Haloperidol Induced Cell Cycle Arrest and Apoptosis in Glioblastoma Cells
Abstract
1. Introduction
2. Experimental Section
2.1. Cell Lines and Treatment Conditions
2.2. Viability Assay
2.3. Flow Cytometric Analysis of Apoptosis, DNA Cell Cycle, Caspase-8 and Cluster of Differentiation (CD) Expression
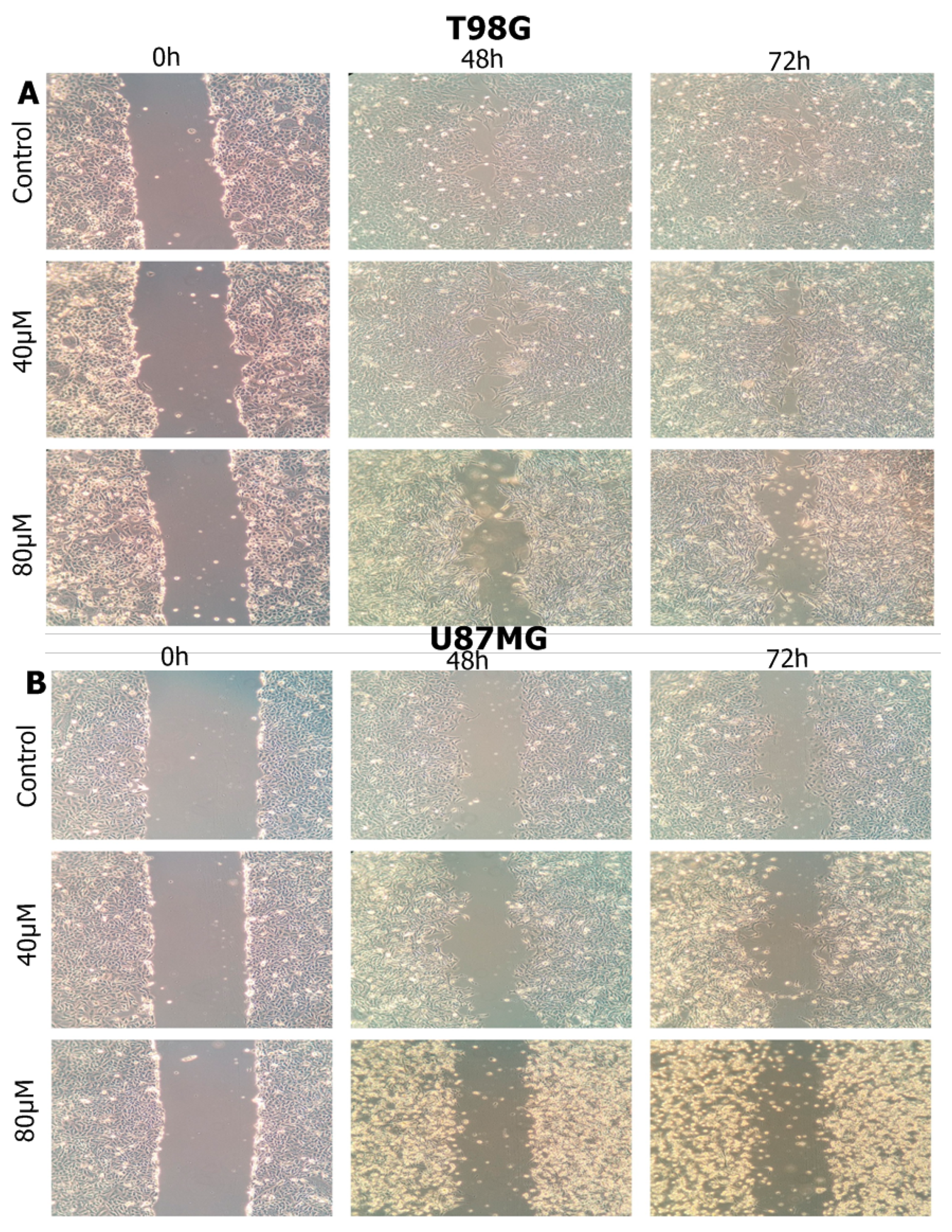
2.4. Wound Healing Assay
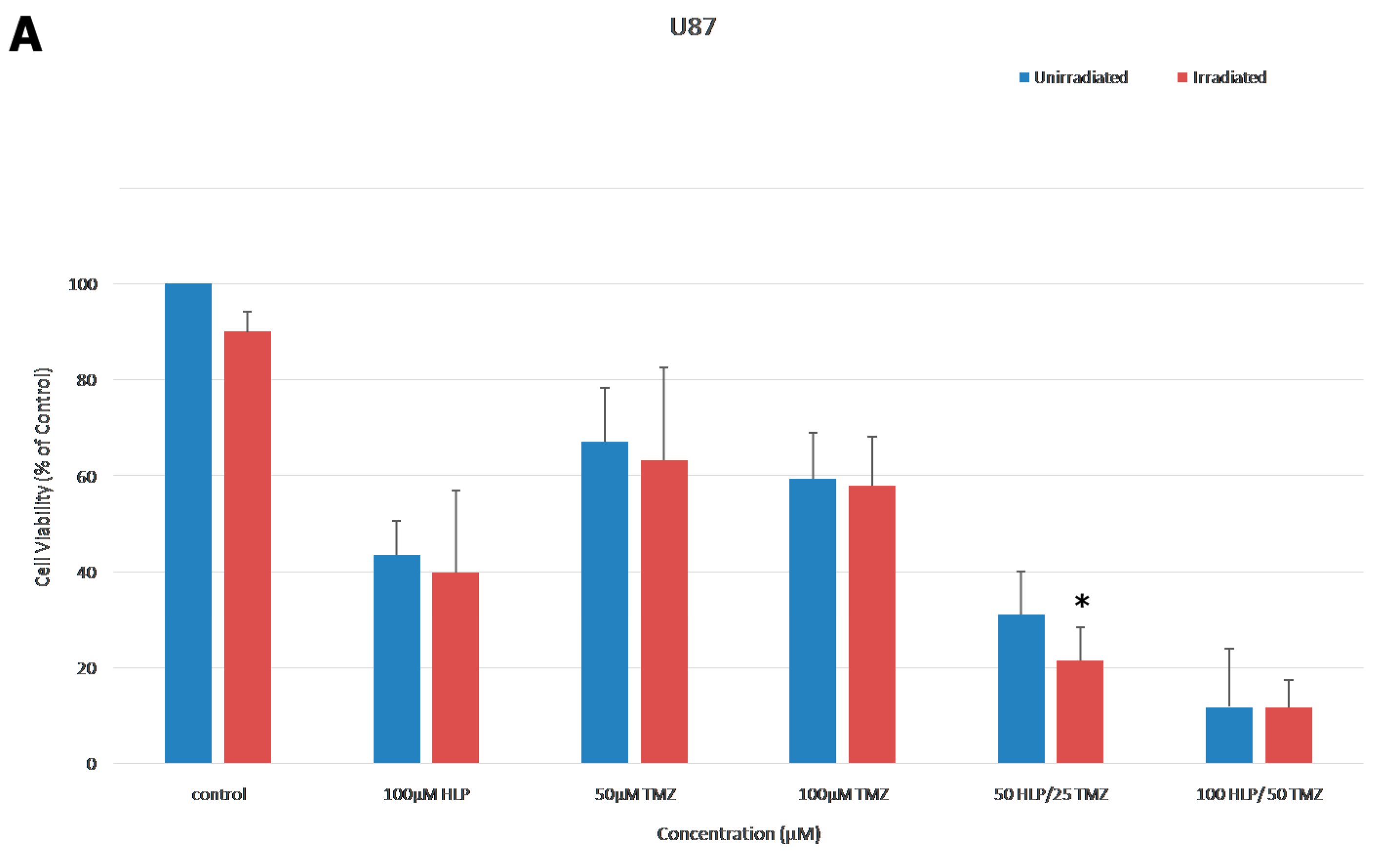
2.5. Combination Treatment with Haloperidol, TMZ and Radiation
2.6. Statistical Analysis
3. Results
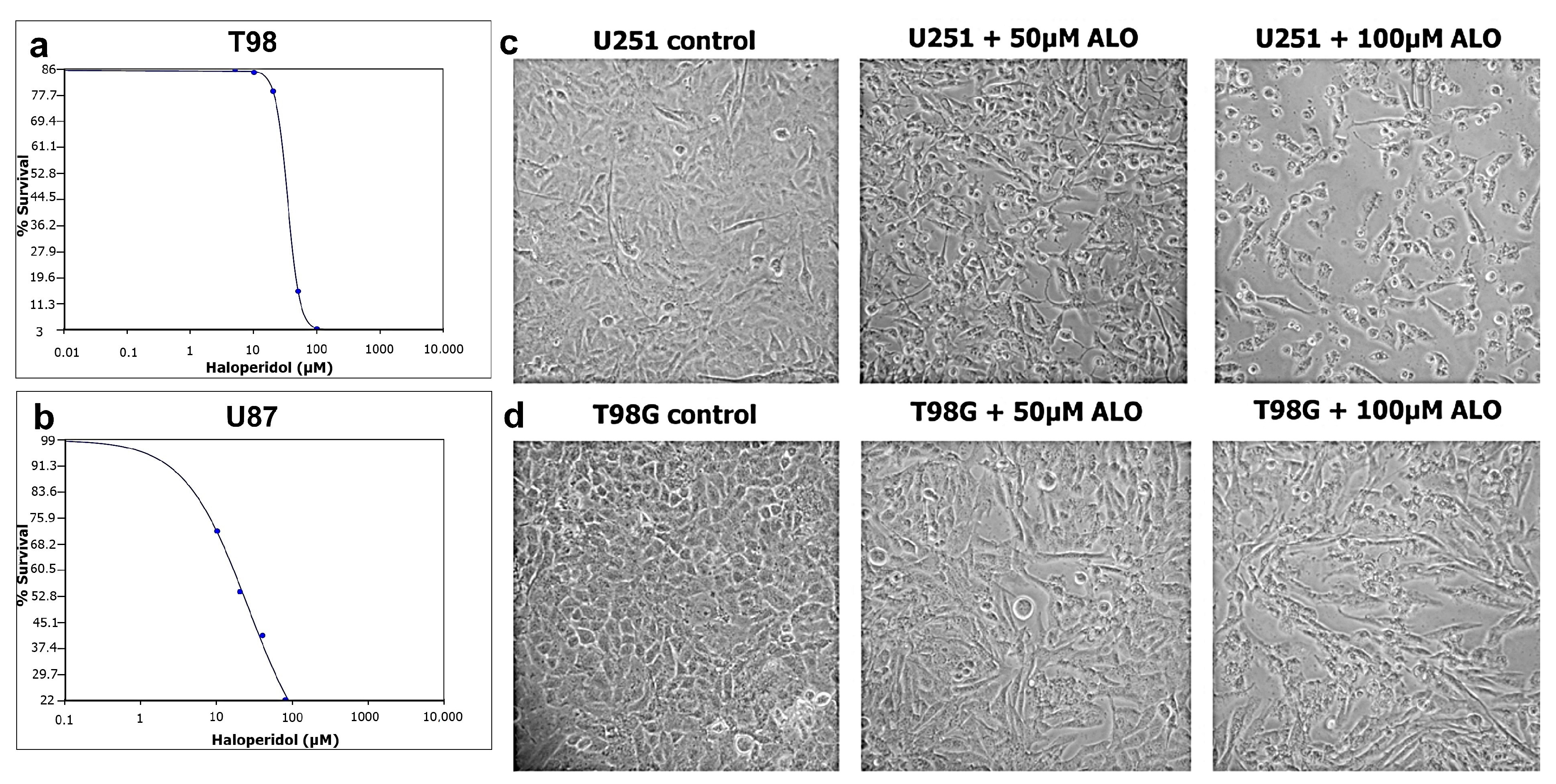
3.1. Sensitivity of GBM Cells to Haloperidol and IC50 Calculation
3.2. Haloperidol Induced G2/M Cell Cycle Arrest and Appearance of subG0/G1 Peak
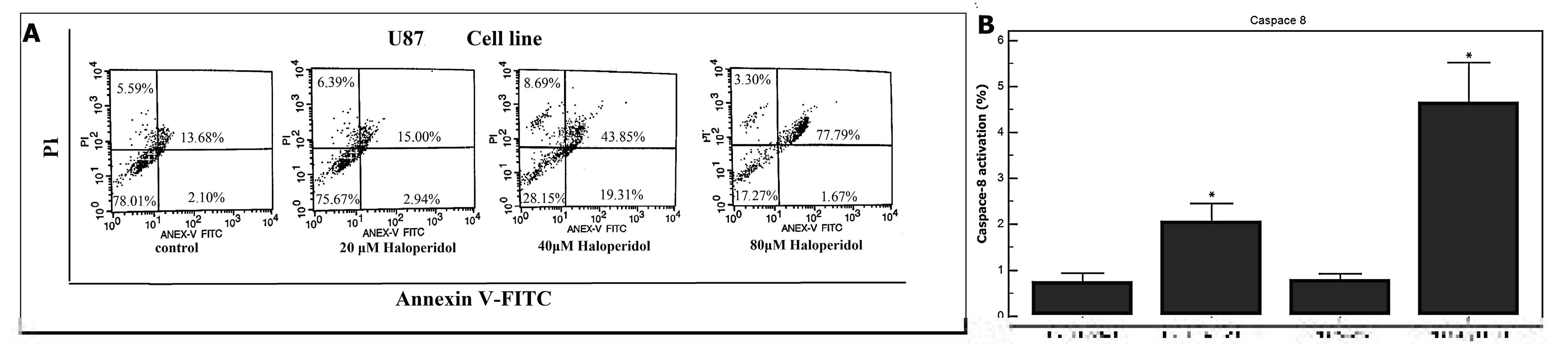
3.3. Haloperidol Induced Apoptosis and Increased Caspase-8 Activation in GBM Cells
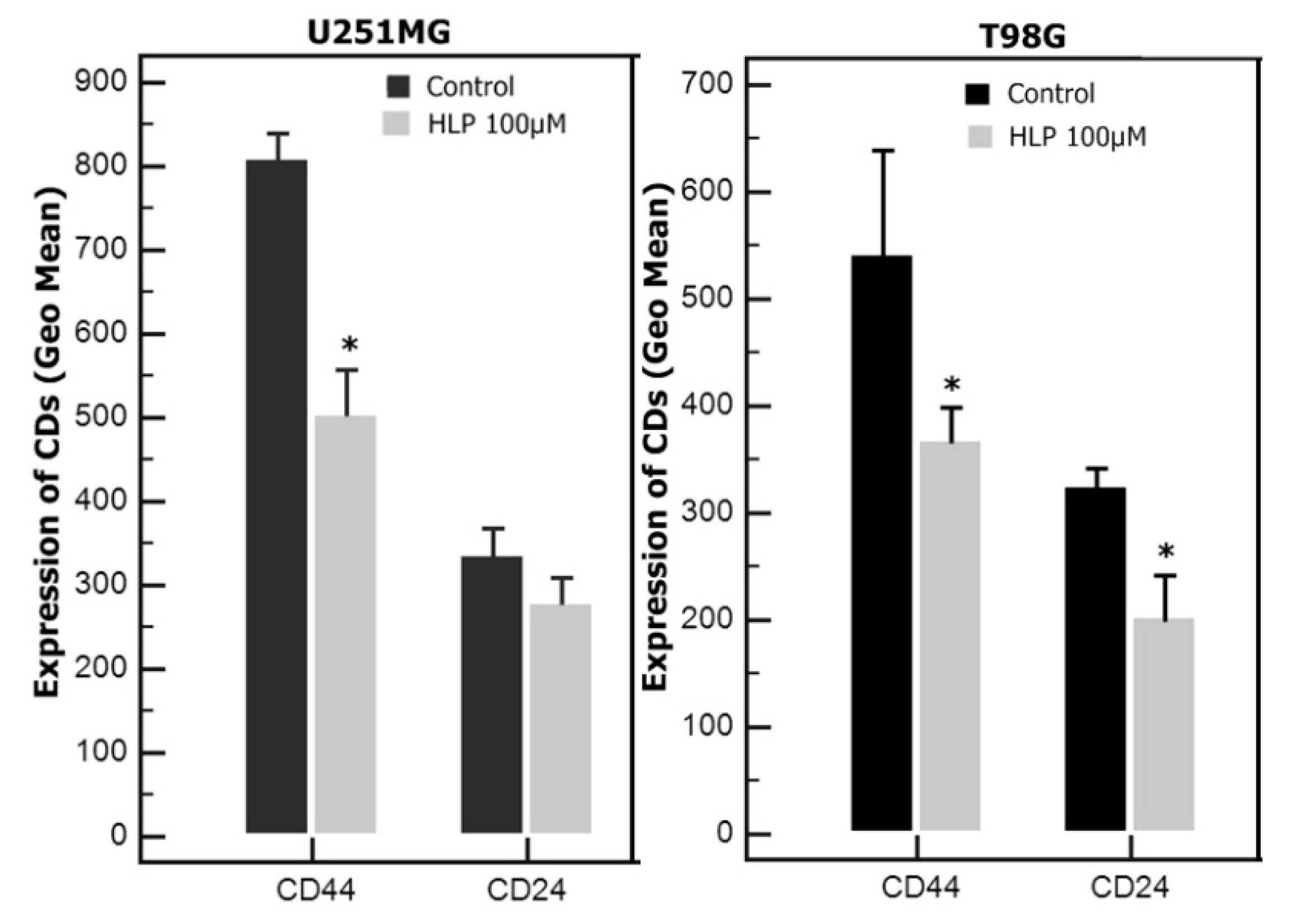
3.4. Haloperidol Induced Changes in CD Expression in U251 and T98 Cells
3.5. Haloperidol Inhibited Cell Migration
3.6. Combination Treatment of Haloperidol, TMZ and Radiation Increased Cell Death
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; Shete, S.; Etzel, C.J.; Scheurer, M.E.; Alexiou, G.; Armstrong, G.; Tsavachidis, S.; Liang, F.-W.; Gilbert, M.; Aldape, K.; et al. Polymorphisms of LIG4, BTBD2, HMGA2, and RTEL1 genes involved in the double-strand break repair pathway predict glioblastoma survival. J. Clin. Oncol. 2010, 10, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.P.; Tirosh, T.; Trombetta, T.T.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 20, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Levin, V.A.; Ellingson, B.M. Understanding brain penetrance of anticancer drugs. Neuro Oncol. 2018, 9, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Faraz, F.; Pannullo, S.; Rosenblum, M.; Smith, A.; Wernicke, A.G. Long-term survival in a patient with glioblastoma on antipsychotic therapy for schizophrenia: A case report and literature review. Ther. Adv. Med. Oncol. 2016, 8, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-K.; Chang, N.; Yoon, Y.; Yang, H.; Cho, H.; Kim, E.; Shin, Y.; Kang, W.; Oh, Y.T.; Mun, G.I.; et al. USP1 targeting impedes GBM growth by inhibiting stem cell maintenance and radioresistance. Neuro Oncol. 2016, 18, 37–47. [Google Scholar] [CrossRef]
- Weissenrieder, J.S.; Reed, J.L.; Green, M.V.; Moldovan, G.L.; Koubek, E.J.; Neighbors, J.D.; Hohl, R.J. The Dopamine D2 Receptor Contributes to the Spheroid Formation Behavior of U87 Glioblastoma Cells. Pharmacology 2020, 105, 19–27. [Google Scholar] [CrossRef]
- Paacella, S.; Fiorito, J.; Cacciatore, I.; Di Giacomo, V.; Patruno, A.; Rapino, M.; Di Stefano, A.; Marinelli, L.; Fornasari, E.; Cataldi, A.; et al. Effect of MRJF4 on C6 Glioma Cells Proliferation and Migration. Cent. Nerv. Syst. Agents Med. Chem. 2017, 17, 129–134. [Google Scholar] [CrossRef]
- Sozio, P.; Fiorito, J.; Di Giacomo, V.; Di Stefano, A.; Marinelli, L.; Cacciatore, I.; Cataldi, A.; Pacella, S.; Türkez, H.; Parenti, C.; et al. Haloperidol metabolite II prodrug: Asymmetric synthesis and biological evaluation on rat C6 glioma cells. Eur. J. Med. Chem. 2015, 90, 1–9. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, X.; Gao, L.; Liu, X.; Li, J.; Huang, X.; Zeng, T. Synergistic Suppression of Glioblastoma Cell Growth by Combined Application of Temozolomide and Dopamine D2 Receptor Antagonists. World Neurosurg. 2019, 128, e468–e477. [Google Scholar] [CrossRef]
- Roh, K.; Roh, S.; Yang, B.-H.; Lee, J.-S.; Chai, Y.G.; Choi, M.R.; Park, Y.C.; Kim, D.-J.; Kim, D.; Choi, J.; et al. Effects of haloperidol and risperidone on the expression of heat shock protein 70 in MK-801-treated rat C6 glioma cells. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 12, 1793–1797. [Google Scholar] [CrossRef]
- Tyler, M.W.; Zaldivar-Diez, J.; Haggarty, S.J. Classics in Chemical Neuroscience: Haloperidol. ACS Chem. Neurosci. 2017, 15, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 11, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Kastamoulas, M.; Chondrogiannis, G.; Kanavaros, P.; Vartholomatos, G.; Bai, M.; Briasoulis, E.; Arvanitis, D.; Galani, V. Cytokine effects on cell survival and death of A549 lung carcinoma cells. Cytokine 2013, 61, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Chondrogiannis, G.; Kastamoulas, M.; Kanavaros, P.; Vartholomatos, G.; Bai, M.; Baltogiannis, D.; Sofikitis, N.; Arvanitis, D.; Galani, V. Cytokine Effects on Cell Viability and Death of Prostate Carcinoma Cells. BioMed Res. Int. 2014, 2014, 1–16. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Tsamis, K.I.; Vartholomatos, E.; Peponi, E.; Tzima, E.; Tasiou, I.; Lykoudis, E.; Tsekeris, P.; Kyritsis, A.P. Combination treatment of TRAIL, DFMO and radiation for malignant glioma cells. J. Neuro-Oncol. 2015, 123, 217–224. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Lazari, D.; Markopoulos, G.S.; Vartholomatos, E.; Hodaj, E.; Galani, V.; Kyritsis, A.P. Moschamine inhibits proliferation of glioblastoma cells via cell cycle arrest and apoptosis. Tumor Biol. 2017, 39, 1010428317705744. [Google Scholar] [CrossRef]
- Alexiou, G.; Vartholomatos, E.; Tsamis, K.I.; Peponi, E.; Markopoulos, G.; A Papathanasopoulou, V.; Tasiou, I.; Ragos, V.; Tsekeris, P.; Kyritsis, A.; et al. Combination treatment for glioblastoma with temozolomide, DFMO and radiation. J. BUON 2019, 24, 397–404. [Google Scholar]
- Hendouei, N.; Saghafi, F.; Shadfar, F.; Hosseinimehr, S.J. Molecular mechanisms of anti-psychotic drugs for improvement of cancer treatment. Eur. J. Pharmacol. 2019, 5, 172402. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, D.; Liu, Z.; Liu, F. Repurposing psychiatric drugs as anti-cancer agents. Cancer Lett. 2018, 10, 257–265. [Google Scholar] [CrossRef]
- Grinshpoon, A.; Barchana, M.; Ponizovsky, A.; Lipshitz, I.; Nahon, D.; Tal, O.; Weizman, A.; Levav, I. Cancer in schizophrenia: Is the risk higher or lower? Schizophr. Res. 2005, 1, 333–341. [Google Scholar] [CrossRef]
- Barak, Y.; Achiron, A.; Mandel, M.; Mirecki, I.; Aizenberg, D. Reduced cancer incidence among patients with schizophrenia. Cancer 2005, 15, 2817–2821. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, J.; van Rooijen, G.; Doedens, P.; Numminen, E.; van Tricht, M.; de Haan, L. Antipsychotic medication and long-term mortality risk in patients with schizophrenia; a systematic review and meta-analysis. Psychol. Med. 2017, 47, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.C.; Gute, B.D.; Drewes, L.R. Predicting Blood-Brain Transport of Drugs: A Computational Approach. Pharm. Res. 1996, 13, 775–778. [Google Scholar] [CrossRef]
- Zhang, G.; Terry, A.V., Jr.; Bartlett, M.G. Sensitive liquid chromatography/tandem mass spectrometry method for the simultaneous determination of olanzapine, risperidone, 9-hydroxyrisperidone, clozapine, haloperidol and ziprasidone in rat brain tissue. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 15, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Lauth, M.; Rohnalter, V.; Bergström, A.; Kooshesh, M.; Svenningsson, P.; Toftgård, R. Antipsychotic drugs regulate hedgehog signaling by modulation of 7-dehydrocholesterol reductase levels. Mol. Pharmacol. 2010, 78, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, M.; Amata, E.; Vinciguerra, S.; Fiorito, J.; Giurdanella, G.; Drago, F.; Caporarello, N.; Prezzavento, O.; Arena, E.; Salerno, L.; et al. Antiangiogenic Effect of (±)-Haloperidol Metabolite II Valproate Ester [(±)-MRJF22] in Human Microvascular Retinal Endothelial Cells. J. Med. Chem. 2016, 10, 9960–9966. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, G.A.; Goussia, A.; Kyritsis, A.P.; Tsiouris, S.; Ntoulia, A.; Malamou-Mitsi, V.; Voulgaris, S.; Fotopoulos, A.D. Influence of glioma’s multidrug resistance phenotype on (99m)Tc-tetrofosmin uptake. Mol. Imaging Biol. 2011, 13, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Szabó, D.; Szabó, G., Jr.; Ocsovszki, I.; Aszalos, A.; Molnár, J. Anti-psychotic drugs reverse multidrug resistance of tumor cell lines and human AML cells ex-vivo. Cancer Lett. 1999, 3, 115–119. [Google Scholar] [CrossRef]
- Ahmed, M.R.; Gurevich, V.V.; Dalby, K.N.; Benovic, J.L.; Gurevich, E.V. Haloperidol and clozapine differentially affect the expression of arrestins, receptor kinases, and extracellular signal-regulated kinase activation. J. Pharmacol. Exp. Ther. 2008, 325, 276–283. [Google Scholar] [CrossRef]
- Arrillaga-Romany, I.; Chi, A.S.; Allen, J.E.; Oster, W.; Wen, P.Y.; Batchelor, T.T. A phase 2 study of the first imipridone ONC201, a selective DRD2 antagonist for oncology, administered every three weeks in recurrent glioblastoma. Oncotarget 2017, 12, 79298–79304. [Google Scholar] [CrossRef]
- Dorsam, R.T.; Gutkind, J.S. G-protein-coupled receptors and cancer. Nat. Rev. Cancer 2007, 7, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Arana, G.W. An overview of side effects caused by typical antipsychotics. J. Clin. Psychiatry 2000, 61, 5–11. [Google Scholar] [PubMed]
- Zhuo, C.; Xun, Z.; Hou, W.; Ji, F.; Lin, X.; Tian, H.; Zheng, W.; Chen, M.; Liu, C.; Wang, W.; et al. Surprising Anticancer Activities of Psychiatric Medications: Old Drugs Offer New Hope for Patients With Brain Cancer. Front. Pharmacol. 2019, 22, 1262. [Google Scholar] [CrossRef]
- Golden, E.B.; Formenti, S.C.; Schiff, P.B. Taxanes as radiosensitizers. Anticancer Drugs 2014, 25, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Levin, V.; Maor, M.; Thall, P.; Yung, W.; Bruner, J.; Sawaya, R.; Kyritsis, A.; Leeds, N.; Woo, S.; Rodríguez, L.; et al. Phase II study of accelerated fractionation radiation therapy with carboplatin followed by vincristine chemotherapy for the treatment of glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 1995, 30, 357–364. [Google Scholar] [CrossRef]
- Deng, J.; Gao, G.; Wang, L.; Wang, T.; Yu, J.; Zhao, Z. CD24 Expression as a Marker for Predicting Clinical Outcome in Human Gliomas. J. Biomed. Biotechnol. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Naor, D.; Sionov, R.V.; Ish-Shalom, D. CD44: Structure, function and association with the malignant process. Adv. Cancer Res. 1997, 71, 241–319. [Google Scholar]
- Knüpfer, M.M.; Hernáiz-Driever, P.; Poppenborg, H.; Wolff, J.E.; Cinatl, J. Valproic acid inhibits proliferation and changes expression of CD44 and CD56 of malignant glioma cells in vitro. Anticancer Res. 1998, 18, 3585. [Google Scholar]
- Zhang, W.; Zhang, C.; Liu, F.; Mao, Y.; Xu, W.; Fan, T.; Sun, Q.; He, S.; Chen, Y.; Guo, W.; et al. Antiproliferative activities of the second-generation antipsychotic drug sertindole against breast cancers with a potential application for treatment of breast-to-brain metastases. Sci. Rep. 2018, 25, 15753. [Google Scholar] [CrossRef]
- Pulkoski-Gross, A.; Li, J.; Zheng, C.; Li, Y.; Ouyang, N.; Rigas, B.; Zucker, S.; Cao, J. Repurposing the antipsychotic trifluoperazine as an antimetastasis agent. Mol. Pharmacol. 2015, 87, 501–512. [Google Scholar] [CrossRef]
- Volavka, J.; Cooper, T.; Czobor, P.; Bitter, I.; Meisner, M.; Laska, E.M.; Gastanaga, P.; Krakowski, M.; Chou, J.C.-Y.; Crowner, M.; et al. Haloperidol Blood Levels and Clinical Effects. Arch. Gen. Psychiatry 1992, 49, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Falvo, P.; Orecchioni, S.; Roma, S.; Raveane, A.; Bertolini, F. Drug repurposing in Oncology, an attractive opportunity for novel combinatorial regimens. Curr. Med. Chem. 2020, 27, 1–20. [Google Scholar] [CrossRef] [PubMed]
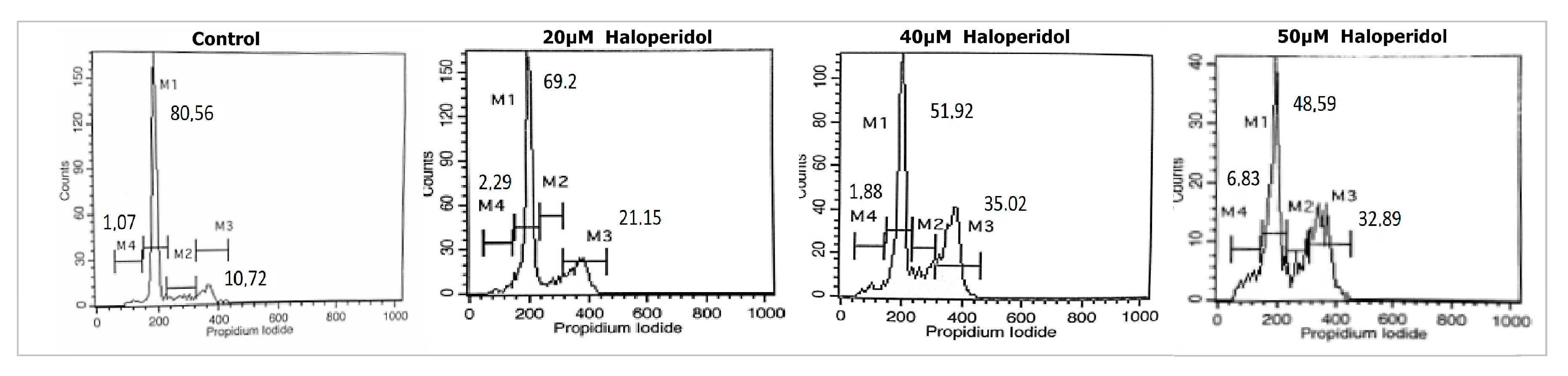


| Treatment | SubG0 (M4) | G0/G1 (M1) | S (M2) | G2/M (M3) |
|---|---|---|---|---|
| Control | 1.07 ± 0.4 | 80.56 ± 4.3 | 8.17 ± 2.6 | 10.72 ± 2.2 |
| 20 μM haloperidol | 1.88 ± 0.5 | 69.2 ± 3.1 | 7.37 ± 1.2 | 21.15 ± 4.7 |
| 40 μM haloperidol | 2.29 ± 0.8 | 51.92 ± 3.5 | 10.73 ± 3.1 | 35.02 ± 2.5 |
| 50 μM haloperidol | 6.83 ± 1.2 | 48.59 ± 4.2 | 12.54 ± 2.9 | 32.89 ± 5.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulos, F.; Isihou, R.; Alexiou, G.A.; Tsalios, T.; Vartholomatos, E.; Markopoulos, G.S.; Sioka, C.; Tsekeris, P.; Kyritsis, A.P.; Galani, V. Haloperidol Induced Cell Cycle Arrest and Apoptosis in Glioblastoma Cells. Biomedicines 2020, 8, 595. https://doi.org/10.3390/biomedicines8120595
Papadopoulos F, Isihou R, Alexiou GA, Tsalios T, Vartholomatos E, Markopoulos GS, Sioka C, Tsekeris P, Kyritsis AP, Galani V. Haloperidol Induced Cell Cycle Arrest and Apoptosis in Glioblastoma Cells. Biomedicines. 2020; 8(12):595. https://doi.org/10.3390/biomedicines8120595
Chicago/Turabian StylePapadopoulos, Fotios, Rafaela Isihou, George A. Alexiou, Thomas Tsalios, Evrysthenis Vartholomatos, Georgios S. Markopoulos, Chrissa Sioka, Pericles Tsekeris, Athanasios P. Kyritsis, and Vasiliki Galani. 2020. "Haloperidol Induced Cell Cycle Arrest and Apoptosis in Glioblastoma Cells" Biomedicines 8, no. 12: 595. https://doi.org/10.3390/biomedicines8120595
APA StylePapadopoulos, F., Isihou, R., Alexiou, G. A., Tsalios, T., Vartholomatos, E., Markopoulos, G. S., Sioka, C., Tsekeris, P., Kyritsis, A. P., & Galani, V. (2020). Haloperidol Induced Cell Cycle Arrest and Apoptosis in Glioblastoma Cells. Biomedicines, 8(12), 595. https://doi.org/10.3390/biomedicines8120595








