Nucleus Accumbens-Associated Protein 1 Binds DNA Directly through the BEN Domain in a Sequence-Specific Manner
Abstract
1. Introduction
2. Experimental Section
2.1. Cloning
2.2. Screening of DNA-Binding Sequences
2.3. Bioinformatics
2.4. Oligonucleotide Pull-Down Assay
2.5. Antibody and Chromatin Immunoprecipitation (ChIP) Assay
2.6. Isothermal Titration Calorimetry (ITC) Experiments
2.7. NMR Spectroscopy
2.8. Structure Calculation
2.9. Cell Culture and siRNA Transfection
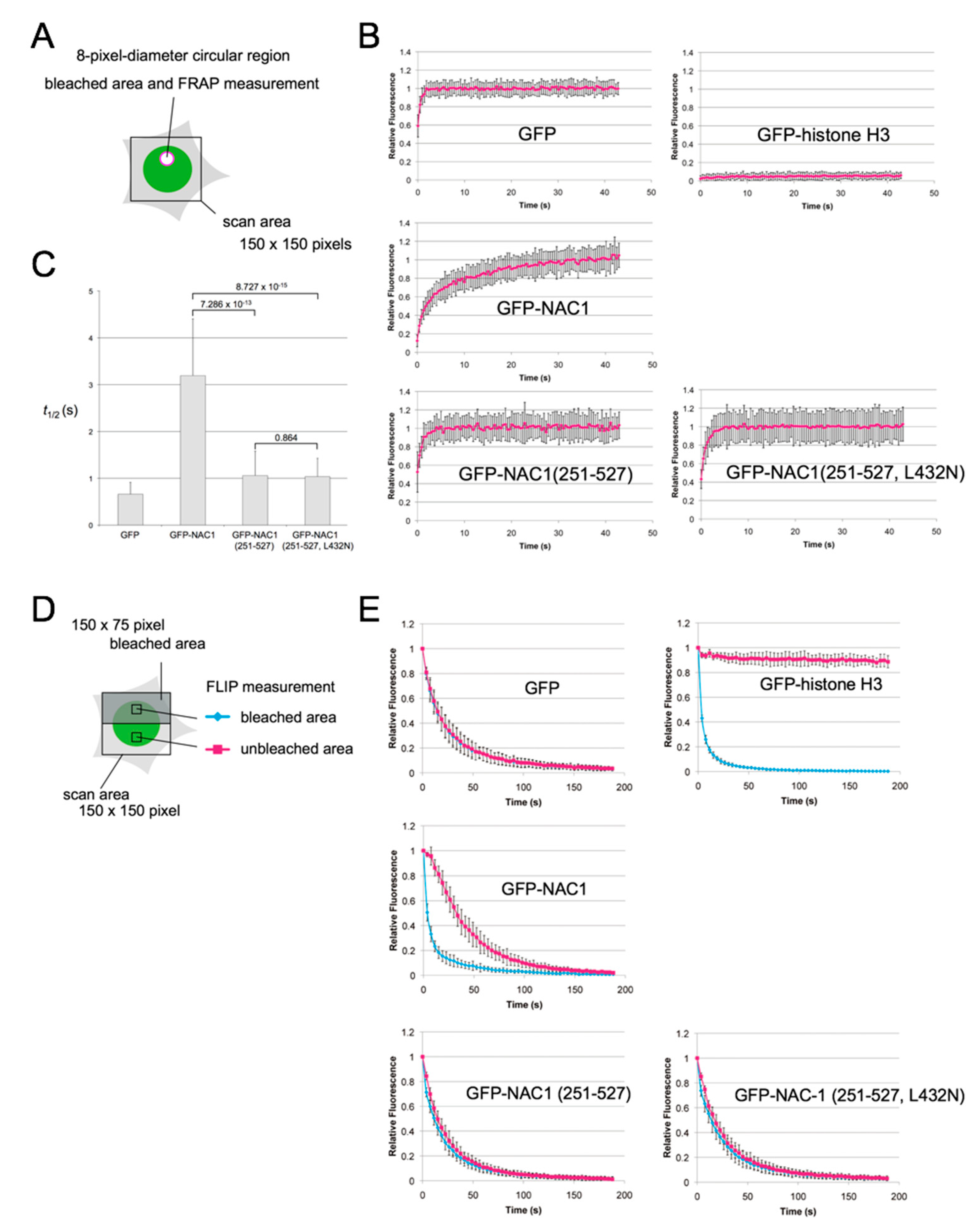
2.10. FRAP and FLIP Analyses
2.11. Accession Numbers
3. Results
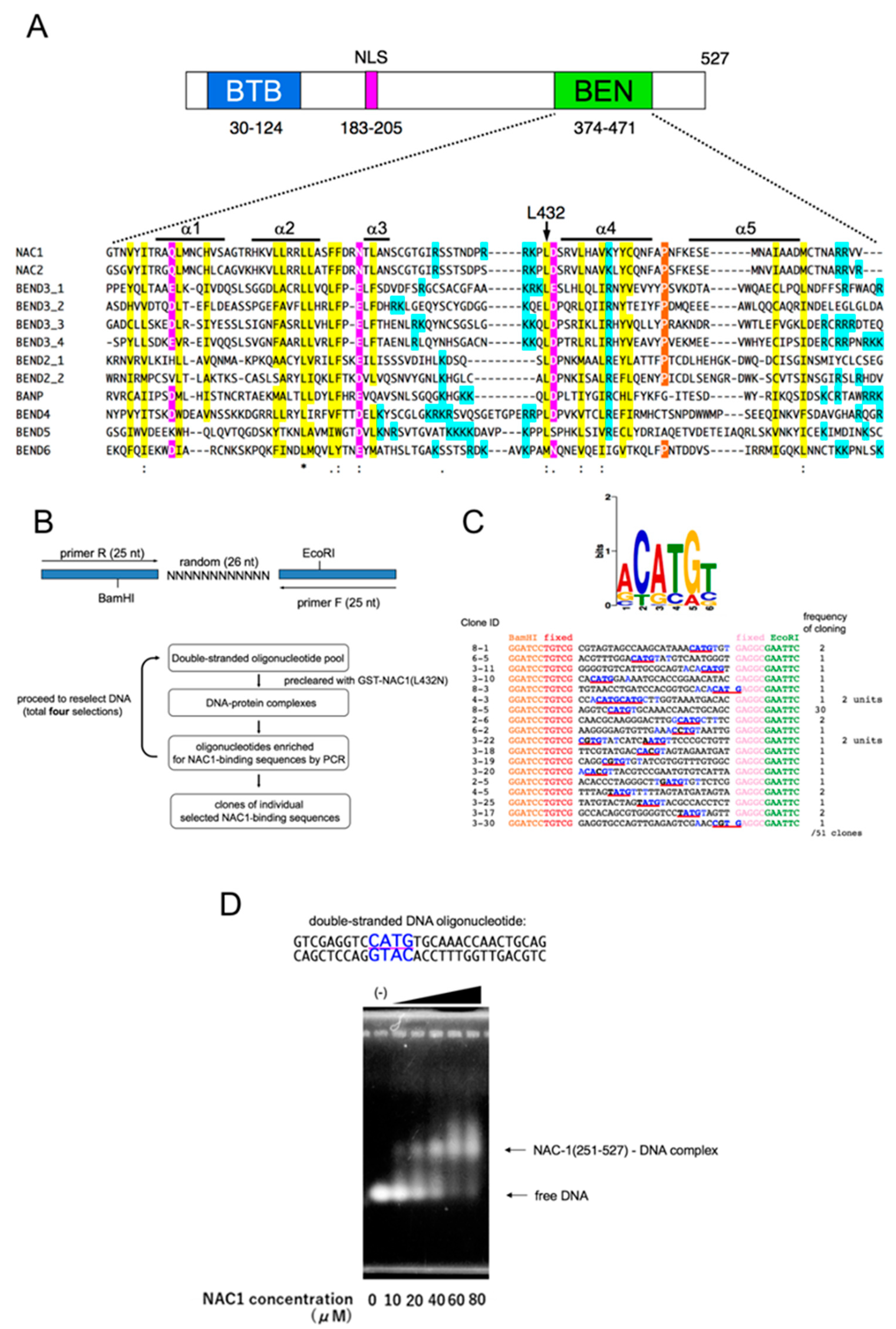
3.1. NAC1 Binds DNA through Its BEN Domain
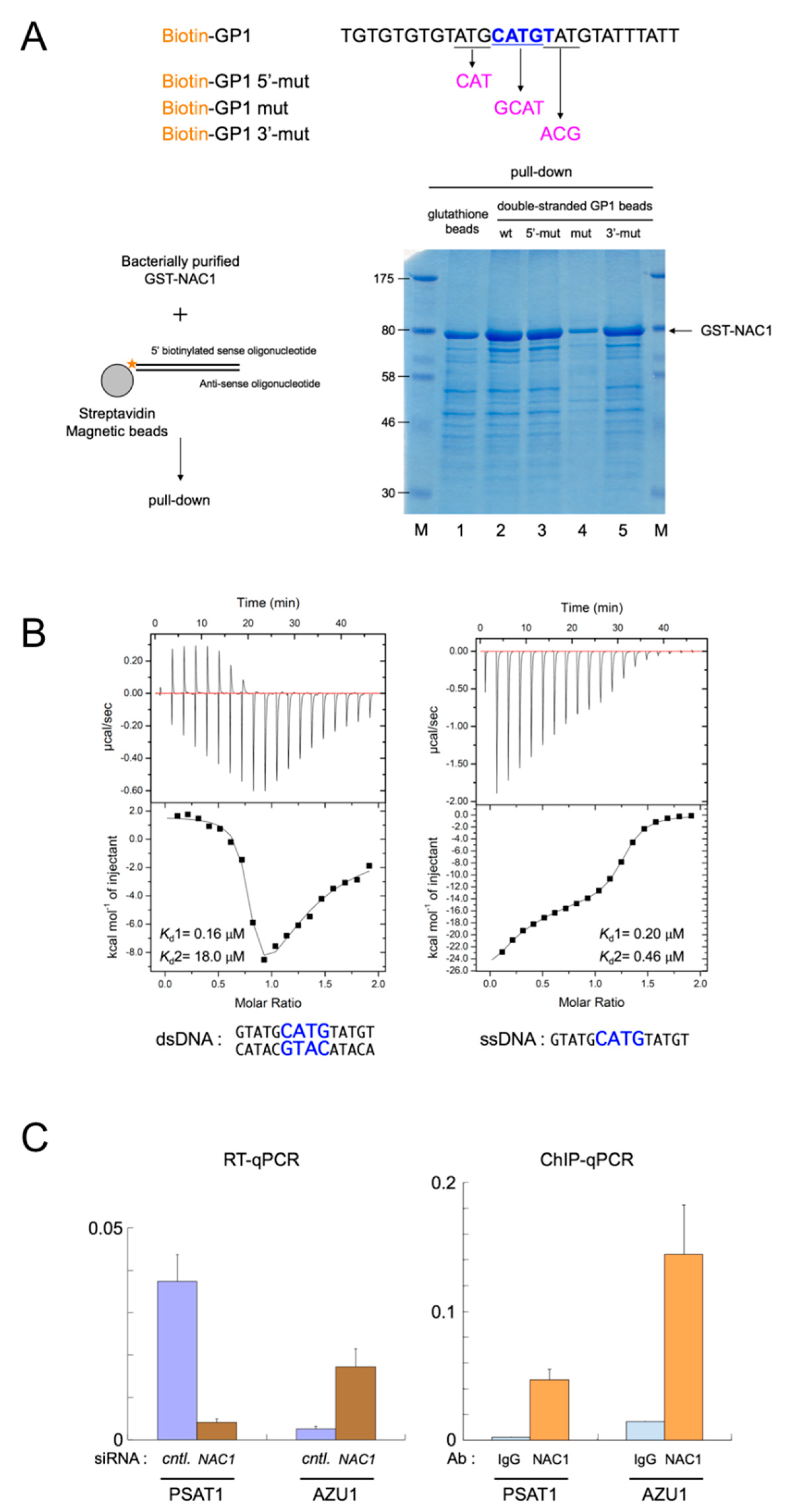
3.2. Identification of the Consensus DNA-Binding Sequence of NAC1
3.3. Solution NMR Structure of the BEN Domain of NAC1
3.4. The BEN Domain of NAC1 Alone Cannot Interact with Chromatin/Other Proteins in Living Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cha, X.Y.; Pierce, R.C.; Kalivas, P.W.; Mackler, S.A. NAC-1, a rat brain mRNA, is increased in the nucleus accumbens three weeks after chronic cocaine self-administration. J. Neurosci. 1997, 17, 6864–6871. [Google Scholar] [CrossRef]
- Nakayama, K.; Nakayama, N.; Davidson, B.; Sheu, J.J.; Jinawath, N.; Santillan, A.; Salani, R.; Bristow, R.E.; Morin, P.J.; Kurman, R.J.; et al. A BTB/POZ protein, NAC-1, is related to tumor recurrence and is essential for tumor growth and survival. Proc. Natl Acad. Sci. USA 2006, 103, 18739–18744. [Google Scholar] [CrossRef]
- Nakayama, K.; Nakayama, N.; Wang, T.-L.; Shih Ie, M. NAC-1 controls cell growth and survival by repressing transcription of Gadd45GIP1, a candidate tumor suppressor. Cancer Res. 2007, 67, 8058–8064. [Google Scholar] [CrossRef] [PubMed]
- Yeasmin, S.; Nakayama, K.; Ishibashi, M.; Katagiri, A.; Iida, K.; Purwana, I.N.; Nakayama, N.; Miyazaki, K. Expression of the bric-a-brac tramtrack broad complex protein NAC-1 in cervical carcinomas seems to correlate with poorer prognosis. Clin. Cancer Res. 2008, 14, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Nakayama, K.; Yeasmin, S.; Katagiri, A.; Iida, K.; Nakayama, N.; Fukumoto, M.; Miyazaki, K. A BTB/POZ gene, NAC-1, a tumor recurrence-associated gene, as a potential target for Taxol resistance in ovarian cancer. Clin. Cancer Res. 2008, 14, 3149–3155. [Google Scholar] [CrossRef] [PubMed]
- Jinawath, N.; Vasoontara, C.; Yap, K.L.; Thiaville, M.M.; Nakayama, K.; Wang, T.-L.; Shih Ie, M. NAC-1, a potential stem cell pluripotency factor, contributes to paclitaxel resistance in ovarian cancer through inactivating Gadd45 pathway. Oncogene 2009, 28, 1941–1948. [Google Scholar] [CrossRef]
- Ishibashi, M.; Nakayama, K.; Yeasmin, S.; Katagiri, A.; Iida, K.; Nakayama, N.; Miyazaki, K. Expression of a BTB/POZ protein, NAC1, is essential for the proliferation of normal cyclic endometrial glandular cells and is up-regulated by estrogen. Clin. Cancer Res. 2009, 15, 804–811. [Google Scholar] [CrossRef]
- Ishikawa, M.; Nakayama, K.; Yeasmin, S.; Katagiri, A.; Iida, K.; Nakayama, N.; Miyazaki, K. NAC1, a potential stem cell pluripotency factor expression in normal endometrium, endometrial hyperplasia and endometrial carcinoma. Int. J. Oncol. 2010, 36, 1097–1103. [Google Scholar]
- Nakayama, K.; Rahman, M.T.; Rahman, M.; Yeasmin, S.; Ishikawa, M.; Katagiri, A.; Iida, K.; Nakayama, N.; Miyazaki, K. Biological role and prognostic significance of NAC1 in ovarian cancer. Gynecol. Oncol. 2010, 119, 469–478. [Google Scholar] [CrossRef]
- Shih Ie, M.; Nakayama, K.; Wu, G.; Nakayama, N.; Zhang, J.; Wang, T.-L. Amplification of the ch19p13.2 NACC1 locus in ovarian high-grade serous carcinoma. Mod. Pathol. 2011, 24, 638–645. [Google Scholar] [CrossRef]
- Yeasmin, S.; Nakayama, K.; Rahman, M.T.; Rahman, M.; Ishikawa, M.; Katagiri, A.; Iida, K.; Nakayama, N.; Otuski, Y.; Kobayashi, H.; et al. Biological and clinical significance of NAC1 expression in cervical carcinomas: A comparative study between squamous cell carcinomas and adenocarcinomas/adenosquamous carcinomas. Hum. Pathol. 2012, 43, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Maruyama, R.; Urano, T.; Nakayama, N.; Kawabata, Y.; Yano, S.; Yoshida, M.; Nakayama, K.; Miyazaki, K.; Takenaga, K.; et al. Low expression of nucleus accumbens-associated protein 1 predicts poor prognosis for patients with pancreatic ductal adenocarcinoma. Pathol. Int. 2012, 62, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, N.; Sakashita, G.; Nariai, Y.; Kato, H.; Sinmyozu, K.; Nakayama, J.I.; Kyo, S.; Urano, T.; Nakayama, K. Cancer-related transcription regulator protein NAC1 forms a protein complex with CARM1 for ovarian cancer progression. Oncotarget 2018, 9, 28408–28420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, M.; Song, Y.; Liu, H.; Xiang, S. NAC1/HMGB1 Signaling Pathway Is Associated with Epithelial-mesenchymal Transition, Invasion, and Metastasis of Lung Cancer Cell Line. Ann. Clin. Lab. Sci. 2018, 48, 559–564. [Google Scholar] [PubMed]
- Wang, J.; Rao, S.; Chu, J.; Shen, X.; Levasseur, D.N.; Theunissen, T.W.; Orkin, S.H. A protein interaction network for pluripotency of embryonic stem cells. Nature 2006, 444, 364–368. [Google Scholar] [CrossRef]
- Kim, J.; Chu, J.; Shen, X.; Wang, J.; Orkin, S.H. An extended transcriptional network for pluripotency of embryonic stem cells. Cell 2008, 132, 1049–1061. [Google Scholar] [CrossRef]
- Ruan, Y.; He, J.; Wu, W.; He, P.; Tian, Y.; Xiao, L.; Liu, G.; Wang, J.; Cheng, Y.; Zhang, S.; et al. Nac1 promotes self-renewal of embryonic stem cells through direct transcriptional regulation of c-Myc. Oncotarget 2017, 8, 47607–47618. [Google Scholar] [CrossRef]
- Malleshaiah, M.; Padi, M.; Rué, P.; Quackenbush, J.; Martinez-Arias, A.; Gunawardena, J. Nac1 coordinates a sub-network of pluripotency factors to regulate embryonic stem cell differentiation. Cell Rep. 2016, 14, 1181–1194. [Google Scholar] [CrossRef]
- Choi, H.; Park, H.J.; Kim, H.; Kim, J.; Lee, Y.K.; Kim, J. Nac1 facilitates pluripotency gene activation for establishing somatic cell reprogramming. Biochem. Biophys. Res. Commun. 2019, 518, 253–258. [Google Scholar] [CrossRef]
- Faiola, F.; Yin, N.; Fidalgo, M.; Huang, X.; Saunders, A.; Ding, J.; Guallar, D.; Dang, B.; Wang, J. NAC1 Regulates Somatic Cell Reprogramming by Controlling Zeb1 and E-cadherin Expression. Stem Cell Rep. 2017, 9, 913–926. [Google Scholar] [CrossRef]
- Yap, K.L.; Sysa-Shah, P.; Bolon, B.; Wu, R.C.; Gao, M.; Herlinger, A.L.; Wang, F.; Faiola, F.; Huso, D.; Gabrielson, K.; et al. Loss of NAC1 expression is associated with defective bony patterning in the murine vertebral axis. PLoS ONE 2013, 8, e69099. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Xu, G.; Nie, L.; Liu, L.; Peng, N.; He, Q.; Zuo, Q.; Zhou, Y.; Cao, Z.; Liu, S.; et al. NAC1 Potentiates Cellular Antiviral Signaling by Bridging MAVS and TBK1. J. Immunol. 2019, 203, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T. Regulation of hematopoietic development by ZBTB transcription factors. Int. J. Hematol. 2016, 104, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Chaharbakhshi, E.; Jemc, J.C. Broad-complex, tramtrack, and bric-à-brac (BTB) proteins: Critical regulators of development. Genesis 2016, 54, 505–518. [Google Scholar] [CrossRef]
- Stead, M.A.; Carr, S.B.; Wright, S.C. Structure of the human Nac1 POZ domain. Acta. Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2009, 65, 445–449. [Google Scholar] [CrossRef]
- Wang, X.; Ji, C.; Zhang, H.; Shan, Y.; Ren, Y.; Hu, Y.; Shi, L.; Guo, L.; Zhu, W.; Xia, Y.; et al. Identification of a small-molecule compound that inhibits homodimerization of oncogenic NAC1 protein and sensitizes cancer cells to anticancer agents. J. Biol. Chem. 2019, 294, 10006–10017. [Google Scholar] [CrossRef]
- Stead, M.A.; Wright, S.C. Nac1 interacts with the POZ-domain transcription factor, Miz1. Biosci. Rep. 2014, 34, e00110. [Google Scholar] [CrossRef]
- Stead, M.A.; Wright, S.C. Structures of heterodimeric POZ domains of Miz1/BCL6 and Miz1/NAC1. Acta Crystallogr. F Struct. Biol. Commun. 2014, 70, 1591–1596. [Google Scholar] [CrossRef]
- Wu, T.; He, P.; Wu, W.; Chen, Y.; Lv, F. Targeting oncogenic transcriptional corepressor Nac1 POZ domain with conformationally constrained peptides by cyclization and stapling. Bioorg. Chem. 2018, 80, 1–10. [Google Scholar] [CrossRef]
- Abhiman, S.; Iyer, L.M.; Aravind, L. BEN: A novel domain in chromatin factors and DNA viral proteins. Bioinformatics 2008, 24, 458–461. [Google Scholar] [CrossRef]
- Duan, H.; Dai, Q.; Kavaler, J.; Bejarano, F.; Medranda, G.; Nègre, N.; Lai, E.C. Insensitive is a corepressor for Suppressor of Hairless and regulates Notch signalling during neural development. EMBO J. 2011, 30, 3120–3133. [Google Scholar] [CrossRef] [PubMed]
- Korutla, L.; Degnan, R.; Wang, P.; Mackler, S.A. NAC1, a cocaine-regulated POZ/BTB protein interacts with CoREST. J. Neurochem. 2007, 101, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Sathyan, K.M.; Shen, Z.; Tripathi, V.; Prasanth, K.V.; Prasanth, S.G. A BEN-domain-containing protein associates with heterochromatin and represses transcription. J. Cell Sci. 2011, 124, 3149–3163. [Google Scholar] [CrossRef] [PubMed]
- Korutla, L.; Wang, P.J.; Mackler, S.A. The POZ/BTB protein NAC1 interacts with two different histone deacetylases in neuronal-like cultures. J. Neurochem. 2005, 94, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Xuan, C.; Wang, Q.; Han, X.; Duan, Y.; Li, L.; Shi, L.; Wang, Y.; Shan, L.; Yao, Z.; Shang, Y. RBB, a novel transcription repressor, represses the transcription of HDM2 oncogene. Oncogene 2013, 32, 3711–3721. [Google Scholar] [CrossRef] [PubMed]
- Rampalli, S.; Pavithra, L.; Bhatt, A.; Kundu, T.K.; Chattopadhyay, S. Tumor suppressor SMAR1 mediates cyclin D1 repression by recruitment of the SIN3/histone deacetylase 1 complex. Mol. Cell. Biol. 2005, 25, 8415–8429. [Google Scholar] [CrossRef]
- Saksouk, N.; Barth, T.K.; Ziegler-Birling, C.; Olova, N.; Nowak, A.; Rey, E.; Mateos-Langerak, J.; Urbach, S.; Reik, W.; Torres-Padilla, M.-E.; et al. Redundant Mechanisms to Form Silent Chromatin at Pericentromeric Regions Rely on BEND3 and DNA Methylation. Mol. Cell 2014, 56, 580–594. [Google Scholar] [CrossRef]
- Khan, A.; Giri, S.; Wang, Y.; Chakraborty, A.; Ghosh, A.K.; Anantharaman, A.; Aggarwal, V.; Sathyan, K.M.; Ha, T.; Prasanth, K.V.; et al. BEND3 represses rDNA transcription by stabilizing a NoRC component via USP21 deubiquitinase. Proc. Natl Acad. Sci. USA 2015, 112, 8338–8343. [Google Scholar] [CrossRef]
- Dai, Q.; Ren, A.; Westholm, J.O.; Serganov, A.A.; Patel, D.J.; Lai, E.C. The BEN domain is a novel sequence-specific DNA-binding domain conserved in neural transcriptional repressors. Genes 2013, 27, 602–614. [Google Scholar] [CrossRef]
- Dai, Q.; Andreu-Agullo, C.; Insolera, R.; Wong, L.C.; Shi, S.H.; Lai, E.C. BEND6 is a nuclear antagonist of Notch signaling during self-renewal of neural stem cells. Development 2013, 140, 1892–1902. [Google Scholar] [CrossRef]
- Yoshida, H.; Park, S.Y.; Oda, T.; Akiyoshi, T.; Sato, M.; Shirouzu, M.; Tsuda, K.; Kuwasako, K.; Unzai, S.; Muto, Y.; et al. A novel 3’ splice site recognition by the two zinc fingers in the U2AF small subunit. Genes Dev. 2015, 29, 1649–1660. [Google Scholar] [CrossRef]
- Delaglio, F.; Grzesiek, S.; Vuister, G.W.; Zhu, G.; Pfeifer, J.; Bax, A. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 1995, 6, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Iwahara, J.; Koshiba, S.; Tomizawa, T.; Tochio, N.; Güntert, P.; Kigawa, T.; Yokoyama, S. KUJIRA, a package of integrated modules for systematic and interactive analysis of NMR data directed to high-throughput NMR structure studies. J. Biomol. NMR 2007, 39, 31–52. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.A. Using NMRView to visualize and analyze the NMR spectra of macromolecules. Methods Mol. Biol. 2004, 278, 313–352. [Google Scholar] [PubMed]
- Nagata, T.; Suzuki, S.; Endo, R.; Shirouzu, M.; Terada, T.; Inoue, M.; Kigawa, T.; Kobayashi, N.; Guntert, P.; Tanaka, A.; et al. The RRM domain of poly(A)-specific ribonuclease has a noncanonical binding site for mRNA cap analog recognition. Nucleic Acids Res. 2008, 36, 4754–4767. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clore, G.M.; Gronenborn, A.M. Determining the structures of large proteins and protein complexes by NMR. Trends Biotechnol. 1998, 16, 22–34. [Google Scholar] [CrossRef]
- Cavanagh, J.; Fairbrother, W.J.; Palmer, A.G.R.; Rance, M.; Skelton, N.J. Protein NMR Spectroscopy, Second Edition: Principles and Practice; Academic Press, Inc.: San Diego, CA, USA, 2006. [Google Scholar]
- Shen, Y.; Delaglio, F.; Cornilescu, G.; Bax, A. TALOS+: A hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J. Biomol. NMR 2009, 44, 213–223. [Google Scholar] [CrossRef]
- Powers, R.; Garrett, D.S.; March, C.J.; Frieden, E.A.; Gronenborn, A.M.; Clore, G.M. The high-resolution, three-dimensional solution structure of human interleukin-4 determined by multidimensional heteronuclear magnetic resonance spectroscopy. Biochemistry 1993, 32, 6744–6762. [Google Scholar] [CrossRef]
- Mulder, F.A.; Schipper, D.; Bott, R.; Boelens, R. Altered flexibility in the substrate-binding site of related native and engineered high-alkaline Bacillus subtilisins. J. Mol. Biol. 1999, 292, 111–123. [Google Scholar] [CrossRef]
- Case, D.A.; Cheatham, T.E., 3rd; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M., Jr.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef]
- Dai, Q.; Ren, A.; Westholm, J.O.; Duan, H.; Patel, D.J.; Lai, E.C. Common and distinct DNA-binding and regulatory activities of the BEN-solo transcription factor family. Genes Dev. 2015, 29, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Lu, X.W.; Kavi, H.; Emelyanov, A.V.; Bernardo, T.J.; Vershilova, E.; Skoultchi, A.I.; Fyodorov, D.V. BEN domain protein Elba2 can functionally substitute for linker histone H1 in Drosophila in vivo. Sci. Rep. 2016, 6, 34354. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Machanick, P. Inferring direct DNA binding from ChIP-seq. Nucleic Acids Res. 2012, 40, e128. [Google Scholar] [CrossRef] [PubMed]
- Lovell, S.C.; Davis, I.W.; Arendall, W.B., 3rd; de Bakker, P.I.; Word, J.M.; Prisant, M.G.; Richardson, J.S.; Richardson, D.C. Structure validation by Cα geometry: φ,ψ and Cβ deviation. Proteins 2003, 50, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.C.; Pettersen, E.F.; Couch, G.S.; Huang, C.C.; Ferrin, T.E. Tools for integrated sequence-structure analysis with UCSF Chimera. BMC Bioinform. 2006, 7, 339. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Okazaki, K.; Nakayama, N.; Nariai, Y.; Kato, H.; Nakayama, K.; Miyazaki, K.; Maruyama, R.; Kosugi, S.; Urano, T.; Sakashita, G. Nuclear localization signal in a cancer-related transcriptional regulator protein NAC1. Carcinogenesis 2012, 33, 1854–1862. [Google Scholar] [CrossRef]
- Nakayama, N.; Kato, H.; Sakashita, G.; Nariai, Y.; Nakayama, K.; Kyo, S.; Urano, T. Protein complex formation and intranuclear dynamics of NAC1 in cancer cells. Arch. Biochem. Biophys. 2016, 606, 10–15. [Google Scholar] [CrossRef]
- Wang, D.; Baumann, A.; Szebeni, A.; Olson, M.O. The nucleic acid binding activity of nucleolar protein B23.1 resides in its carboxyl-terminal end. J. Biol. Chem. 1994, 269, 30994–30998. [Google Scholar]
- Pollock, R.M. Determination of protein-DNA sequence specificity by PCR-assisted binding-site selection. Curr. Protoc. Mol. Biol. 2001, 12. [Google Scholar] [CrossRef]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1994, 2, 28–36. [Google Scholar]
- Zerbino, D.R.; Wilder, S.P.; Johnson, N.; Juettemann, T.; Flicek, P.R. The ensembl regulatory build. Genome Biol. 2015, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Boeva, V. Analysis of Genomic Sequence Motifs for Deciphering Transcription Factor Binding and Transcriptional Regulation in Eukaryotic Cells. Front. Genet. 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakayama, N.; Sakashita, G.; Nagata, T.; Kobayashi, N.; Yoshida, H.; Park, S.-Y.; Nariai, Y.; Kato, H.; Obayashi, E.; Nakayama, K.; et al. Nucleus Accumbens-Associated Protein 1 Binds DNA Directly through the BEN Domain in a Sequence-Specific Manner. Biomedicines 2020, 8, 608. https://doi.org/10.3390/biomedicines8120608
Nakayama N, Sakashita G, Nagata T, Kobayashi N, Yoshida H, Park S-Y, Nariai Y, Kato H, Obayashi E, Nakayama K, et al. Nucleus Accumbens-Associated Protein 1 Binds DNA Directly through the BEN Domain in a Sequence-Specific Manner. Biomedicines. 2020; 8(12):608. https://doi.org/10.3390/biomedicines8120608
Chicago/Turabian StyleNakayama, Naomi, Gyosuke Sakashita, Takashi Nagata, Naohiro Kobayashi, Hisashi Yoshida, Sam-Yong Park, Yuko Nariai, Hiroaki Kato, Eiji Obayashi, Kentaro Nakayama, and et al. 2020. "Nucleus Accumbens-Associated Protein 1 Binds DNA Directly through the BEN Domain in a Sequence-Specific Manner" Biomedicines 8, no. 12: 608. https://doi.org/10.3390/biomedicines8120608
APA StyleNakayama, N., Sakashita, G., Nagata, T., Kobayashi, N., Yoshida, H., Park, S.-Y., Nariai, Y., Kato, H., Obayashi, E., Nakayama, K., Kyo, S., & Urano, T. (2020). Nucleus Accumbens-Associated Protein 1 Binds DNA Directly through the BEN Domain in a Sequence-Specific Manner. Biomedicines, 8(12), 608. https://doi.org/10.3390/biomedicines8120608





