Regulatory Effects of Skate Skin-Derived Collagen Peptides with Different Molecular Weights on Lipid Metabolism in the Liver and Adipose Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Collagen Hydrolysate Sample
2.2. Animal Study
2.3. Plasma Lipid, Aminotransferase, and Adipokine Levels
2.4. Hepatic Lipid Concentration
2.5. Histological Analysis
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
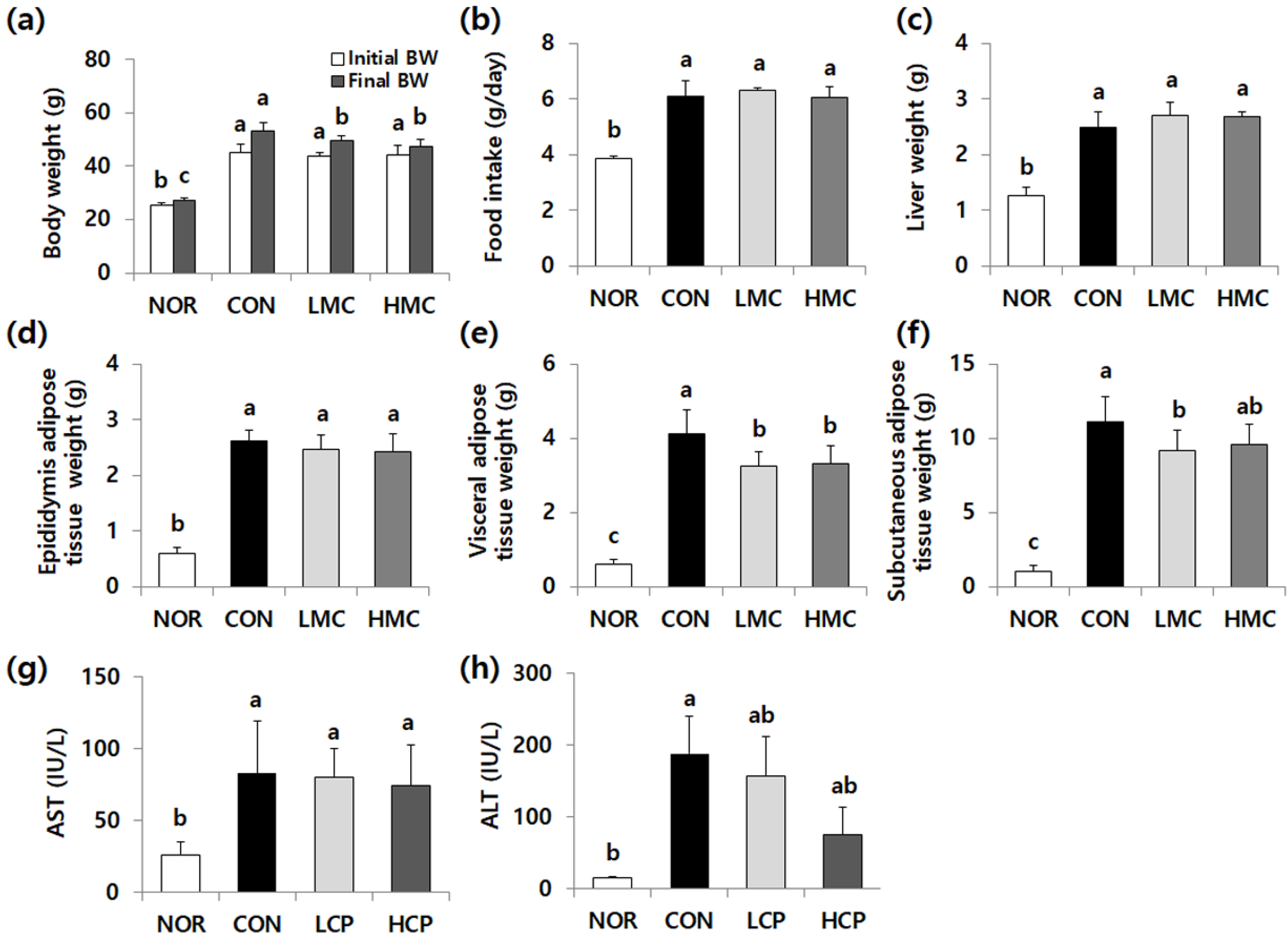
3.1. Body Weight Gain, Organ Weight, Food Intake, and Aminotransferase Activity
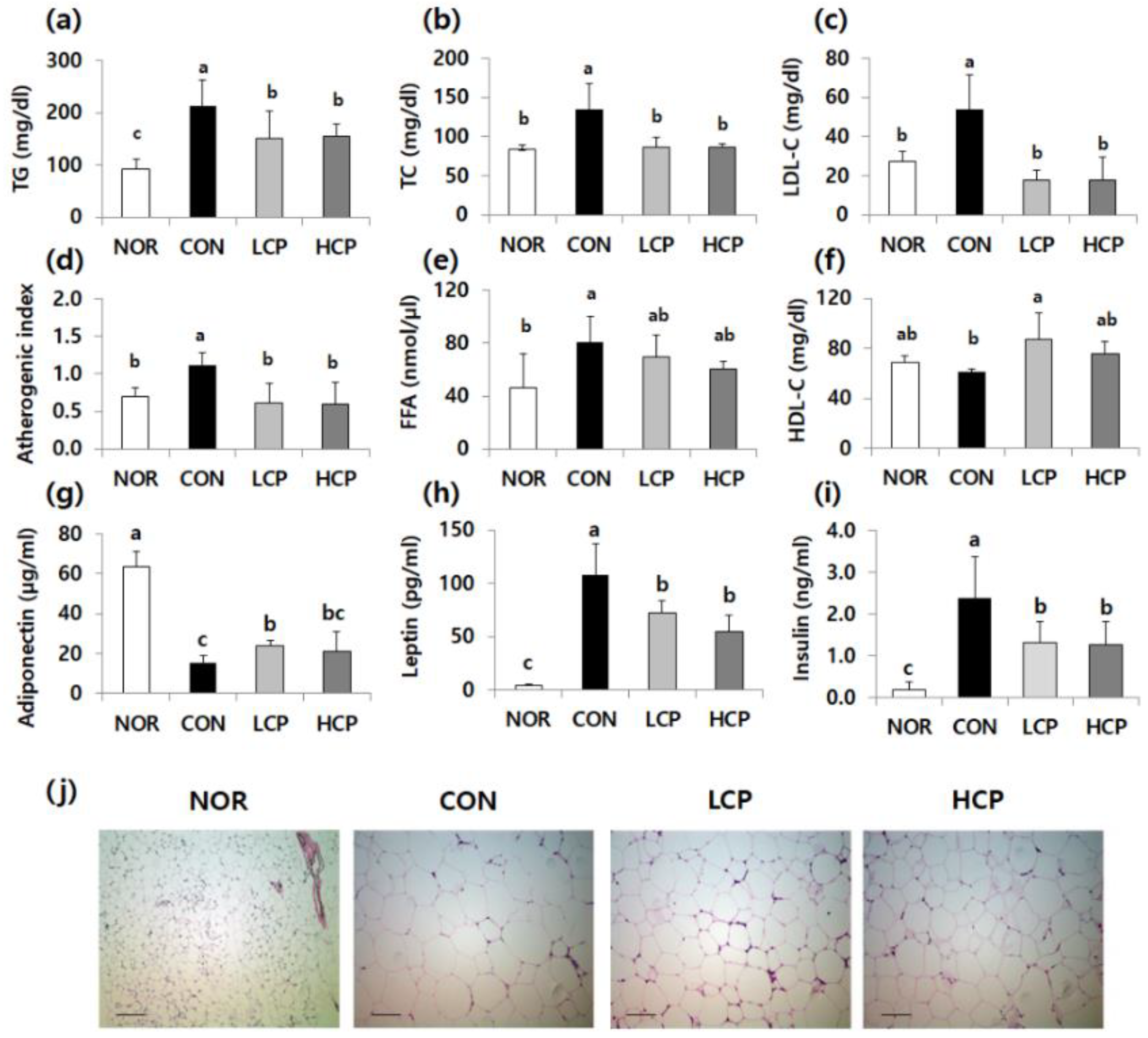
3.2. Effects of Skate Skin Collagen Peptides on Plasma Lipid, Adiponectin, and Leptin Levels, and Lipid Droplets in Adipose Tissue
3.3. Effects of Skate Skin Collagen Peptides on Hepatic Lipid Levels and the Degree of Lipid Accumulation in Liver Tissue
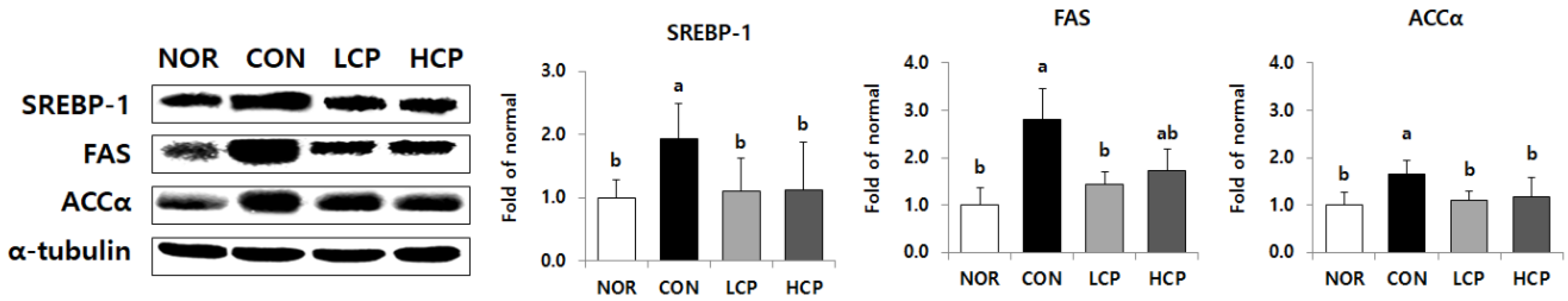
3.4. Protein Expression of Fatty Acid Synthesis in the Liver
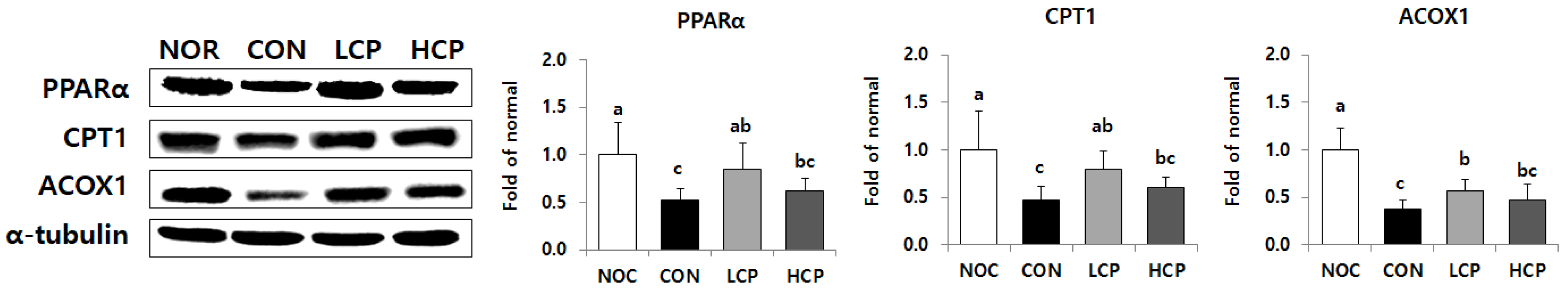
3.5. Protein Expression of Beta-Oxidation in the Liver
3.6. Protein Expression of Cholesterol Metabolism in the Liver
3.7. Protein Expression of Lipogenesis-Related Molecules in Adipose Tissue
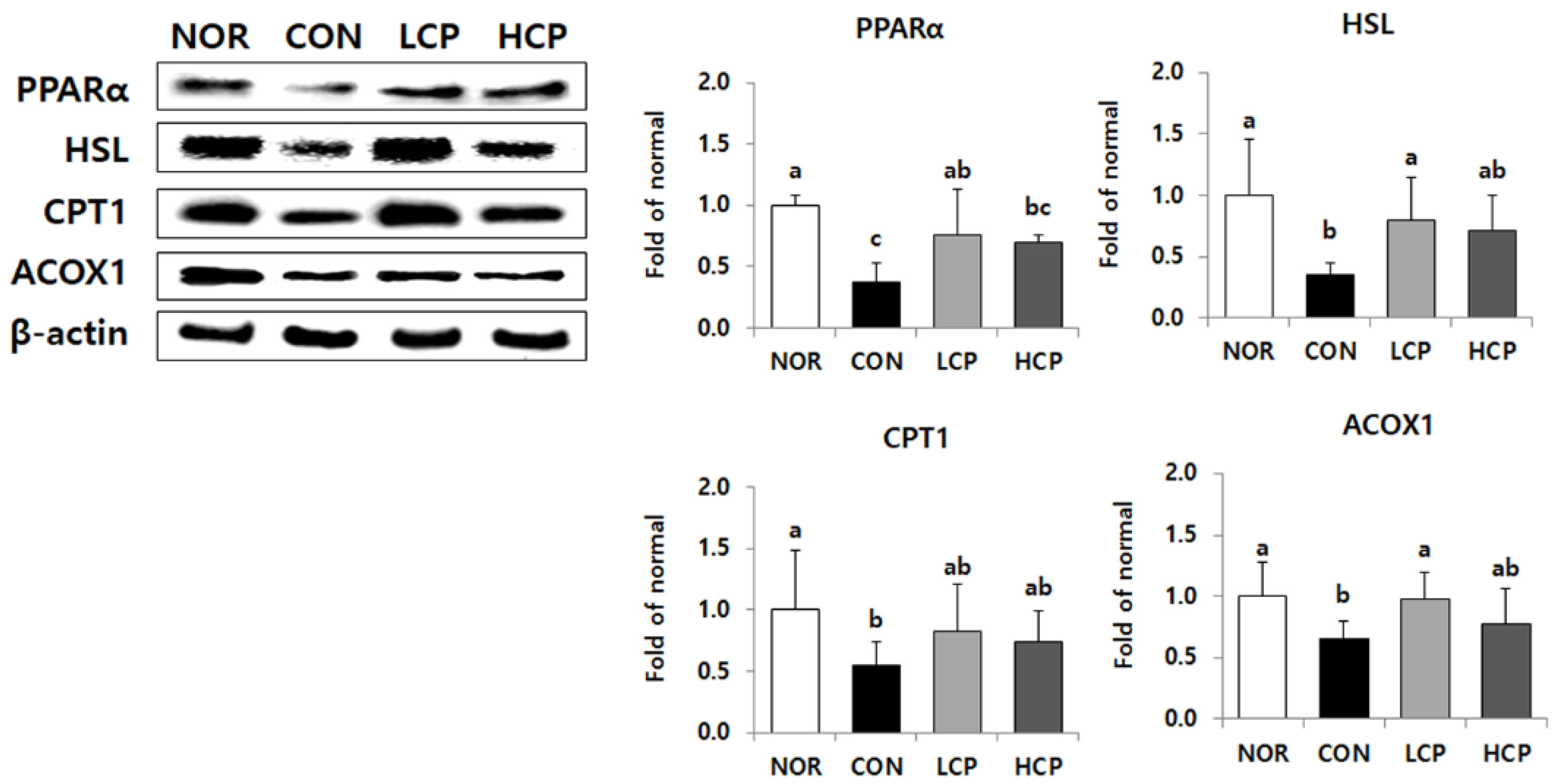
3.8. Protein Expression of Lipolysis-Related Molecules in Adipose Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MW | Molecular weight |
| SREBP | Sterol regulatory element binding protein |
| FAS | Fatty acid synthase |
| ACC | Acetyl-CoA carboxylase |
| HMGCR | 3-hydroxy-3-methylglutaryl-CoA reductase |
| AP2 | Adipocyte protein 2 |
| PPARα | Peroxisome proliferator activated receptor α |
| CPT1 | Carnitine palmitoyltransferase 1A |
| ACOX1 | Acyl-CoA oxidase 1 |
| HSL | Hormone-sensitive lipase |
| TG | Triglyceride |
| HFD | High fat diet |
| LCP | Low MW collagen peptide |
| HCP | High MW collagen peptide |
| AST | Aspartic acid transaminase |
| CON | Control |
| ALT | Alanine transaminase |
| TC | Total cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| FFA | Free fatty acid |
| HDL-C | High-density lipoprotein cholesterol |
| NOR | Normal |
| CYP7A1 | Microsomal cytochrome P450 family 7 subfamily A member 1 |
| PBS | Phosphate-buffered saline |
| ANOVA | One-way analysis of variance |
References
- Blanco, M.; Vázquez, J.; Pérez-Martín, R.; Sotelo, C. Hydrolysates of fish skin collagen: An opportunity for valorizing fish industry byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Y.; Pei, X.; Yang, R.; Zhang, Z.; Li, Y. The lipid-lowering and antioxidative effects of marine collagen peptides. Chin. J. Prev. Med. 2008, 42, 226–230. [Google Scholar]
- Pan, X.; Zhao, Y.-Q.; Hu, F.-Y.; Wang, B. Preparation and identification of antioxidant peptides from protein hydrolysate of skate (Raja porosa) cartilage. J. Funct. Foods 2016, 25, 220–230. [Google Scholar] [CrossRef]
- Zhu, C.-F.; Li, G.-Z.; Peng, H.-B.; Zhang, F.; Chen, Y.; Li, Y. Treatment with marine collagen peptides modulates glucose and lipid metabolism in Chinese patients with type 2 diabetes mellitus. Appl. Physiol. Nutr. Metab. 2010, 35, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Yoon, H.-D.; Seong, J.-H.; Lee, Y.-G.; Xie, C.-L.; Kim, S.-H.; Choi, W.-S. Effects of soluble collagen peptides extract derived from Mugil cephalus scale on the blood glucose and lipid metabolism in diabetic rats. J. Life Sci. 2009, 19, 1794–1801. [Google Scholar]
- Pei, X.; Yang, R.; Zhang, Z.; Gao, L.; Wang, J.; Xu, Y.; Zhao, M.; Han, X.; Liu, Z.; Li, Y. Marine collagen peptide isolated from Chum Salmon (Oncorhynchus keta) skin facilitates learning and memory in aged C57BL/6J mice. Food Chem. 2010, 118, 333–340. [Google Scholar] [CrossRef]
- Liu, J.; Si, S.; Qin, Y.; Zhang, B.; Song, S.; Guo, Y. The effect of different molecular weight collagen peptides on MC3T3-E1 cells differentiation. Bio-Med. Mater. Eng. 2015, 26, S2041–S2047. [Google Scholar] [CrossRef]
- Erdmann, K.; Cheung, B.W.; Schröder, H. The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. J. Nutr. Biochem. 2008, 19, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Jeevithan, E.; Jingyi, Z.; Bao, B.; Shujun, W.; JeyaShakila, R.; Wu, W.H. Biocompatibility assessment of type-II collagen and its polypeptide for tissue engineering: Effect of collagen’s molecular weight and glycoprotein content on tumor necrosis factor (Fas/Apo-1) receptor activation in human acute T-lymphocyte leukemia cell line. RSC Adv. 2016, 6, 14236–14246. [Google Scholar]
- Chen, J.; Ma, X.; Yang, Y.; Dai, Z.; Wu, Z.; Wu, G. Glycine enhances expression of adiponectin and IL-10 in 3T3-L1 adipocytes without affecting adipogenesis and lipolysis. Amino Acids 2018, 50, 629–640. [Google Scholar] [CrossRef]
- Abranches, M.V.; de Oliveira, F.C.E.; da Conceição, L.L.; Peluzio, M.d.C.G. Obesity and diabetes: The link between adipose tissue dysfunction and glucose homeostasis. Nutr. Res. Rev. 2015, 28, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D. AMPK: A key regulator of energy balance in the single cell and the whole organism. Int. J. Obes. 2008, 32, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Bruckbauer, A.; Li, F.; Cao, Q.; Cui, X.; Wu, R.; Shi, H.; Zemel, M.B.; Xue, B. Interaction between metformin and leucine in reducing hyperlipidemia and hepatic lipid accumulation in diet-induced obese mice. Metabolism 2015, 64, 1426–1434. [Google Scholar] [CrossRef]
- Eberlé, D.; Hegarty, B.; Bossard, P.; Ferré, P.; Foufelle, F. SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef]
- Jump, D.B. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr. Opin. Lipidol. 2008, 19, 242. [Google Scholar] [CrossRef]
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101. [Google Scholar] [CrossRef]
- Zechner, R.; Zimmermann, R.; Eichmann, T.O.; Kohlwein, S.D.; Haemmerle, G.; Lass, A.; Madeo, F. FAT SIGNALS-lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012, 15, 279–291. [Google Scholar] [CrossRef]
- Shon, J.; Eo, J.-H.; Hwang, S.J.; Eun, J.-B. Effect of processing conditions on functional properties of collagen powder from skate (Raja kenojei) skins. Food Sci. Biotechnol. 2011, 20, 99–106. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Mizuta, S.; Yokoyama, Y.; Yoshinaka, R. Purification and characterization of molecular species of collagen in the skin of skate (Raja kenojei). Food Chem. 2007, 100, 921–925. [Google Scholar] [CrossRef]
- Woo, M.; Song, Y.; Kang, K.-H.; Noh, J. Anti-Obesity Effects of Collagen Peptide Derived from Skate (Raja kenojei) Skin Through Regulation of Lipid Metabolism. Mar. Drugs 2018, 16, 306. [Google Scholar] [CrossRef]
- Lee, H.; Woo, M.; Song, Y.; Noh, J. Inhibitory effect of skate skin collagen on hepatic lipid accumulation through regulation of lipid metabolism. J. Korean Soc. Food Sci. Nutr. 2018, 47, 235–242. [Google Scholar] [CrossRef]
- Baek, J.-M.; Kang, K.-H.; Kim, S.-H.; Noh, J.-S.; Jeong, K.-S. Development of high functional collagen peptide materials using skate skins. J. Environ. Sci. Int. 2016, 25, 579–588. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Park, C.H.; Xu, F.H.; Roh, S.-S.; Song, Y.O.; Uebaba, K.; Noh, J.S.; Yokozawa, T. Astaxanthin and Corni Fructus protect against diabetes-induced oxidative stress, inflammation, and advanced glycation end product in livers of streptozotocin-induced diabetic rats. J. Med. Food 2015, 18, 337–344. [Google Scholar] [CrossRef]
- Lee, E.J.; Hur, J.; Ham, S.A.; Jo, Y.; Lee, S.; Choi, M.-J.; Seo, H.G. Fish collagen peptide inhibits the adipogenic differentiation of preadipocytes and ameliorates obesity in high fat diet-fed mice. Int. J. Biol. Macromol. 2017, 104, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Berillis, P. Marine collagen: Extraction and applications. In Research Trends in Biochemistry, Molecular Biology and Microbiology, Madhukar, S., Ed.; SM Group: Dover, DE, USA, 2015; pp. 1–13. [Google Scholar]
- Ranathunga, S.; Rajapakse, N.; Kim, S.-K. Purification and characterization of antioxidative peptide derived from muscle of conger eel (Conger myriaster). Eur. Food Res. Technol. 2006, 222, 310–315. [Google Scholar] [CrossRef]
- Deeslie, W.D.; Cheryan, M. Fractionation of soy protein hydrolysates using ultrafiltration membranes. J. Food Sci. 1992, 57, 411–413. [Google Scholar] [CrossRef]
- Astre, G.; Deleruyelle, S.; Dortignac, A.; Bonnet, C.; Valet, P.; Dray, C. Diet-induced obesity and associated disorders are prevented by natural bioactive type 1 fish collagen peptides (Naticol®) treatment. J. Physiol. Biochem. 2018, 74, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.F.; Wauters, M.A.; De Leeuw, I.H. The beneficial effects of modest weight loss on cardiovascular risk factors. Int. J. Obes. Relat. Metab. Disord. 1997, 21, S5–S9. [Google Scholar]
- Saito, M.; Kiyose, C.; Higuchi, T.; Uchida, N.; Suzuki, H. Effect of collagen hydrolysates from salmon and trout skins on the lipid profile in rats. J. Agric. Food Chem. 2009, 57, 10477–10482. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, W.; Mu, B.; Zhang, F.; Lai, N.; Zhou, J.; Xu, A.; Liu, J.; Li, Y. Effects of marine collagen peptides on glucose metabolism and insulin resistance in type 2 diabetic rats. J. Food Sci. Technol. 2017, 54, 2260–2269. [Google Scholar] [CrossRef] [PubMed]
- Tak, Y.J.; Kim, Y.J.; Lee, J.G.; Yi, Y.H.; Cho, Y.H.; Kang, G.H.; Lee, S.Y. Effect of Oral Ingestion of Low-Molecular Collagen Peptides Derived from Skate (Raja Kenojei) Skin on Body Fat in Overweight Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Mar. Drugs 2019, 17, 157. [Google Scholar] [CrossRef]
- Mizuta, S.; Hwang, J.-H.; Yoshinaka, R. Molecular species of collagen in pectoral fin cartilage of skate (Raja kenojei). Food Chem. 2003, 80, 1–7. [Google Scholar] [CrossRef]
- Wijekoon, E.P.; Skinner, C.; Brosnan, M.E.; Brosnan, J.T. Amino acid metabolism in the Zucker diabetic fatty rat: Effects of insulin resistance and of type 2 diabetes. Can. J. Physiol. Pharmacol. 2004, 82, 506–514. [Google Scholar] [CrossRef]
- Takashina, C.; Tsujino, I.; Watanabe, T.; Sakaue, S.; Ikeda, D.; Yamada, A.; Sato, T.; Ohira, H.; Otsuka, Y.; Oyama-Manabe, N. Associations among the plasma amino acid profile, obesity, and glucose metabolism in Japanese adults with normal glucose tolerance. Nutr. Metab. 2016, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.T.; Mighiu, P.I.; Naples, M.; Adeli, K.; Lam, T.K. Glycine normalizes hepatic triglyceride-rich VLDL secretion by triggering the CNS in high-fat fed rats. Circ. Res. 2012, 110, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Hafidi, M.E.; Pérez, I.; Zamora, J.; Soto, V.; Carvajal-Sandoval, G.; Banos, G. Glycine intake decreases plasma free fatty acids, adipose cell size, and blood pressure in sucrose-fed rats. Am. J. Physiol. 2004, 287, R1387–R1393. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Chi, C.; Gong, Y.; Luo, H.; Ding, G. Influence of average molecular weight on antioxidant and functional properties of cartilage collagen hydrolysates from Sphyrna lewini, Dasyatis akjei and Raja porosa. Food Res. Int. 2013, 51, 283–293. [Google Scholar] [CrossRef]
- Jeon, Y.-J.; Byun, H.-G.; Kim, S.-K. Improvement of functional properties of cod frame protein hydrolysates using ultrafiltration membranes. Process Biochem. 1999, 35, 471–478. [Google Scholar] [CrossRef]
- Cudennec, B.; Ravallec-Plé, R.; Courois, E.; Fouchereau-Peron, M. Peptides from fish and crustacean by-products hydrolysates stimulate cholecystokinin release in STC-1 cells. Food Chem. 2008, 111, 970–975. [Google Scholar] [CrossRef]
- Roberts, P.R.; Burney, J.; Black, K.W.; Zaloga, G.P. Effect of chain length on absorption of biologically active peptides from the gastrointestinal tract. Digestion 1999, 60, 332–337. [Google Scholar] [CrossRef]
- Gbogouri, G.; Linder, M.; Fanni, J.; Parmentier, M. Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. J. Food Sci. 2004, 69, C615–C622. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, M.; Noh, J.S. Regulatory Effects of Skate Skin-Derived Collagen Peptides with Different Molecular Weights on Lipid Metabolism in the Liver and Adipose Tissue. Biomedicines 2020, 8, 187. https://doi.org/10.3390/biomedicines8070187
Woo M, Noh JS. Regulatory Effects of Skate Skin-Derived Collagen Peptides with Different Molecular Weights on Lipid Metabolism in the Liver and Adipose Tissue. Biomedicines. 2020; 8(7):187. https://doi.org/10.3390/biomedicines8070187
Chicago/Turabian StyleWoo, Minji, and Jeong Sook Noh. 2020. "Regulatory Effects of Skate Skin-Derived Collagen Peptides with Different Molecular Weights on Lipid Metabolism in the Liver and Adipose Tissue" Biomedicines 8, no. 7: 187. https://doi.org/10.3390/biomedicines8070187
APA StyleWoo, M., & Noh, J. S. (2020). Regulatory Effects of Skate Skin-Derived Collagen Peptides with Different Molecular Weights on Lipid Metabolism in the Liver and Adipose Tissue. Biomedicines, 8(7), 187. https://doi.org/10.3390/biomedicines8070187




