in the pathogenesis of intestinal Behçet’s disease. Clin. Exp. Immunol. 2005, 139, 371–378. [Google Scholar] [CrossRef]
- Verdejo, C.; Hervías, D.; Roncero, Ó.; Arias, Á.; Bouhmidi, A.; Lorente, R.; Salueña, I.; Lucendo, A.J. Fecal calprotectin is not superior to serum C-reactive protein or the Harvey–Bradshaw index in predicting postoperative endoscopic recurrence in Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Zittan, E.; Kabakchiev, B.; Kelly, O.B.; Milgrom, R.; Nguyen, G.C.; Croitoru, K.; Steinhart, A.H.; Silverberg, M.S. Development of the Harvey-Bradshaw Index-pro (HBI-PRO) Score to Assess Endoscopic Disease Activity in Crohn’s Disease. J. Crohns Colitis 2016, 11, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.W.; Stewart, S.A.; Heisler, C.; Sandborn, W.J.; Loftus, E.V., Jr.; Zello, G.A.; Fowler, S.A.; Jones, J.L. Biomarker-Based Models Outperform Patient-Reported Scores in Predicting Endoscopic Inflammatory Disease Activity. Inflamm. Bowel Dis. 2018, 24, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Zittan, E.; Kelly, O.B.; Gralnek, I.M.; Silverberg, M.S.; Steinhart, A.H. Fecal calprotectin correlates with active colonic inflammatory bowel disease but not with small intestinal Crohn’s disease activity. JGH Open 2018, 2, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Zittan, E.; Kelly, O.B.; Kirsch, R.; Milgrom, R.; Burns, J.; Nguyen, G.C.; Croitoru, K.; Van Assche, G.; Silverberg, M.S.; Steinhart, A.H. Low Fecal Calprotectin Correlates with Histological Remission and Mucosal Healing in Ulcerative Colitis and Colonic Crohnʼs Disease. Inflamm. Bowel Dis. 2016, 22, 623–630. [Google Scholar] [CrossRef]
- Filik, L.; Dagli, U.; Ulker, A. C-reactive protein and monitoring the activity of Crohn’s disease. Adv. Ther. 2006, 23, 655–662. [Google Scholar] [CrossRef]
- Yang, D.-H.; Yang, S.-K.; Park, S.H.; Lee, H.-S.; Boo, S.-J.; Park, J.-H.; Na, S.Y.; Jung, K.W.; Kim, K.-J.; Ye, B.D.; et al. Usefulness of C-Reactive Protein as a Disease Activity Marker in Crohn’s Disease according to the Location of Disease. Gut Liver 2014, 9, 80–86. [Google Scholar] [CrossRef]
- Panés, J.; Bouhnik, Y.; Reinisch, W.; Stoker, J.; A Taylor, S.; Baumgart, D.; Danese, S.; Halligan, S.; Marincek, B.; Matos, C.; et al. Imaging techniques for assessment of inflammatory bowel disease: Joint ECCO and ESGAR evidence-based consensus guidelines. J. Crohns Colitis 2013, 7, 556–585. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, R.; Sanders, D.S.; Morris, A.J.; E McAlindon, M. Guidelines on small bowel enteroscopy and capsule endoscopy in adults. Gut 2007, 57, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Ladas, S.D.; Triantafyllou, K.; Spada, C.; Riccioni, M.E.; Rey, J.-F.; Niv, Y.; Delvaux, M.; De Franchis, R.; Costamagna, G. European Society of Gastrointestinal Endoscopy (ESGE): Recommendations (2009) on clinical use of video capsule endoscopy to investigate small-bowel, esophageal and colonic diseases. Endoscopy 2010, 42, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Nathan, T.; Rafaelsen, S.; Kjeldsen, J. Diagnostic Accuracy of Capsule Endoscopy for Small Bowel Crohn’s Disease Is Superior to That of MR Enterography or CT Enterography. Clin. Gastroenterol. Hepatol. 2011, 9, 124–129.e1. [Google Scholar] [CrossRef] [PubMed]
- Rezapour, M.; Amadi, C.; Gerson, L.B. Retention associated with video capsule endoscopy: Systematic review and meta-analysis. Gastrointest. Endosc. 2017, 85, 1157–1168.e2. [Google Scholar] [CrossRef]
- Gee, M.S.; Harisinghani, M.G. MRI in patients with inflammatory bowel disease. J. Magn. Reson. Imaging 2011, 33, 527–534. [Google Scholar] [CrossRef]
- Qiu, Y.; Mao, R.; Chen, B.-L.; Li, X.-H.; He, Y.; Zeng, Z.-R.; Li, Z.-P.; Chen, M.-H. Systematic review with meta-analysis: Magnetic resonance enterography vs. computed tomography enterography for evaluating disease activity in small bowel Crohn’s disease. Aliment. Pharmacol. Ther. 2014, 40, 134–146. [Google Scholar] [CrossRef]
- Quon, J.S.; Quon, P.R.; Lim, C.S.; Abdeen, N.; Schieda, N. Magnetic resonance enterography in post-operative inflammatory bowel disease. Abdom. Imaging 2015, 40, 1034–1049. [Google Scholar] [CrossRef]
- Desai, D.; Faubion, W.A.; Sandborn, W.J. Review article: Biological activity markers in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2006, 25, 247–255. [Google Scholar] [CrossRef]
- Jensen, M.D.; Kjeldsen, J.; Nathan, T. Fecal calprotectin is equally sensitive in Crohn’s disease affecting the small bowel and colon. Scand. J. Gastroenterol. 2011, 46, 694–700. [Google Scholar] [CrossRef]
- Cerrillo, E.; Beltrán, B.; Pous, S.; Echarri, A.; Gallego, J.C.; Iborra, M. Fecal Calprotectin in Ileal Crohn’s Disease: Relationship with Magnetic Resonance Enterography and a Pathology Score. Inflamm. Bowel Dis. 2015, 21, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Sipponen, T.; Haapamäki, J.; Savilahti, E.; Alfthan, H.; Hämäläinen, E.; Rautiainen, H.; Koskenpato, J.; Nuutinen, H.; Färkkilä, M. Fecal calprotectin and S100A12 have low utility in prediction of small bowel Crohn’s disease detected by wireless capsule endoscopy. Scand. J. Gastroenterol. 2012, 47, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Gecse, K.B.; Brandse, J.F.; Van Wilpe, S.; Lowenberg, M.; Ponsioen, C.; Brink, G.V.D.; D’Haens, G. Impact of disease location on fecal calprotectin levels in Crohn’s disease. Scand. J. Gastroenterol. 2015, 50, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Maréchal, C.; Aimone-Gastin, I.; Baumann, C.; Dirrenberger, B.; Guéant, J.-L.; Peyrin-Biroulet, L. Compliance with the faecal calprotectin test in patients with inflammatory bowel disease. United Eur. Gastroenterol. J. 2017, 5, 702–707. [Google Scholar] [CrossRef]
- De Macario, E.C.; De Macario, E.C. Chaperonopathies and chaperonotherapy. FEBS Lett. 2007, 581, 3681–3688. [Google Scholar] [CrossRef]
- Macario, A.J.L.; Cappello, F.; Zummo, G.; De Macario, E.C. Chaperonopathies of senescence and the scrambling of interactions between the chaperoning and the immune systems. Ann. N. Y. Acad. Sci. 2010, 1197, 85–93. [Google Scholar] [CrossRef]
- Rodolico, V.; Tomasello, G.; Zerilli, M.; Martorana, A.; Pitruzzella, A.; Gammazza, A.M.; David, S.; Zummo, G.; Damiani, P.; Accomando, S.; et al. Hsp60 and Hsp10 increase in colon mucosa of Crohn’s disease and ulcerative colitis. Cell Stress Chaperon 2010, 15, 877–884. [Google Scholar] [CrossRef]
- Vocka, M.; Langer, D.; Fryba, V.; Petrtyl, J.; Hanus, T.; Kalousova, M.; Zima, T.; Petruzelka, L. Novel serum markers HSP60, CHI3L1, and IGFBP-2 in metastatic colorectal cancer. Oncol. Lett. 2019, 18, 6284–6292. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Hanauer, S.B.; Lochs, H.; Löfberg, R.; Modigliani, R.; Present, D.H.; Rutgeerts, P.; Schölmerich, J.; Stange, E.F.; et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology 2002, 122, 512–530. [Google Scholar] [CrossRef]
- Gomes, P.; Du Boulay, C.; Smith, C.L.; Holdstock, G. Relationship between disease activity indices and colonoscopic findings in patients with colonic inflammatory bowel disease. Gut 1986, 27, 92–95. [Google Scholar] [CrossRef]
- Aguilera-Castro, L.; Ferre-Aracil, C.; De Paredes, A.G.G.; Rodriguez-De-Santiago, E.; López-Sanromán, A. Management of complex perianal Crohn’s disease. Ann. Gastroenterol. 2016, 30, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Reiss, M.; Sandborn, W.J. The Role of Psychosocial Care in Adapting to Health Care Reform. Clin. Gastroenterol. Hepatol. 2015, 13, 2219–2224. [Google Scholar] [CrossRef] [PubMed]
- Nigro, G.; Angelini, G.; Grosso, S.B.; Caula, G.; Guidetti, C.S. Psychiatric predictors of noncompliance in inflammatory bowel disease: Psychiatry and compliance. J. Clin. Gastroenterol. 2001, 32, 66–68. [Google Scholar] [CrossRef]
- Van Langenberg, D.R.; Lange, K.; Hetzel, D.; Holtmann, G.J.; Andrews, J.M. Adverse clinical phenotype in inflammatory bowel disease: A cross sectional study identifying factors potentially amenable to change. J. Gastroenterol. Hepatol. 2010, 25, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Mikocka-Walus, A.; Anderegg, C.; Bauerfeind, P.; Beglinger, C.; Begré, S.; Belli, D.; Bengoa, J.M.; Biedermann, L.; Bigler, B.; Binek, J.; et al. Symptoms of Depression and Anxiety Are Independently Associated With Clinical Recurrence of Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 829–835.e1. [Google Scholar] [CrossRef] [PubMed]
- Yanartas, O.; Kani, H.T.; Bicakci, E.; Kilic, I.; Banzragch, M.; Acikel, C.; Atug, O.; Kuşçu, K.; Imeryuz, N.; Akin, H. The effects of psychiatric treatment on depression, anxiety, quality of life, and sexual dysfunction in patients with inflammatory bowel disease. Neuropsychiatr. Dis. Treat. 2016, 12, 673–683. [Google Scholar] [CrossRef]
- Mikocka-Walus, A.; Bampton, P.A.; Hetzel, D.; A Hughes, P.; Esterman, A.; Andrews, J.M. Cognitive-behavioural therapy has no effect on disease activity but improves quality of life in subgroups of patients with inflammatory bowel disease: A pilot randomised controlled trial. BMC Gastroenterol. 2015, 15, 54. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Iheozor-Ejiofor, Z.; Gjuladin-Hellon, T.; Parian, A.; E Matarese, L.; Bracewell, K.; Macdonald, J.K.; Gordon, M.; Mullin, G.E. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef]
- Levine, A.; Wine, E.; Assa, A.; Boneh, R.S.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8. [Google Scholar] [CrossRef]
- Boneh, R.S.; Chermesh, I.; Ben-Avraham, S.; Boaz, M.; Levine, A.; Shabat, C.S.; Yanai, H. Dietary Therapy With the Crohn’s Disease Exclusion Diet is a Successful Strategy for Induction of Remission in Children and Adults Failing Biological Therapy. J. Crohns Colitis 2017, 11, 1205–1212. [Google Scholar] [CrossRef]
- Sigall-Boneh, R.; Pfeffer-Gik, T.; Segal, I.; Zangen, T.; Boaz, M.; Levine, A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Haskey, N.; Gibson, D.L. An Examination of Diet for the Maintenance of Remission in Inflammatory Bowel Disease. Nutrients 2017, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Packey, C.D.; Sartor, R.B. Interplay of commensal and pathogenic bacteria, genetic mutations, and immunoregulatory defects in the pathogenesis of inflammatory bowel diseases. J. Intern. Med. 2008, 263, 597–606. [Google Scholar] [CrossRef]
- Sartor, R.B. Microbial Influences in Inflammatory Bowel Diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, V.D.; Romeo, M.; Gammazza, A.M.; Carini, F.; Damiani, P.; Damiano, G.; Buscemi, S.; Monte, A.I.L.; Gerges-Geagea, A.; Jurjus, A.; et al. The long-term effects of probiotics in the therapy of ulcerative colitis: A clinical study. Biomed. Pap. 2016, 160, 372–377. [Google Scholar] [CrossRef]
- Chapman, T.M.; Plosker, G.L.; Figgitt, D.P. VSL#3 probiotic mixture: A review of its use in chronic inflammatory bowel diseases. Drugs 2006, 66, 1371–1387. [Google Scholar]
- Gionchetti, P.; Rizzello, F.; Venturi, A.; Brigidi, P.; Matteuzzi, D.; Bazzocchi, G.; Poggioli, G.; Miglioli, M.; Campieri, M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: A double-blind, placebo-controlled trial. Gastroenterology 2000, 119, 305–309. [Google Scholar] [CrossRef]
- Seksik, P.; Dray, X.; Sokol, H.; Marteau, P. Is there any place for alimentary probiotics, prebiotics or synbiotics, for patients with inflammatory bowel disease? Mol. Nutr. Food Res. 2008, 52, 906–912. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D. Risk and Safety of Probiotics. Clin. Infect. Dis. 2015, 60, S129–S134. [Google Scholar] [CrossRef]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohns Colitis 2016, 11, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J. Crohn’s Disease Evaluation and Treatment: Clinical Decision Tool. Gastroenterology 2014, 147, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.M.; Gaywood, I.; Scott, B.B. Guidelines for osteoporosis in coeliac disease and inflammatory bowel disease. Gut 2000, 46, I1–I8. [Google Scholar] [CrossRef]
- Compston, J. Osteoporosis in inflammatory bowel disease. Gut 2003, 52, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Bemelman, W.A.; Allez, M. The surgical intervention: Earlier or never? Best Pr. Res. Clin. Gastroenterol. 2014, 28, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Ponsioen, C.Y.; De Groof, E.J.; Eshuis, E.J.; Gardenbroek, T.J.; Bossuyt, P.M.M.; Hart, A.; Warusavitarne, J.; Buskens, C.; Van Bodegraven, A.A.; Brink, M.A.; et al. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn’s disease: A randomised controlled, open-label, multicentre trial. Lancet Gastroenterol. Hepatol. 2017, 2, 785–792. [Google Scholar] [CrossRef]
- Gionchetti, P.; Dignass, A.; Danese, S.; Dias, F.J.M.; Rogler, G.; Lakatos, P.L.; Adamina, M.; Ardizzone, S.; Buskens, C.; Sebastian, S.; et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 2: Surgical Management and Special Situations. J. Crohns Colitis 2016, 11, 135–149. [Google Scholar] [CrossRef]
- Campbell, L.; Ambe, R.; Weaver, J.; Marcus, S.M.; Cagir, B. Comparison of Conventional and Nonconventional Strictureplasties in Crohn’s Disease. Dis. Colon Rectum 2012, 55, 714–726. [Google Scholar] [CrossRef]
- Guzmán, A.R.; Wehkamp, J.; Kirschniak, A.; Naumann, A.; Malek, N.P.; Goetz, M. Endoscopic balloon dilatation of Crohn’s-associated intestinal strictures: High patient satisfaction and long-term efficacy. United Eur. Gastroenterol. J. 2016, 4, 794–799. [Google Scholar] [CrossRef]
- Navaneethan, U.; Lourdusamy, V.; Njei, B.; Shen, B. Endoscopic balloon dilation in the management of strictures in Crohn’s disease: A systematic review and meta-analysis of non-randomized trials. Surg. Endosc. 2016, 30, 5434–5443. [Google Scholar] [CrossRef]
- Cosnes, J.; Nion-Larmurier, I.; Beaugerie, L.; Afchain, P.; Tiret, E.; Gendre, J.-P. Impact of the increasing use of immunosuppressants in Crohn’s disease on the need for intestinal surgery. Gut 2005, 54, 237–241. [Google Scholar] [CrossRef]
- Rieder, F.; Zimmermann, E.M.; Remzi, F.H.; Sandborn, W.J. Crohn’s disease complicated by strictures: A systematic review. Gut 2013, 62, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Bettenworth, D.; Nowacki, T.M.; Cordes, F.; Buerke, B.; Lenze, F. Assessment of stricturing Crohn’s disease: Current clinical practice and future avenues. World J. Gastroenterol. 2016, 22, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.; Perrey, A.; Lambert, C.; Pereira, B.; Goutte, M.; Dubois, A.; Goutorbe, F.; Dapoigny, M.; Bommelaer, G.; Hordonneau, C.; et al. Medical Therapies for Stricturing Crohn’s Disease: Efficacy and Cross-Sectional Imaging Predictors of Therapeutic Failure. Dig. Dis. Sci. 2017, 105, 289–1636. [Google Scholar] [CrossRef]
- Ding, N.S.; Yip, W.M.; Thomas-Gibson, S.; Humphries, A.; Hart, A.; Choi, C.H.; Saunders, B.; Arebi, N. Endoscopic Dilatation of Crohn’s Anastomotic Strictures is Effective in the Long Term, and Escalation of Medical Therapy Improves Outcomes in the Biologic Era. J. Crohns Colitis 2016, 10, 1172–1178. [Google Scholar] [CrossRef]
- Holtmann, M.H.; Neurath, M.F. Anti-TNF strategies in stenosing and fistulizing Crohn’s disease. Int. J. Color. Dis. 2004, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zittan, E.; Gralnek, I.M.; Hatoum, O.A.; Sakran, N.; Kolonimos, N. Preoperative Exclusive Total Parental Nutrition is Associated with Clinical and Laboratory Remission in Severe Active Crohn’s Disease—A Pilot Study. Nutrients 2020, 12, 1244. [Google Scholar] [CrossRef]
- Bloomgren, G.; Richman, S.; Hotermans, C.; Subramanyam, M.; Goelz, S.; Natarajan, A. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N. Engl. J. Med. 2012, 366, 1870–1880. [Google Scholar] [CrossRef]
- Nelson, S.M.; Nguyen, T.M.; McDonald, J.W.; Macdonald, J.K. Natalizumab for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2018, 8, CD006097. [Google Scholar] [CrossRef]
- Lega, S.; Phan, B.L.; Rosenthal, C.; Gordon, J.; Haddad, N.; Pittman, N.; Benkov, K.J.; Dubinsky, M. Proactively Optimized Infliximab Monotherapy Is as Effective as Combination Therapy in IBD. Inflamm. Bowel Dis. 2018, 25, 134–141. [Google Scholar] [CrossRef]
- Restellini, S.; Chao, C.-Y.; Lakatos, P.L.; Aruljothy, A.; Aziz, H.; Kherad, O.; Bitton, A.; Wild, G.; Afif, W.; Bessissow, T. Therapeutic Drug Monitoring Guides the Management of Crohn’s Patients with Secondary Loss of Response to Adalimumab. Inflamm. Bowel Dis. 2018, 24, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H.; Motoya, S.; Matsumoto, T.; Watanabe, K.; Hisamatsu, T.; Yoshimura, N.; Ishida, T.; Kato, S.; Nakagawa, T.; Esaki, M.; et al. Significance of measurement of serum trough level and anti-drug antibody of adalimumab as personalised pharmacokinetics in patients with Crohn’s disease: A subanalysis of the DIAMOND trial. Aliment. Pharmacol. Ther. 2017, 46, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Belaiche, J.; Desager, J.P.; Horsmans, Y.; Louis, E. Therapeutic drug monitoring of azathioprine and 6-mercaptopurine metabolites in Crohn disease. Scand. J. Gastroenterol. 2001, 36, 71–76. [Google Scholar] [CrossRef]
- Gilissen, L.P.L.; Wong, D.R.; Engels, L.G.J.B.; Bierau, J.; Bakker, J.A.; Paulussen, A.D.C.; Romberg-Camps, M.J.; Stronkhorst, A.; Bus, P.; Bos, L.P.; et al. Therapeutic drug monitoring of thiopurine metabolites in adult thiopurine tolerant IBD patients on maintenance therapy. J. Crohns Colitis 2012, 6, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Abreu, M.T.; Cohen, R.; Tremaine, W. American Gastroenterological Association Institute Medical Position Statement on Corticosteroids, Immunomodulators, and Infliximab in Inflammatory Bowel Disease. Gastroenterology 2006, 130, 935–939. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Nunes, P.B.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohns Colitis 2018, 13, 144K–164K. [Google Scholar] [CrossRef]
- Zittan, E.; Kelly, O.; Kabakchiev, B.; Nguyen, G.C.; Croitoru, K.; Steinhart, A.H.; Silverberg, M. Sa1968 Post-Induction Adalimumab Drug Levels Predict Clinical and Laboratory Remission at Week 24 in Patients With Crohn’s Disease. Gastroenterology 2016, 150, S419. [Google Scholar] [CrossRef]
- Graham, M.F.; Willey, A.; Adams, J.; Diegelmann, R.F. Corticosteroids increase procollagen gene expression, synthesis, and secretion by human intestinal smooth muscle cells. Gastroenterology 1995, 109, 1454–1461. [Google Scholar] [CrossRef]
- Louis, E.; Boverie, J.; Dewit, O.; Baert, F.; De Vos, M.; D’Haens, G. Treatment of small bowel subocclusive Crohn’s disease with infliximab: An open pilot study. Acta Gastro-Enterol. Belg. 2007, 70, 15–19. [Google Scholar]
- Vasilopoulos, S.; Kugathasan, S.; Saeian, K.; Emmons, J.E.; Hogan, W.J.; Otterson, M.F. Intestinal strictures complicating initially successful infliximab treatment for luminal Crohn’s disease. Am. J. Gastroenterol. 2000, 95, 2503. [Google Scholar] [CrossRef]
- Toy, L.S.; Scherl, E.J.; Kornbluth, A.; Marion, J.F.; Greenstein, A.J.; Agus, S.; Gerson, C.; Fox, N.; Present, D.H. Complete bowel obstruction following initial response to infliximab therapy for crohn’s disease: A series of a newly described complication. Gastroenterology 2000, 118, A569. [Google Scholar] [CrossRef]
- Pallotta, N.; Barberani, F.; Hassan, N.A.; Guagnozzi, D.; Vincoli, G.; Corazziari, E. Effect of infliximab on small bowel stenoses in patients with Crohn’s disease. World J. Gastroenterol. 2008, 14, 1885–1890. [Google Scholar] [CrossRef] [PubMed]
- Bouhnik, Y.; Carbonnel, F.; Laharie, D.; Stefanescu, C.; Hébuterne, X.; Abitbol, V. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: A multicentre, prospective, observational cohort (CREOLE) study. Gut 2018, 67, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, A.-L.; Kalisazan, B.; Wienckiewicz, J.; Bouarioua, N.; Soule, J.-C. Infliximab treatment for symptomatic Crohn’s disease strictures. Aliment. Pharmacol. Ther. 2009, 29, 279–285. [Google Scholar] [CrossRef]
- Collins, F.S.; Varmus, H. A New Initiative on Precision Medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef]

| Study | Country | Design | Cohort | Total Patients | Treat-to-Target Protocol | Primary Endpoint | Results |
|---|---|---|---|---|---|---|---|
| CALM | Multiple | prospective RCT | Adults with active endoscopic CD | 244 | Clinically based (standard care) vs. tight control (proactive care) ADA dosing based on FC, CRP, and CDAI | Mucosal healing (CDEIS < 4) without deep ulcers at 48 weeks | 46% proactive care vs. 30% standard care |
| TAXIT | Belgium | prospective RCT | Adults with moderate-to-severe CD or UC | 263 (CD = 178, UC = 85) | Clinically based (standard care) vs. concentration-based IFX (proactive care) dosing | Clinical remission at 1 year | 62.6% proactive care vs. 54.9% standard care (CD) |
| POCER | Australia & New Zealand | prospective RCT | Adults with CD undergoing intestinal resection | 184 | Clinically based (standard care) vs. endoscopic disease monitoring (proactive care) | Post-surgical endoscopic remission at 18 months | 51% proactive care vs. 33% standard care |
| Assessment | Cutoff Value | Sensitivity | Specificity | References |
|---|---|---|---|---|
| Questionnaires | ||||
| CDAI | ≥150 | 24–38% | 72–100% | [9,37,40,43] |
| HBI | >4 | 57% | 76% | [40,44,45] |
| PRO | 61% | 55% | [45,46] | |
| PRO+ | 63% | 88% | [45,46] | |
| Biomarkers | ||||
| FC | >100 μg/g | 67–100% | 57–64% | [35,36,37,38,39,40,42,44,47,48] |
| FC | >200 μg/g | 47–86% | 78–93% | [35,36,37,38,39,40,42,44,47,48] |
| FC | Pooled | 88% | 73% | [35,36,37,38,39,40,42,44,47,48] |
| CRP | ≥5 mg/L | 24–70% | 64–100% | [36,37,38,39,41,42,44,49,50] |
| CRP | Pooled | 49% | 92% | [37,39,41,44,49,50] |
| Lactoferrin | >0.725 | 85% | 77% | [37,39,40,41] |
| Lactoferrin | Pooled | 82% | 79% | [37,39,40,41] |
| Imaging | ||||
| VCE | 100% | 91% | [51,52,53,54,55] | |
| MRE | 81% | 86% | [51,54,56,57,58] | |
| CTE | 76% | 85% | [51,54,57] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zittan, E.; Gralnek, I.M.; Berns, M.S. The New Proactive Approach and Precision Medicine in Crohn’s Disease. Biomedicines 2020, 8, 193. https://doi.org/10.3390/biomedicines8070193
Zittan E, Gralnek IM, Berns MS. The New Proactive Approach and Precision Medicine in Crohn’s Disease. Biomedicines. 2020; 8(7):193. https://doi.org/10.3390/biomedicines8070193
Chicago/Turabian StyleZittan, Eran, Ian M. Gralnek, and Marc S. Berns. 2020. "The New Proactive Approach and Precision Medicine in Crohn’s Disease" Biomedicines 8, no. 7: 193. https://doi.org/10.3390/biomedicines8070193
APA StyleZittan, E., Gralnek, I. M., & Berns, M. S. (2020). The New Proactive Approach and Precision Medicine in Crohn’s Disease. Biomedicines, 8(7), 193. https://doi.org/10.3390/biomedicines8070193






