Mesenchymal Stem Cells: A Trump Card for the Treatment of Diabetes?
Abstract
1. Introduction
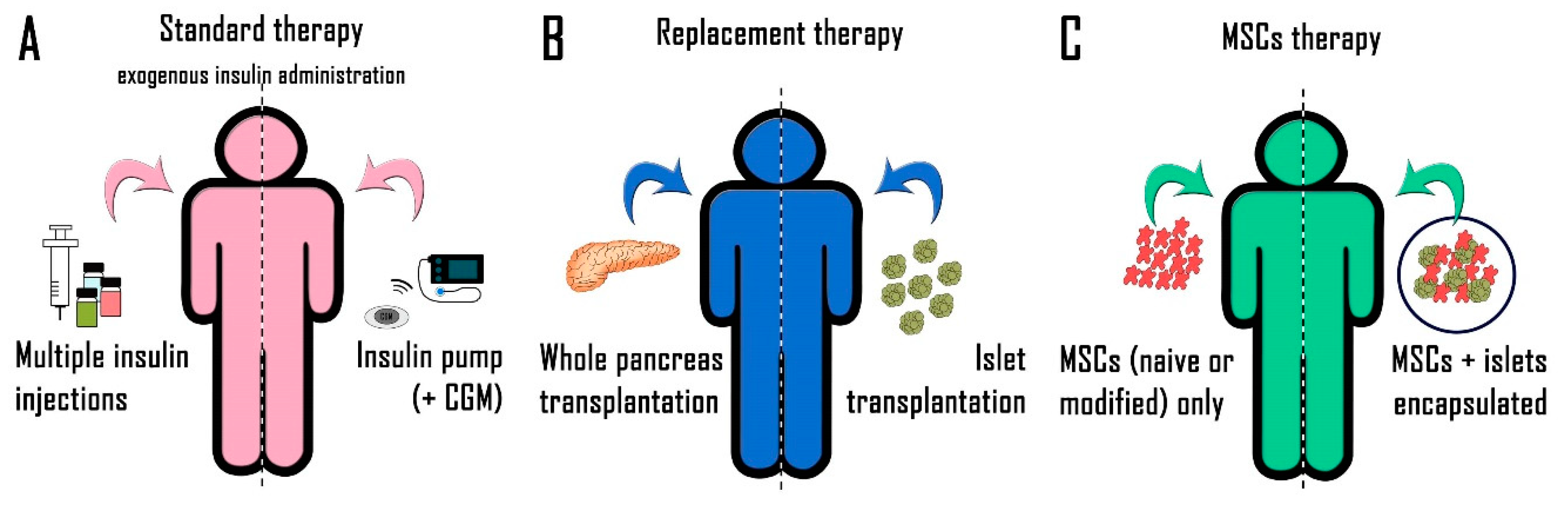
2. Diabetes Treatment
2.1. Exogenous Insulin Administration
2.2. New Therapeutic Approaches
2.2.1. Whole Pancreas Transplantation
2.2.2. Pancreatic Islet Transplantation
2.2.3. Beta-Cell Replacement
3. Mesenchymal Stem Cells
3.1. Administration of MSCs Alone
3.1.1. In Vitro Studies
3.1.2. In Vivo Studies
3.1.3. MSC Manipulation
3.2. Co-Transplantation of MSCs and Pancreatic Islets
3.2.1. Role of Soluble Factors
3.2.2. Role of Direct Contact
3.3. Clinical Use of MSCs
4. Conclusions
Funding
Conflicts of Interest
References
- Association, A.D. Standards of medical care in diabetes--2014. Diabetes Care 2014, 37, S14–S80. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Mahato, R.I. Mesenchymal stem cell-based therapy for type 1 diabetes. Discov. Med. 2014, 17, 139–143. [Google Scholar] [PubMed]
- Ramírez-Domínguez, M. Historical Background of Pancreatic Islet Isolation. Adv. Exp. Med. Biol. 2016, 938, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.; Pathak, N.M.; O’Neill, C.L.; Guduric-Fuchs, J.; Medina, R.J. Therapies for Type 1 Diabetes: Current Scenario and Future Perspectives. Clin. Med. Insights Endocrinol. Diabetes 2019, 12, 1179551419844521. [Google Scholar] [CrossRef]
- Premkumar, L.S.; Pabbidi, R.M. Diabetic peripheral neuropathy: Role of reactive oxygen and nitrogen species. Cell Biochem. Biophys. 2013, 67, 373–383. [Google Scholar] [CrossRef]
- Maffi, P.; Secchi, A. Islet Transplantation Alone Versus Solitary Pancreas Transplantation: An Outcome-Driven Choice? Curr. Diab. Rep. 2019, 19, 26. [Google Scholar] [CrossRef]
- Tjernberg, J.; Ekdahl, K.N.; Lambris, J.D.; Korsgren, O.; Nilsson, B. Acute antibody-mediated complement activation mediates lysis of pancreatic islets cells and may cause tissue loss in clinical islet transplantation. Transplantation 2008, 85, 1193–1199. [Google Scholar] [CrossRef]
- Vaithilingam, V.; Bal, S.; Tuch, B.E. Encapsulated Islet Transplantation: Where Do We Stand? Rev. Diabet. Stud. 2017, 14, 51–78. [Google Scholar] [CrossRef]
- Millman, J.R.; Pagliuca, F.W. Autologous Pluripotent Stem Cell-Derived β-Like Cells for Diabetes Cellular Therapy. Diabetes 2017, 66, 1111–1120. [Google Scholar] [CrossRef]
- Rezania, A.; Bruin, J.E.; Arora, P.; Rubin, A.; Batushansky, I.; Asadi, A.; O’Dwyer, S.; Quiskamp, N.; Mojibian, M.; Albrecht, T.; et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 1121–1133. [Google Scholar] [CrossRef]
- Pagliuca, F.W.; Millman, J.R.; Gürtler, M.; Segel, M.; Van Dervort, A.; Ryu, J.H.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of functional human pancreatic β cells in vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Sackett, S.D.; Rodriguez, A.; Odorico, J.S. The Nexus of Stem Cell-Derived Beta-Cells and Genome Engineering. Rev. Diabet. Stud. 2017, 14, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.J. Gearing Up for Stem Cell-derived Beta Cells-Are We Ready? Transplantation 2018, 102, 1207–1208. [Google Scholar] [CrossRef] [PubMed]
- van der Torren, C.R.; Zaldumbide, A.; Duinkerken, G.; Brand-Schaaf, S.H.; Peakman, M.; Stangé, G.; Martinson, L.; Kroon, E.; Brandon, E.P.; Pipeleers, D.; et al. Immunogenicity of human embryonic stem cell-derived beta cells. Diabetologia 2017, 60, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Velazco-Cruz, L.; Song, J.; Maxwell, K.G.; Goedegebuure, M.M.; Augsornworawat, P.; Hogrebe, N.J.; Millman, J.R. Acquisition of Dynamic Function in Human Stem Cell-Derived β Cells. Stem. Cell Reports 2019, 12, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, D.M.; Mitchell, S.A.; Sackett, S.D.; Odorico, J.S. Mimicking nature-made beta cells: Recent advances towards stem cell-derived islets. Curr. Opin. Organ. Transplant. 2019, 24, 574–581. [Google Scholar] [CrossRef]
- Cho, J.; D’Antuono, M.; Glicksman, M.; Wang, J.; Jonklaas, J. A review of clinical trials: Mesenchymal stem cell transplant therapy in type 1 and type 2 diabetes mellitus. Am. J. Stem. Cells 2018, 7, 82–93. [Google Scholar]
- Scuteri, A.; Donzelli, E.; Rodriguez-Menendez, V.; Ravasi, M.; Monfrini, M.; Bonandrini, B.; Figliuzzi, M.; Remuzzi, A.; Tredici, G. A double mechanism for the mesenchymal stem cells’ positive effect on pancreatic islets. PLoS ONE 2014, 9, e84309. [Google Scholar] [CrossRef]
- Moreira, A.; Kahlenberg, S.; Hornsby, P. Therapeutic potential of mesenchymal stem cells for diabetes. J. Mol. Endocrinol. 2017, 59, R109–R120. [Google Scholar] [CrossRef]
- Stiner, R.; Alexander, M.; Liu, G.; Liao, W.; Liu, Y.; Yu, J.; Pone, E.J.; Zhao, W.; Lakey, J.R.T. Transplantation of stem cells from umbilical cord blood as therapy for type I diabetes. Cell Tissue Res. 2019, 378, 155–162. [Google Scholar] [CrossRef]
- Nojehdehi, S.; Soudi, S.; Hesampour, A.; Rasouli, S.; Soleimani, M.; Hashemi, S.M. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J. Cell Biochem. 2018, 119, 9433–9443. [Google Scholar] [CrossRef] [PubMed]
- Kuljanin, M.; Bell, G.I.; Sherman, S.E.; Lajoie, G.A.; Hess, D.A. Proteomic characterisation reveals active Wnt-signalling by human multipotent stromal cells as a key regulator of beta cell survival and proliferation. Diabetologia 2017, 60, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Mesples, A.; Majeed, N.; Zhang, Y.; Hu, X. Early immunotherapy using autologous adult stem cells reversed the effect of anti-pancreatic islets in recently diagnosed type 1 diabetes mellitus: Preliminary results. Med. Sci. Monit. 2013, 19, 852–857. [Google Scholar] [CrossRef]
- Ezquer, F.; Ezquer, M.; Contador, D.; Ricca, M.; Simon, V.; Conget, P. The antidiabetic effect of mesenchymal stem cells is unrelated to their transdifferentiation potential but to their capability to restore Th1/Th2 balance and to modify the pancreatic microenvironment. Stem. Cells 2012, 30, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem. Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Hao, H.; Tong, C.; Cheng, Y.; Liu, J.; Pang, Y.; Si, Y.; Guo, Y.; Zang, L.; Mu, Y.; et al. Human umbilical cord-derived mesenchymal stem cells elicit macrophages into an anti-inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem Cells 2016, 34, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Hao, H.; Cheng, Y.; Gao, J.; Liu, J.; Xie, Z.; Zhang, Q.; Zang, L.; Han, W.; Mu, Y. The homing of human umbilical cord-derived mesenchymal stem cells and the subsequent modulation of macrophage polarization in type 2 diabetic mice. Int. Immunopharmacol. 2018, 60, 235–245. [Google Scholar] [CrossRef]
- Rackham, C.L.; Amisten, S.; Persaud, S.J.; King, A.J.F.; Jones, P.M. Mesenchymal stromal cell secretory factors induce sustained improvements in islet function pre- and post-transplantation. Cytotherapy 2018, 20, 1427–1436. [Google Scholar] [CrossRef]
- Monfrini, M.; Donzelli, E.; Rodriguez-Menendez, V.; Ballarini, E.; Carozzi, V.A.; Chiorazzi, A.; Meregalli, C.; Canta, A.; Oggioni, N.; Crippa, L.; et al. Therapeutic potential of Mesenchymal Stem Cells for the treatment of diabetic peripheral neuropathy. Exp. Neurol. 2017, 288, 75–84. [Google Scholar] [CrossRef]
- Banerjee, M.; Kumar, A.; Bhonde, R.R. Reversal of experimental diabetes by multiple bone marrow transplantation. Biochem. Biophys. Res. Commun. 2005, 328, 318–325. [Google Scholar] [CrossRef]
- Domouky, A.M.; Hegab, A.S.; Al-Shahat, A.; Raafat, N. Mesenchymal stem cells and differentiated insulin producing cells are new horizons for pancreatic regeneration in type I diabetes mellitus. Int. J. Biochem. Cell Biol. 2017, 87, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Zhang, Z.; Qian, G.S. Mesenchymal stem cells to treat diabetic neuropathy: A long and strenuous way from bench to the clinic. Cell Death Discov. 2016, 2, 16055. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.R.; Zhang, N.K.; Zhang, Y.; Chen, Y.; Wang, L.; Zhu, Y.; Tang, H.H. Overexpression of apelin in Wharton’ jelly mesenchymal stem cell reverses insulin resistance and promotes pancreatic β cell proliferation in type 2 diabetic rats. Stem. Cell Res. Ther. 2018, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Mahdipour, E.; Salmasi, Z.; Sabeti, N. Potential of stem cell-derived exosomes to regenerate β islets through Pdx-1 dependent mechanism in a rat model of type 1 diabetes. J. Cell Physiol. 2019, 234, 20310–20321. [Google Scholar] [CrossRef]
- Montanari, E.; Meier, R.P.H.; Mahou, R.; Seebach, J.D.; Wandrey, C.; Gerber-Lemaire, S.; Buhler, L.H.; Gonelle-Gispert, C. Multipotent mesenchymal stromal cells enhance insulin secretion from human islets via N-cadherin interaction and prolong function of transplanted encapsulated islets in mice. Stem. Cell Res. Ther. 2017, 8, 199. [Google Scholar] [CrossRef]
- Rackham, C.L.; Dhadda, P.K.; Chagastelles, P.C.; Simpson, S.J.; Dattani, A.A.; Bowe, J.E.; Jones, P.M.; King, A.J. Pre-culturing islets with mesenchymal stromal cells using a direct contact configuration is beneficial for transplantation outcome in diabetic mice. Cytotherapy 2013, 15, 449–459. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, H.; Yin, S.; Ji, C.; Zhang, X.; Zhang, B.; Wu, P.; Shi, Y.; Mao, F.; Yan, Y.; et al. Human Mesenchymal Stem Cell Derived Exosomes Alleviate Type 2 Diabetes Mellitus by Reversing Peripheral Insulin Resistance and Relieving β-Cell Destruction. ACS Nano 2018, 12, 7613–7628. [Google Scholar] [CrossRef]
- Xiang, C.; Xie, Q.P. Protection of mouse pancreatic islet function by co-culture with hypoxia pre-treated mesenchymal stromal cells. Mol. Med. Rep. 2018, 18, 2589–2598. [Google Scholar] [CrossRef]
- Xu, L.; Xu, C.; Zhou, S.; Liu, X.; Wang, J.; Qian, S.; Xin, Y.; Gao, Y.; Zhu, Y.; Tang, X. PAX4 promotes PDX1-induced differentiation of mesenchymal stem cells into insulin-secreting cells. Am. J. Transl. Res. 2017, 9, 874–886. [Google Scholar]
- Bader, A.M.; Klose, K.; Bieback, K.; Korinth, D.; Schneider, M.; Seifert, M.; Choi, Y.H.; Kurtz, A.; Falk, V.; Stamm, C. Hypoxic Preconditioning Increases Survival and Pro-Angiogenic Capacity of Human Cord Blood Mesenchymal Stromal Cells In Vitro. PLoS ONE 2015, 10, e0138477. [Google Scholar] [CrossRef]
- Xu, T.; Lv, Z.; Chen, Q.; Guo, M.; Wang, X.; Huang, F. Vascular endothelial growth factor over-expressed mesenchymal stem cells-conditioned media ameliorate palmitate-induced diabetic endothelial dysfunction through PI-3K/AKT/m-TOR/eNOS and p38/MAPK signaling pathway. Biomed. Pharmacother 2018, 106, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Yoo, K.H.; Sym, S.J.; Khang, D. Mesenchymal stem cell therapy assisted by nanotechnology: A possible combinational treatment for brain tumor and central nerve regeneration. Int. J. Nanomedicine 2019, 14, 5925–5942. [Google Scholar] [CrossRef] [PubMed]
- Arzouni, A.A.; Vargas-Seymour, A.; Dhadda, P.K.; Rackham, C.L.; Huang, G.C.; Choudhary, P.; King, A.J.F.; Jones, P.M. Characterization of the Effects of Mesenchymal Stromal Cells on Mouse and Human Islet Function. Stem. Cells Transl. Med. 2019, 8, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Borg, D.J.; Welzel, P.B.; Grimmer, M.; Friedrichs, J.; Weigelt, M.; Wilhelm, C.; Prewitz, M.; Stißel, A.; Hommel, A.; Kurth, T.; et al. Macroporous biohybrid cryogels for co-housing pancreatic islets with mesenchymal stromal cells. Acta Biomater 2016, 44, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, P.O.; Schwarcz, E.; Korsgren, O.; Le Blanc, K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes 2015, 64, 587–592. [Google Scholar] [CrossRef]
- Sbano, P.; Cuccia, A.; Mazzanti, B.; Urbani, S.; Giusti, B.; Lapini, I.; Rossi, L.; Abbate, R.; Marseglia, G.; Nannetti, G.; et al. Use of donor bone marrow mesenchymal stem cells for treatment of skin allograft rejection in a preclinical rat model. Arch. Dermatol. Res. 2008, 300, 115–124. [Google Scholar] [CrossRef][Green Version]
- van de Vyver, M. Intrinsic Mesenchymal Stem Cell Dysfunction in Diabetes Mellitus: Implications for Autologous Cell Therapy. Stem. Cells Dev. 2017, 26, 1042–1053. [Google Scholar] [CrossRef]
- Kornicka, K.; Houston, J.; Marycz, K. Dysfunction of Mesenchymal Stem Cells Isolated from Metabolic Syndrome and Type 2 Diabetic Patients as Result of Oxidative Stress and Autophagy may Limit Their Potential Therapeutic Use. Stem Cell Rev. Rep. 2018, 14, 337–345. [Google Scholar] [CrossRef]
- El-Badawy, A.; El-Badri, N. Clinical Efficacy of Stem Cell Therapy for Diabetes Mellitus: A Meta-Analysis. PLoS ONE 2016, 11, e0151938. [Google Scholar] [CrossRef]
- Foudah, D.; Redaelli, S.; Donzelli, E.; Bentivegna, A.; Miloso, M.; Dalpra, L.; Tredici, G. Monitoring the genomic stability of in vitro cultured rat bone-marrow-derived mesenchymal stem cells. Chromosome Res. 2009, 17, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Tappenbeck, N.; Schröder, H.M.; Niebergall-Roth, E.; Hassinger, F.; Dehio, U.; Dieter, K.; Kraft, K.; Kerstan, A.; Esterlechner, J.; Frank, N.Y.; et al. In vivo safety profile and biodistribution of GMP-manufactured human skin-derived ABCB5-positive mesenchymal stromal cells for use in clinical trials. Cytotherapy 2019, 21, 546–560. [Google Scholar] [CrossRef] [PubMed]
| Authors | Source of MSCs | Application | Suggested Mechanism |
|---|---|---|---|
| Banerjee et al., 2005 [30] | Mouse BM-MSCs | T1D | Islet regeneration |
| Domouky et al., 2017 [31] | Rat BM-MSCs | T1D | Islet regeneration |
| Ezquer et al., 2012 [24] | Mouse BM-MSCs | T1D | |
| Gao et al., 2018 [33] | Human Wharton’s jelly MSCs | T2D | Immunomodulatory effect |
| Kuljanin et al., 2017 [22] | Human BM-MSCs | T1D | Immunomodulatory effect |
| Mahdipour et al., 2019 [34] | Human menstrual blood-derived MSCs | T1D | Islet regeneration |
| Monfrini et al., 2017 [29] | Rat BM-MSCs | T1D | Islet regeneration |
| Montanari et al., 2017 [35] | Human BM-MSCs | T1D | Increased Islet function |
| Nojehdehi et al., 2018 [21] | Mouse Adipose MSCs | T1D | Islet regeneration |
| Rackham et al., 2013 [36] | Mouse Adipose MSCs | T1D | Increased Islet function |
| Rackham et al., 2018 [28] | Mouse Adipose MSCs | T1D | Islet regeneration |
| Sun et al., 2018 [37] | Human umbilical cord-derived MSCs | T2D | Increased Islet function |
| Xiang et al., 2018 [38] | Mouse BM-MSCs | T1D | Islet regeneration |
| Xie et al., 2016 [26] | Human umbilical cord-derived MSCs | T2D | Increased Islet function |
| Yin et al., 2018 [27] | Human umbilical cord-derived MSCs | T2D | Immunomodulatory effect |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donzelli, E.; Scuteri, A. Mesenchymal Stem Cells: A Trump Card for the Treatment of Diabetes? Biomedicines 2020, 8, 112. https://doi.org/10.3390/biomedicines8050112
Donzelli E, Scuteri A. Mesenchymal Stem Cells: A Trump Card for the Treatment of Diabetes? Biomedicines. 2020; 8(5):112. https://doi.org/10.3390/biomedicines8050112
Chicago/Turabian StyleDonzelli, Elisabetta, and Arianna Scuteri. 2020. "Mesenchymal Stem Cells: A Trump Card for the Treatment of Diabetes?" Biomedicines 8, no. 5: 112. https://doi.org/10.3390/biomedicines8050112
APA StyleDonzelli, E., & Scuteri, A. (2020). Mesenchymal Stem Cells: A Trump Card for the Treatment of Diabetes? Biomedicines, 8(5), 112. https://doi.org/10.3390/biomedicines8050112





