Abstract
Dysregulation of glucose and lipid metabolism increases plasma levels of lipoproteins and triglycerides, resulting in vascular endothelial damage. Remarkably, the oxidation of lipid and lipoprotein particles generates electronegative lipoproteins that mediate cellular deterioration of atherosclerosis. In this review, we examined the core of atherosclerotic plaque, which is enriched by byproducts of lipid metabolism and lipoproteins, such as oxidized low-density lipoproteins (oxLDL) and electronegative subfraction of LDL (LDL(−)). We also summarized the chemical properties, receptors, and molecular mechanisms of LDL(−). In combination with other well-known markers of inflammation, namely metabolic diseases, we concluded that LDL(−) can be used as a novel prognostic tool for these lipid disorders. In addition, through understanding the underlying pathophysiological molecular routes for endothelial dysfunction and inflammation, we may reassess current therapeutics and might gain a new direction to treat atherosclerotic cardiovascular diseases, mainly targeting LDL(−) clearance.
1. Introduction
Approximately 1.9 billion people are obese or overweight worldwide [1]. Obesity is associated with excessive calorific intake and microvasculature damage, resulting in atherosclerosis, diabetes, and cardiovascular diseases (CVDs) [2]. The prevalence of CVDs has significantly increased in the past few decades [3]. Current strategies against CVDs mainly focus on lowering the level of low-density lipoprotein cholesterol (LDL-C) [4,5]. Intensive-dose statin therapy has been endorsed for clinical atherosclerotic vascular disease (ASCVD); however, it also increases statin-related side effects and intolerance [6,7]. To figure out this dilemma and find a balanced solution, here we address the mechanistic players behind these metabolic disturbances through the following disease progression steps: unhealthy lifestyle and unbalanced diet lead to obesity, chronic inflammation, and development of atherosclerosis and CVDs [8,9,10].
The onset of atherosclerosis initiates vascular lipid deposition, luminal narrowing, and plaque expansion. Unstable plaque deposits further lead to myocardial infarction and stroke [11]. Plaque consists of LDL-C variants, lipids, leukocytes, and inflammasomes in the vascular walls (Figure 1) [11,12]. In addition, several mediators of vasoconstriction, platelet aggregation, inflammatory chemokines, leukocyte adherence, and nitric oxide (NO) disturb the endothelial homeostasis [13]. LDL variants such as oxidized LDL (oxLDL) are essential constituents in the pathogenesis of atherosclerosis and CVDs [14,15,16]. Differing from the in vitro preparation of oxLDL, electronegative LDL (LDL(−)) is separated from human plasma using fast-protein liquid chromatography equipped with an anion exchange column [17]. According to the physical properties of LDL(−), it can be defined as the minimized oxLDL [18,19].
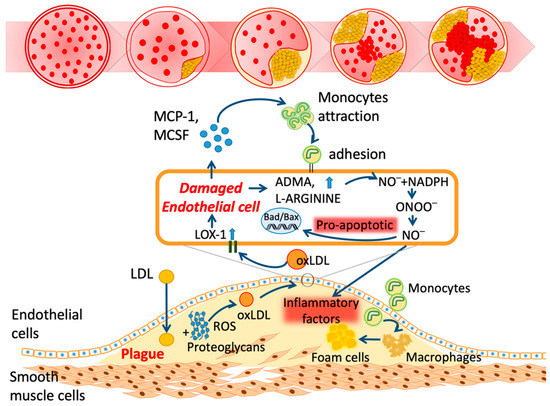
Figure 1.
Schematic mechanism of atherosclerosis. LDL: low-density lipoprotein; ROS: reactive oxygen species; oxLDL: oxidized LDL; LOX-1: lectin-like oxidized LDL receptor-1; ADMA: asymmetric dimethylarginine; NO: nitric oxide; NADPH: nicotinamide adenine dinucleotide phosphate; ONOO: peroxynitrite; Bad: BCL2-associated agonist of cell death; Bax: Bcl-2-associated X protein; MCP-1: monocyte chemoattractant protein-1; MCSF: macrophage colony-stimulating factor.
Accumulating evidence shows that LDL(−) could be a novel marker for ASCVD, and levels of LDL(−) are positively correlated with the increasing severity of CVDs [20,21,22]. LDL(−) serves as a pivotal target for further studies and clinical development strategies beyond statins therapies. By targeting LDL(−), we summarize its pathophysiological links and highlight the molecular mechanisms of atherogenic lipids in the current review.
2. Properties of Electronegative Low-Density Lipoprotein (LDL(−))
2.1. Chemical Properties of LDL(−)
LDL(−) differs from LDL(+) in many aspects [23]. Regarding the lipid components, LDL(−) contains higher concentrations of triglycerides, non-esterified fatty acids (NEFA), lysophosphatidylcholine (LPC), platelet-activating factor (PAF), and ceramide [24,25,26,27]. Notably, lipid extracts of LDL(−) contribute to the atherogenic effects on endothelial cells and immune cells [27,28]. Regarding its protein composition, LDL(−) shows additional proteins such as apolipoprotein AI (apoAI), apolipoprotein E (apoE), and apolipoprotein CIII (apoCIII) [29]. Furthermore, the conformation of apoB100 in LDL(−) is altered and has higher competency to bind with proteoglycans [30,31,32]. Based on the sodium chloride gradient, Chen et al. successfully divided LDL into five subfractions, L1–L5, with increasing electronegativity [29,33,34]. L1 LDL is unmodified; in contrast, L5 LDL is highly O-glycosylated on the apoB100 and apoE [28,35]. The terminal glycan of apoE glycosylation (94S, 194T, 289T) in L5 LDL is sialic acid. This sialic-acid-containing glycan increases the electronegativity and hydrophilicity [35]. However, by dividing human plasma LDL into either two subfractions ((+) and (−)) or five (L1–L5), the most electronegative subfractions show similar properties and apoptotic effects on endothelial cells. Thus, we will be using LDL(−) throughout this review.
2.2. Receptors of LDL(−)
LDL(−) is not recognized by the LDL receptor. Instead, it goes through lectin-like oxLDL receptor-1 (LOX-1), which is highly expressed in endothelial cells, immune cells, platelets, and adipocytes [36,37,38,39]. Transfection with LOX-1-specific small interfering RNAs (siLOX-1) to endothelial cells may attenuate LDL(−)-induced downstream signaling [36]. LOX-1-neutralizing antibodies such as TS20 (for bovine) [40], TS58 (for mouse) [41], and TS92 (for human) [42,43] can inhibit the internalization of LDL(−). Genetic knockout LOX-1 also protects against the harmful effects of LDL(−) [37,38]. Higher content of PAF on LDL(−) activates the PAF receptor (PAFR) and leads to endothelial cell apoptosis [33]. Incubating PAF acetylhydrolase (PAF-AH) with LDL(−) or pretreatment of WEB-2086 attenuates LDL(−)-induced apoptosis [33]. In addition, ceramide-rich LDL(−) activates toll-like receptor 4 (TLR4) and the cluster of differentiation 14 (CD14) on monocytes that results in cytokine release. Using the TLR4 inhibitor, the viral inhibitory peptide of TLR4 (VIPER), reduces these effects [44,45].
2.3. Structure Modifications and Enzymatic Functions of Electronegative LDL
Electronegativity and apolipoprotein misfolding are two independent features of LDL(−) [46]. The misfolded apoB100 of LDL(−) shows an increased binding affinity to proteoglycans, which may prolong LDL retention in the arterial wall and trigger inflammatory responses [31]. Stabilizing the LDL’s structure through the use of 17-β-estradiol (E2) prevents aggregation; however, it cannot prevent the generation of LDL(−) [46,47]. The structural modifications of apoB100 are associated with phospholipolytic activities and exchange of lipid components [28,48,49]. The sphingomyelinase (SMase)-like activity of LDL(−) may hydrolyze sphingomyelin, which produces apoptotic factor, a ceramide [28,48]. The phospholipase D (PLD) activity of LDL(−) degrades phosphorylcholine, LPC, and sphingomyelin, which is associated with self-aggregation and atherogenic properties. Treatment with 400 μM of chlorpromazine may effectively inhibit both the SMase and PLD activities of LDL(−) [48].
2.4. Animal Models Showing Elevated Electronegative LDL
The overproduction of LDL(−) was demonstrated in animal models that consumed a high-fat diet. Lai et al. gave either a standard chow diet or high-fat & high-cholesterol (HFC) diet to each group of 8-week-old male golden Syrian hamsters for six weeks. Plasma LDL-C levels in HFC-diet-fed hamsters were significantly higher than for the control group. Additionally, LDL(−) accounted for 12.5% of all lipoproteins in control hamsters, whereas the value was drastically increased to 42% in HFC-diet-fed hamsters [50]. Recently, Chang et al. distributed an atherogenic diet to sixteen-week-old male New Zealand White rabbits. After six weeks, the LDL(−) from HFC-diet-fed rabbits accounted for about 17.2 ± 5.5% of the LDL fraction. On the other hand, it was almost undetectable in rabbits fed with a control chow diet [51]. Moreover, from the recent publication by Chan et al., LDL(+) and LDL(−) isolated from SLE patients’ LDL samples were then injected into eight-week-old apoE knockout mice. Their results showed that only the LDL(−)-injected mice experienced a significant increase in the plasma CX3CL1 level. By observing histological staining results, LDL(−) can trigger endothelial dysfunction and the formation of atherosclerotic lesions in apoE knockout mice [27]. Taken together, we summarized that LDL(−) plays a vital role in atherosclerosis and plaque formation.
3. Mechanisms of Electronegative LDL on Endothelial Cells
The endothelium regulates fluid and molecule trafficking between the bloodstream and tissues for metabolism [52]. In addition, it inhibits platelet aggregation and adhesions by secreting prostacyclin, NO, and exosomes [53,54]. With LDL(−), the atherogenic components lead to endothelial activation and vascular inflammation. Chemokines such as monocyte chemotactic protein-1 (MCP-1) and interleukin-8 (IL-8) are released from the damaged endothelium. The vascular adhesion molecules are highly expressed to promote plaque formation [55]. The mechanisms behind this are listed below.
3.1. Phosphatidylinositol-3 Kinase (PI3K)-Serine/Threonine Kinase (Akt) Signaling
The phosphatidylinositol-3 kinase (PI3K)-serine/threonine kinase (Akt) signaling involves the proliferation and survival of endothelial cells through inhibiting pro-apoptotic proteins [56]. Both fibroblast growth factor 2 (FGF2) and vascular endothelial growth factor (VEGF) activate PI3K/Akt signaling [57,58]; in contrast, LDL(−) disrupts Akt phosphorylation, impairing the FGF2 mRNA expression, as well as induces endothelial cell apoptosis [40,59]. In their study, Lu et al. also demonstrated that the apoptotic effects of LDL(−) on endothelial cells could be attenuated by treatment with FGF2 or constitutively expressing active Akt [59]. LDL(−) inhibits B-cell lymphoma 2 (Bcl-2); in contrast, it triggers the expression of Bad/Bax (Bcl-2-associated agonist cell death) and inflammatory factor tumor necrosis factor-α (TNF-α). These actions result in the release of cytochrome c from mitochondria [36,59].
3.2. Lectin-Like oxLDL Receptor-1 (LOX-1) Signaling
Lectin-like oxLDL receptor-1 (LOX-1) reacts with multiple ligands in response to danger signals [60]. Patients with cerebral stroke and coronary artery diseases exhibited elevated levels of soluble-form LOX-1 (sLOX-1) [61,62]. Furthermore, patients with ST segment elevation myocardial infarction (STEMI) and rheumatoid arthritis (RA) showed increased sLOX-1 expression in the aspirated coronary thrombi [63,64]. Due to earlier release than biochemical markers of myocardial injury, sLOX-1 could be a novel biomarker for plaque instability [65]. In a hypercholesteremic mice model, the LOX-1 knockout reduced the plaque size and atherosclerotic lesions [66,67,68].
For the detailed mechanisms, LDL(−) leads to the overexpressed changes of LOX-1 on endothelial cells by inducing the expression changes of the pro-inflammatory molecules nuclear factor of kappa light polypeptide gene enhancer in B-cells (NF-κB), vascular cell adhesion molecule (1VCAM-1), and MCP-1 [69,70]. Recently, a similar cohort study was completed to show similar results of LOX-1-mediated inflammation in SLE patients [71]. In addition, the expression of LOX-1 dependents on vasoconstrictors (angiotensin II, endothelin-1) and inflammatory factors such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and IL-1β was observed [72]. In vitro, oxidized LDL may enhance the production of angiotensin-converting enzyme (ACE) and endothelin-1 [73,74].
Through LOX-1, LDL(−) downregulates the phosphorylation of Akt and endothelial nitric oxide synthase (eNOS) but increases C-reactive protein (CRP) [11,36,42]. LOX-1 activates Ras homolog family member A (RhoA) and the Ras-related C3 botulinum toxin substrate 1 (Rac1) pathway, leading to the inhibition of intracellular endothelial NO synthesis and overproduction of ROS [75]. Recently, NOS was reported to influence miR-122 expression in hypertension cases, leading to endothelial dysfunction; however, the expression changes of miR-122-mediating endothelial dysfunction remains unanswered. We, therefore, predict LOX-1 signaling of LDL(−) in such cases [76]. Similarly, ROS overproduction leads to p66shc protein phosphorylation, which further deteriorates mitochondrial DNA and contributes to plaque formation [77,78,79]. The phenomenon mentioned above can be attenuated by knocking out the LOX-1 gene [80,81].
3.3. Mitochondria Damage
The basal physiological mechanism of mitochondrial ROS formation is dependent on several factors such as NO, cytosolic Ca2+, and fatty acids [82]. NADPH oxidase 4 (NOX4) in vascular cells inhibits mitochondrial complex I and promotes ROS generation [83]. During the pro-apoptotic conditions, ROS formation is also boosted by growth factor adaptor protein p66Shc, which facilitates the cytochrome c oxidation. Moreover, ROS formation can be further increased by the expression and activation of p66Shc during hyperglycemic conditions [84,85]. LDL(−) inhibits endothelial nitric oxide synthase (eNOS) expression via the Akt signaling pathway, resulting in decreased NO production and leading to endothelial cell apoptosis [86]. Recently, Chen et al. demonstrated that apoE in LDL(−) is responsible for LDL-induced mitochondrial dysfunction. After LDL(−) internalization, apoE translocates from the lysosome to the mitochondria, leading to mitochondrial permeability transition pore (mPTP) opening, dynamin-related protein 1 (DRP1) phosphorylation, and mitochondrial fission [41].
3.4. Endoplasmic Reticulum Stress
The intraluminal oxidation in the endoplasmic reticulum (ER) plays a critical role in maintaining calcium concentration and proper folding of transmembrane proteins. The increased amount of lipoprotein promotes a condition known as ER stress, defined by the accumulation of unfolded protein in the ER lumen [87,88]. The molecular mechanism between LDL oxidation and UPR (unfolded protein response)-mediated expression of IL-8, IL-6, and MCP-1 in endothelial cells, which contributes to endothelial dysfunction, is poorly explained [89,90]. Apart from oxidation, glycation of LDL is also found to be a potent marker for dyslipidemia. Studies showed that glycated LDL could initiate nicotinamide adenine dinucleotide phosphate (NADPH) oxidation via ROS production and could induce apoptosis in endothelial cells [91,92]. Therefore, the LDL oxidation and glycation are involved in amplifying endothelial dysfunction and contributing to atherosclerosis.
4. Mechanisms of Electronegative LDL on Immune Cells
Alongside endothelial cells, immune cells play a significant role in the pathogenesis of atherosclerosis. Monocytes and T lymphocytes create an inflammatory milieu by releasing several cytokines and growth factors. As LDL(−) concentration is elevated in the blood plasma, it tend to interacts with these monocytes and lymphocytes via cytokines and growth factors [93,94]. LDL(−) impregnates the process of oxidation via the feedback loop mechanism shown in Figure 2 and enhances inflammation. The NEFA and ceramide in LDL(−) also show atherogenic properties [93,95,96,97]. The detailed mechanisms behind this are listed below.
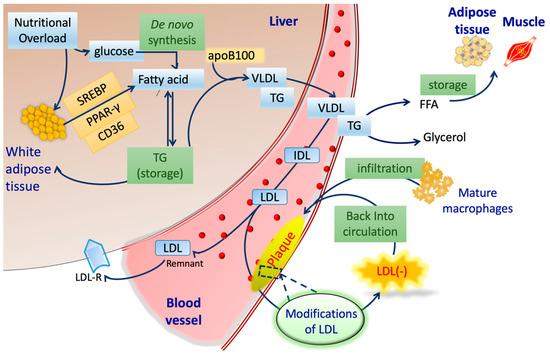
Figure 2.
Schematic procedures of lipoprotein metabolism and LDL(−) formation. SREBP: sterol regulatory element-binding protein; PPAR-γ: peroxisome proliferator-activated receptor; CD36: cluster of differentiation 36; TG: triglycerides; apoB100: apolipoprotein B100; VLDL: very low-density lipoprotein; IDL: intermediate-density lipoprotein; LDL: low-density lipoprotein; LDLR: LDL receptor; FFA: free fatty acid.
4.1. Monocytes
Numerous studies have described the effects of LDL(−) on inducing cytokine release from monocytes, which may be important in atherosclerosis [25,98]. Remodeling of the vascular extracellular matrix (ECM) seemed to be an important landmark of atherosclerosis. LDL(−) induces the release of matrix metalloproteinase (MMP)-9 and tissue inhibitors of metalloproteinase (TIMP)-1 from monocytes through the TLR4/CD14 inflammatory pathway [45]. Additionally, the downstream signal cascade of TLR4/CD14 will then trigger PI3K/Akt signaling and promote p38 mitogen-activated protein kinase (p38 MAPK) phosphorylation, leading to LDL(−)-induced cytokine release from monocytes [99]. The elevated levels of those cytokines may regulate and contribute to vascular plaque formation.
4.2. Macrophages
Macrophages play a crucial role in the early stage pathogenesis of atherosclerosis [100]. Circulating monocytes undergo differentiation into macrophages and further polarization into classically activated (M1) or alternatively activated (M2) states in order to withstand environmental stimuli. M1 macrophages are responsible for pro-inflammatory properties, whereas M2 macrophages exert opposing anti-inflammatory properties [101].
According to Yang et al., LDL(+) and LDL(−) isolated from patients with ST segment elevation myocardial infarction (STEMI) were treated with THP-1 macrophages. Their results indicated that only LDL(−) could induce the overproduction of interleukin (IL)-1β [102], granulocyte colony-stimulating factor (G-CSF), and granulocyte–macrophage colony-stimulating factor (GM-CSF) in macrophages through LOX-1-, extracellular signal-regulated kinase (ERK)1/2-, and NF-κB-dependent pathways. Inhibition of ERK1/2 and NF-κB activation can prevent G-CSF and GM-CSF production induced by LDL(−) [103].
In 2020, Chang et al. treated THP-1 with LDL(−), which resulted in increased pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, and TNF-α, as well as M1 surface marker CD86; however, M2-related cytokines and surface marker CD206 were not changed by LDL(−) [39]. Additionally, the expression of CD11c, a marker of M1 macrophages, can also be induced by LDL(−) [104]. LDL(−) can induce M1 polarization of human macrophages responsible for secreting pro-inflammatory cytokines, resulting in foam cell formation and vascular plaque formation.
In addition to human macrophages, in treating LDL(+) and LDL(−) with RAW264.7 cell, the results showed that only LDL(−) can induce the expression of CD95 death receptor (Fas), its ligand CD95 L (FasL), and tumor necrosis factor ligand member 10 (Tnfsf10), which stimulate the activation of the caspases, resulting in cell apoptosis [105].
4.3. Platelets
Apart from monocytes and macrophages, accumulating evidence has shown that LDL(−) may trigger platelet activation and aggregation. Platelet hyperreactivity is the most direct evidence contributing to thrombosis in the leading causes of cardiovascular diseases, such as STEMI [106] and stroke [43,107]. As above, Chan et al. separated LDL(+) and LDL(−) from patients with STEMI, with the results illustrating that only LDL(−) was augmented in patients compared to healthy controls. Treating LDL(−) to platelets enhanced their aggregation and adhesion to damaged human aortic endothelial cells (HAECs), which was through LOX-1 and PAFR activation [37]. Furthermore, LDL(−)-induced amyloid β (Aβ) release via IκB kinase 2 (IKK2) in human platelets was reported by Shen et al. in 2016. Besides, LDL(−) works synergistically with Aβ to induce glycoprotein IIb/IIIa receptor activation and phosphorylation of IKK2, IkBa, p65, and c-Jun N-terminal kinase 1 in order to enhance platelet aggregation. These results can be attenuated by inhibiting IKK2, LOX-1, or NF-kB with their inhibitors BMS-345541, TS92, and Bay 117-82, respectively [43]. To conclude, high levels of LDL(−) in patients can trigger platelet activation and aggregation through LOX-1 and PAFR receptors.
5. Electronegative LDL in Vascular Diseases
Figure 2 demonstrates the lipid and lipoprotein metabolism in the liver, blood, and peripheral tissues. Nutritional overload increases fatty acids via the overexpression of cluster of differentiation 36 (CD36) and peroxisome proliferator-activated receptor (PPAR-γ) [108,109,110]. This phenomenon is highly contrasted to the de novo synthesis pathway, although FFAs from either source in the liver are indistinguishable. The elevated level of free fatty acids ultimately increases triglyceride through esterification. Combined with apoB100 and triglyceride, the efflux of VLDL into circulation promotes the pro-atherogenic metabolic state. VLDL particles deliver lipids hydrolyzed by lipoprotein lipase (LPL) and release FFAs in plasma [111,112,113].
With the increasing incidence of LDL retention in endothelial cells [114,115,116,117,118], the LDL particles reportedly undergo oxidative modifications by macrophages and endothelial cells within arterial walls (Figure 2) [119,120,121,122,123,124]. The accumulation of oxLDL further boosts the electronegativity, ultimately generating LDL(−) in circulation [33]. LDL(−) is highly atherogenic and pro-apoptotic to the vascular system, including the endothelium of the blood–brain barrier (BBB). Wang et al. in 2017 explored the role of LDL(−) in pheochromocytoma-derived cell line (PC12) cells, where deliberate dosages of LDL(−) induced neurotoxic stress in a LOX-1-dependent manner [125].
The presence of LDL(−) in circulation correlates with atherosclerosis progression and endothelial dysfunction-mediated cardiovascular diseases. LDL(−) levels are significantly higher in frequent smokers, diabetic patients, and hypercholesterolemia patients [33,34,40,59]. In addition, LDL(−) levels were 10-times higher in STEMI and stroke patients, even though the LDL-C levels were similar to healthy controls [37,43].
6. Current Treatment Strategies Targeting Electronegative LDL
The diagnosis and treatment for endothelial damage are dependent on the ankle–brachial index, vascular imaging, surgery, and revascularization [126,127,128]. Currently, treatment for dyslipidemia and the prevention of microvasculature damage mainly revolve around reducing LDL-C levels [129,130,131]. A plethora of studies have demonstrated that excessive levels of lipids lead to endothelial damage; however, only a few studies have outlined strong mechanistic interactions between lipid alterations and endothelial dysfunction (Table 1).

Table 1.
Primary dyslipidemia markers and pathways involved in different diseases.
Statins, the inhibitors of β-hydroxy β-methylglutaryl-CoA (HMG-CoA), are successful in lowering cholesterol loadings and expression of LOX-1; they also inhibit atherosclerotic progression and acute atherothrombosis [162,163,164]. Additionally, statins effectively reduce the proportion of LDL(−) [165,166,167,168]; discontinuation leads to LDL(−) approaching baseline levels [42]. However, the mechanisms of LDL(−) reduction are still not clear. Ezetimibe inhibits the Niemann–Pick C1-like 1 transporter (NPC1L1), which leads to decreased cholesterol absorption [169]. Proprotein convertase subtilisin kexin type 9 (PCSK9) is an enzyme for the degradation of LDL receptor (LDLR); blocking PCSK9 may increase LDLR, therefore lowering blood LDL-C concentrations. PCSK9 inhibitors such as alirocumab and evolocumab aggressively reduce the degradation of LDL receptors and increase the clearance of LDL cholesterol in hepatic cells [170]. They increase plaque stability but decrease the necrotic lipid core, as shown in Figure 1 [171,172,173,174,175]. However, other than statins, whether these drugs can decrease LDL(−) or not is currently unclear.
Several anti-inflammatory approaches were taken here to study the management of dyslipidemia, such as cell therapy using mesenchymal stem cells [176], leukotriene inhibitors [177], chemokine ligands (CC motif ligand), MCP-1, IL-1, and TNF-α blockers for the prevention of atherosclerotic plaque formation [178,179,180,181,182,183,184]. The currently used drugs significantly decrease LDL-C levels, stabilize vascular plaque, and slowdown atherosclerotic progression; however, new therapeutic strategies for LDL(−) and biomarkers are still needed.
7. Perspective
LDL(−) plays a critical role in the pathophysiology of atherogenesis. It triggers the dysfunction of endothelium by macrophage differentiation, monocyte migration, and platelet aggregation. Moreover, LDL(−) impairs endothelial cells by superoxide overproduction and platelet activation [185,186,187]. In combination with other well-known markers of inflammation, namely metabolic diseases, we concluded that LDL(−) can be a novel prognostic tool for these lipid disorders. Regarding treatment for the prevention of ASCVD, even though statins can partially reduce the concentration, finding a way to clear LDL(−) remains of utmost importance [22]. In particular, a method involving hydrolyzing atherogenic lipids in LDL(−) and producing harmless metabolites might be a novel therapeutic approach in the future.
Author Contributions
Conceptualization, C.-S.C.; validation, L.-Y.K.; resources, Y.-H.L.; data curation, H.-C.C.; writing—original draft preparation, S.H.L., V.K.M., F.P.; writing—review and editing, S.H.L., L.-Y.K.; visualization, F.P., H.-C.C.; supervision, C.-S.C., Y.-H.L.; project administration, L.-Y.K.; funding acquisition, C.-S.C., H.-C.C., L.-Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by grants from the Kaohsiung Medical University (KMU-TC108A03-0), Kaohsiung Medical University Hospital (KMUH-M109017), Kaohsiung Municipal Ta-Tung Hospital (kmtth-101-001), Taiwan Ministry of Science and Technology (MOST109-2320-B-037-028-, 109-2628-B-037-010).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Aβ | Amyloid β |
| ACE | Angiotensin-converting enzyme |
| ADP | Adenosine diphosphate |
| ADPase | Ecto-Adenosine diphosphate |
| ApoB100 | Apolipoprotein B100 |
| ApoCIII | Apolipoprotein CIII |
| ApoE | Apolipoprotein E |
| ASCVD | Atherosclerotic cardiovascular diseases |
| Bad/Bax | BCL2-associated agonist of cell death |
| Bcl-2 | B-cell lymphoma 2 |
| BBB | Blood–brain barrier |
| BP | Blood pressure |
| CAD | Coronary artery disease |
| CCL | Chemokine ligand |
| CD | Cluster of differentiation |
| CER | Ceramide |
| cIMTPWV | Carotid intermedia thickness and pulse wave velocity |
| COX | Cyclooxygenase |
| CD36 | Cluster of differentiation 36 |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| EC | Endothelial cell |
| ECM | Extracellular matrix |
| ED | Endothelial dysfunction |
| ERK | Extracellular signal-regulated kinase |
| eNOS | Endothelial nitric oxide synthase |
| ER | Endoplasmic reticulum |
| Fas | CD95 death receptor |
| FasL | Ligand CD95 L |
| FFA | Free fatty acids |
| FGF2 | Fibroblast growth factor 2 |
| FPLC | Fast-protein liquid chromatography |
| G-CSF | Granulocyte colony-stimulating factor |
| GDF | Growth differentiation factor |
| GM-CSF | Granulocyte–macrophage colony-stimulating factor |
| HDL | High-density lipoprotein |
| HFC | High-fat, high-cholesterol |
| HIF-1α | Hypoxia-inducible factor-1α |
| HMGCoA | β-hydroxy β-methylglutaryl-CoA |
| HUVECs | Human umbilical vein endothelial cells |
| ICAM | Intracellular adhesion molecule 1 |
| IDL | Intermediate-density lipoprotein |
| IFN-γ | Interferon- γ |
| IKK2 | IκB kinase 2 |
| IL | Interleukin |
| iNOS | Inducible NO synthase |
| IR | Insulin resistance |
| IRAK2 | Interleukin-1 receptor-associated kinase-2 |
| IRE-1 | Inositol requiring enzyme-1 |
| Lp(a) | Lipoprotein (a) |
| LDL | Low-density lipoprotein |
| LDL(−) | Electronegative LDL |
| LDL-C | LDL cholesterol |
| LPC | Lysophosphatidylcholine |
| LPL | Lipoprotein lipase |
| LOX-1 | Lectin-like oxidized low-density lipoprotein receptor-1 |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemotactic protein-1 |
| MetS | Metabolic syndrome |
| MMP | Metalloproteinase |
| MSC | Mesenchymal stem cell |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NEFA | Non-esterified fatty acids |
| NF-κB | Nuclear factor kappa light-chain enhancer of activated B cells |
| NO | Nitric oxide |
| Nox | NADPH oxidase |
| NPC1L1 | Niemann–pick C1-like |
| oxLDL | Oxidized LDL |
| PAFR | Platelet activating factor |
| PAFR | Platelet activating factor receptor |
| PC12 | Pheochromocytoma cell-derived cell line |
| PGI2 | Prostacyclin 2 |
| PI3K | Phosphatidylinositol-3 kinase |
| PLD | Phospholipase D |
| PSCK9 | Proprotein convertase subtilisin kexin type 9 |
| RhoA | Ras homology family member A |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| Smase | Sphingomyelinase |
| STEMI | ST segment elevation myocardial infarction |
| TGF-β | Transforming growth factor- β |
| TIMP | Tissue inhibitors of metalloproteinase |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor-α |
| Tnfsf10 | Tumor necrosis factor ligand, member 10 |
| UCP 2 | Uncoupling protein 2 |
| UPR | Unfolded protein response |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VEGF | Vascular endothelial growth factor |
| VIPER | Viral inhibitory peptide of TLR4 |
| VLDL | Very low-density lipoprotein |
| VSMCs | Vascular smooth muscle cells |
References
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Hinton, W.; McGovern, A.; Coyle, R.; Han, T.S.; Sharma, P.; Correa, A.; Ferreira, F.; de Lusignan, S. Incidence and prevalence of cardiovascular disease in English primary care: A cross-sectional and follow-up study of the Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC). BMJ Open 2018, 8, e020282. [Google Scholar] [CrossRef]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S49–S73. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Friera, L.; Fuster, V.; Lopez-Melgar, B.; Oliva, B.; Garcia-Ruiz, J.M.; Mendiguren, J.; Bueno, H.; Pocock, S.; Ibanez, B.; Fernandez-Ortiz, A.; et al. Normal LDL-Cholesterol Levels Are Associated With Subclinical Atherosclerosis in the Absence of Risk Factors. J. Am. Coll. Cardiol. 2017, 70, 2979–2991. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.P.; Patti, A.M.; Giglio, R.V.; Nikolic, D.; Castellino, G.; Rizzo, M.; Banach, M. Management of Statin Intolerance in 2018: Still More Questions Than Answers. Am. J. Cardiovasc. Drugs 2018, 18, 157–173. [Google Scholar] [CrossRef]
- Schwandt, P.; Liepold, E.; Bertsch, T.; Haas, G.M. Lifestyle, Cardiovascular Drugs and Risk Factors in Younger and Elder Adults: The PEP Family Heart Study. Int. J. Prev. Med. 2010, 1, 56–61. [Google Scholar]
- Yasue, H.; Hirai, N.; Mizuno, Y.; Harada, E.; Itoh, T.; Yoshimura, M.; Kugiyama, K.; Ogawa, H. Low-grade inflammation, thrombogenicity, and atherogenic lipid profile in cigarette smokers. Circ. J. 2006, 70, 8–13. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Stancel, N.; Chen, C.C.; Ke, L.Y.; Chu, C.S.; Lu, J.; Sawamura, T.; Chen, C.H. Interplay between CRP, Atherogenic LDL, and LOX-1 and Its Potential Role in the Pathogenesis of Atherosclerosis. Clin. Chem. 2016, 62, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Mudau, M.; Genis, A.; Lochner, A.; Strijdom, H. Endothelial dysfunction: The early predictor of atherosclerosis. Cardiovasc. J. Afr. 2012, 23, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Carmena, R.; Duriez, P.; Fruchart, J.C. Atherogenic lipoprotein particles in atherosclerosis. Circulation 2004, 109, III-2–III-7. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Witztum, J.L.; Steinberg, D. The oxidative modification hypothesis of atherosclerosis: Does it hold for humans? Trends Cardiovasc. Med. 2001, 11, 93–102. [Google Scholar] [CrossRef]
- Estruch, M.; Sanchez-Quesada, J.L.; Ordonez Llanos, J.; Benitez, S. Electronegative LDL: A circulating modified LDL with a role in inflammation. Mediators Inflamm. 2013, 2013, 181324. [Google Scholar] [CrossRef]
- Nyyssonen, K.; Kaikkonen, J.; Salonen, J.T. Characterization and determinants of an electronegatively charged low-density lipoprotein in human plasma. Scand. J. Clin. Lab. Investig. 1996, 56, 681–689. [Google Scholar] [CrossRef]
- Barros, M.R.; Bertolami, M.C.; Abdalla, D.S.; Ferreira, W.P. Identification of mildly oxidized low-density lipoprotein (electronegative LDL) and its auto-antibodies IgG in children and adolescents hypercholesterolemic offsprings. Atherosclerosis 2006, 184, 103–107. [Google Scholar] [CrossRef]
- Ivanova, E.A.; Bobryshev, Y.V.; Orekhov, A.N. LDL electronegativity index: A potential novel index for predicting cardiovascular disease. Vasc. Health Risk Manag. 2015, 11, 525–532. [Google Scholar] [CrossRef][Green Version]
- Chu, C.S.; Chan, H.C.; Tsai, M.H.; Stancel, N.; Lee, H.C.; Cheng, K.H.; Tung, Y.C.; Chan, H.C.; Wang, C.Y.; Shin, S.J.; et al. Range of L5 LDL levels in healthy adults and L5’s predictive power in patients with hyperlipidemia or coronary artery disease. Sci. Rep. 2018, 8, 11866. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; Law, S.H.; Lenzen, D.; Tan, Y.H.; Weng, S.F.; Ito, E.; Wu, J.C.; Chen, C.H.; Chan, H.C.; Ke, L.Y. Clinical Significance of Electronegative Low-Density Lipoprotein Cholesterol in Atherothrombosis. Biomedicines 2020, 8, 254. [Google Scholar] [CrossRef]
- Sanchez-Quesada, J.L.; Benitez, S.; Ordonez-Llanos, J. Electronegative low-density lipoprotein. Curr. Opin. Lipidol. 2004, 15, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Benitez, S.; Camacho, M.; Arcelus, R.; Vila, L.; Bancells, C.; Ordonez-Llanos, J.; Sanchez-Quesada, J.L. Increased lysophosphatidylcholine and non-esterified fatty acid content in LDL induces chemokine release in endothelial cells. Relationship with electronegative LDL. Atherosclerosis 2004, 177, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Estruch, M.; Sanchez-Quesada, J.L.; Beloki, L.; Ordonez-Llanos, J.; Benitez, S. The Induction of Cytokine Release in Monocytes by Electronegative Low-Density Lipoprotein (LDL) Is Related to Its Higher Ceramide Content than Native LDL. Int. J. Mol. Sci. 2013, 14, 2601–2616. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.Y.; Stancel, N.; Bair, H.; Chen, C.H. The underlying chemistry of electronegative LDL’s atherogenicity. Curr. Atheroscler. Rep. 2014, 16, 428. [Google Scholar] [CrossRef]
- Chan, H.C.; Chan, H.C.; Liang, C.J.; Lee, H.C.; Su, H.; Lee, A.S.; Shiea, J.; Tsai, W.C.; Ou, T.T.; Wu, C.C.; et al. Role of Low-Density Lipoprotein in Early Vascular Aging Associated With Systemic Lupus Erythematosus. Arthritis Rheumatol. 2020, 72, 972–984. [Google Scholar] [CrossRef]
- Ke, L.Y.; Chan, H.C.; Chen, C.C.; Lu, J.; Marathe, G.K.; Chu, C.S.; Chan, H.C.; Wang, C.Y.; Tung, Y.C.; McIntyre, T.M.; et al. Enhanced Sphingomyelinase Activity Contributes to the Apoptotic Capacity of Electronegative Low-Density Lipoprotein. J. Med. Chem. 2016, 59, 1032–1040. [Google Scholar] [CrossRef]
- Ke, L.Y.; Engler, D.A.; Lu, J.; Matsunami, R.K.; Chan, H.C.; Wang, G.J.; Yang, C.Y.; Chang, J.G.; Chen, C.H. Chemical composition-oriented receptor selectivity of L5, a naturally occurring atherogenic low-density lipoprotein. Pure Appl. Chem. 2011, 83. [Google Scholar] [CrossRef]
- Jayaraman, S.; Chavez, O.R.; Perez, A.; Minambres, I.; Sanchez-Quesada, J.L.; Gursky, O. Binding to heparin triggers deleterious structural and biochemical changes in human low-density lipoprotein, which are amplified in hyperglycemia. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158712. [Google Scholar] [CrossRef]
- Bancells, C.; Benitez, S.; Ordonez-Llanos, J.; Oorni, K.; Kovanen, P.T.; Milne, R.W.; Sanchez-Quesada, J.L. Immunochemical analysis of the electronegative LDL subfraction shows that abnormal N-terminal apolipoprotein B conformation is involved in increased binding to proteoglycans. J. Biol. Chem. 2011, 286, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J.; Villegas, S.; Benitez, S.; Bancells, C.; Diercks, T.; Ordonez-Llanos, J.; Sanchez-Quesada, J.L. 2D-NMR reveals different populations of exposed lysine residues in the apoB-100 protein of electronegative and electropositive fractions of LDL particles. J. Lipid Res. 2010, 51, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Jiang, T.; Yang, J.H.; Jiang, W.; Lu, J.; Marathe, G.K.; Pownall, H.J.; Ballantyne, C.M.; McIntyre, T.M.; Henry, P.D.; et al. Low-density lipoprotein in hypercholesterolemic human plasma induces vascular endothelial cell apoptosis by inhibiting fibroblast growth factor 2 transcription. Circulation 2003, 107, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Raya, J.L.; Chen, H.H.; Chen, C.H.; Abe, Y.; Pownall, H.J.; Taylor, A.A.; Smith, C.V. Isolation, characterization, and functional assessment of oxidatively modified subfractions of circulating low-density lipoproteins. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.Y.; Chan, H.C.; Chen, C.C.; Chang, C.F.; Lu, P.L.; Chu, C.S.; Lai, W.T.; Shin, S.J.; Liu, F.T.; Chen, C.H. Increased APOE glycosylation plays a key role in the atherogenicity of L5 low-density lipoprotein. FASEB J. 2020, 34, 9802–9813. [Google Scholar] [CrossRef]
- Lu, J.; Yang, J.H.; Burns, A.R.; Chen, H.H.; Tang, D.; Walterscheid, J.P.; Suzuki, S.; Yang, C.Y.; Sawamura, T.; Chen, C.H. Mediation of electronegative low-density lipoprotein signaling by LOX-1: A possible mechanism of endothelial apoptosis. Circ. Res. 2009, 104, 619–627. [Google Scholar] [CrossRef]
- Chan, H.C.; Ke, L.Y.; Chu, C.S.; Lee, A.S.; Shen, M.Y.; Cruz, M.A.; Hsu, J.F.; Cheng, K.H.; Chan, H.C.; Lu, J.; et al. Highly electronegative LDL from patients with ST-elevation myocardial infarction triggers platelet activation and aggregation. Blood 2013, 122, 3632–3641. [Google Scholar] [CrossRef]
- Ke, L.Y.; Chan, H.C.; Chan, H.C.; Kalu, F.C.U.; Lee, H.C.; Lin, I.L.; Jhuo, S.J.; Lai, W.T.; Tsao, C.R.; Sawamura, T.; et al. Electronegative Low-Density Lipoprotein L5 Induces Adipose Tissue Inflammation Associated With Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2017, 102, 4615–4625. [Google Scholar] [CrossRef]
- Chang, S.F.; Chang, P.Y.; Chou, Y.C.; Lu, S.C. Electronegative LDL Induces M1 Polarization of Human Macrophages Through a LOX-1-Dependent Pathway. Inflammation 2020, 43, 1524–1535. [Google Scholar] [CrossRef]
- Tang, D.; Lu, J.; Walterscheid, J.P.; Chen, H.H.; Engler, D.A.; Sawamura, T.; Chang, P.Y.; Safi, H.J.; Yang, C.Y.; Chen, C.H. Electronegative LDL circulating in smokers impairs endothelial progenitor cell differentiation by inhibiting Akt phosphorylation via LOX-1. J. Lipid Res. 2008, 49, 33–47. [Google Scholar] [CrossRef]
- Chen, W.Y.; Chen, Y.F.; Chan, H.C.; Chung, C.H.; Peng, H.Y.; Ho, Y.C.; Chen, C.H.; Chang, K.C.; Tang, C.H.; Lee, A.S. Role of apolipoprotein E in electronegative low-density lipoprotein-induced mitochondrial dysfunction in cardiomyocytes. Metab. Clin. Exp. 2020, 107, 154227. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; Wang, Y.C.; Lu, L.S.; Walton, B.; Yilmaz, H.R.; Huang, R.Y.; Sawamura, T.; Dixon, R.A.; Lai, W.T.; Chen, C.H.; et al. Electronegative low-density lipoprotein increases C-reactive protein expression in vascular endothelial cells through the LOX-1 receptor. PLoS ONE 2013, 8, e70533. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.Y.; Chen, F.Y.; Hsu, J.F.; Fu, R.H.; Chang, C.M.; Chang, C.T.; Liu, C.H.; Wu, J.R.; Lee, A.S.; Chan, H.C.; et al. Plasma L5 levels are elevated in ischemic stroke patients and enhance platelet aggregation. Blood 2016, 127, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Estruch, M.; Sanchez-Quesada, J.L.; Ordonez-Llanos, J.; Benitez, S. Ceramide-enriched LDL induces cytokine release through TLR4 and CD14 in monocytes. Similarities with electronegative LDL. Clin. Investig. Arterioscler. 2014, 26, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Ligi, D.; Benitez, S.; Croce, L.; Rivas-Urbina, A.; Puig, N.; Ordonez-Llanos, J.; Mannello, F.; Sanchez-Quesada, J.L. Electronegative LDL induces MMP-9 and TIMP-1 release in monocytes through CD14 activation: Inhibitory effect of glycosaminoglycan sulodexide. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3559–3567. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, R.; Balogh, G.; Costa, G.; De Spirito, M.; Greco, G.; Mei, G.; Nicolai, E.; Vigh, L.; Ursini, F.; Parasassi, T. Estradiol binding prevents ApoB-100 misfolding in electronegative LDL(−). Biochemistry 2010, 49, 7297–7302. [Google Scholar] [CrossRef]
- Brunelli, R.; De Spirito, M.; Mei, G.; Papi, M.; Perrone, G.; Stefanutti, C.; Parasassi, T. Misfolding of apoprotein B-100, LDL aggregation and 17-beta -estradiol in atherogenesis. Curr. Med. Chem. 2014, 21, 2276–2283. [Google Scholar] [CrossRef]
- Bancells, C.; Benitez, S.; Villegas, S.; Jorba, O.; Ordonez-Llanos, J.; Sanchez-Quesada, J.L. Novel phospholipolytic activities associated with electronegative low-density lipoprotein are involved in increased self-aggregation. Biochemistry 2008, 47, 8186–8194. [Google Scholar] [CrossRef]
- Sanchez-Quesada, J.L.; Villegas, S.; Ordonez-Llanos, J. Electronegative low-density lipoprotein. A link between apolipoprotein B misfolding, lipoprotein aggregation and proteoglycan binding. Curr. Opin. Lipidol. 2012, 23, 479–486. [Google Scholar] [CrossRef]
- Lai, Y.S.; Yang, T.C.; Chang, P.Y.; Chang, S.F.; Ho, S.L.; Chen, H.L.; Lu, S.C. Electronegative LDL is linked to high-fat, high-cholesterol diet-induced nonalcoholic steatohepatitis in hamsters. J. Nutr. Biochem. 2016, 30, 44–52. [Google Scholar] [CrossRef]
- Chang, P.Y.; Pai, J.H.; Lai, Y.S.; Lu, S.C. Electronegative LDL from Rabbits Fed with Atherogenic Diet Is Highly Proinflammatory. Mediators Inflamm. 2019, 2019, 6163130. [Google Scholar] [CrossRef] [PubMed]
- Kruger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Hamilos, M.; Petousis, S.; Parthenakis, F. Interaction between platelets and endothelium: From pathophysiology to new therapeutic options. Cardiovasc. Diagn. Ther. 2018, 8, 568–580. [Google Scholar] [CrossRef]
- Ziouzenkova, O.; Asatryan, L.; Sahady, D.; Orasanu, G.; Perrey, S.; Cutak, B.; Hassell, T.; Akiyama, T.E.; Berger, J.P.; Sevanian, A.; et al. Dual roles for lipolysis and oxidation in peroxisome proliferation-activator receptor responses to electronegative low density lipoprotein. J. Biol. Chem. 2003, 278, 39874–39881. [Google Scholar] [CrossRef]
- Rommel, C.; Clarke, B.A.; Zimmermann, S.; Nunez, L.; Rossman, R.; Reid, K.; Moelling, K.; Yancopoulos, G.D.; Glass, D.J. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science 1999, 286, 1738–1741. [Google Scholar] [CrossRef]
- Kanda, S.; Hodgkin, M.N.; Woodfield, R.J.; Wakelam, M.J.; Thomas, G.; Claesson-Welsh, L. Phosphatidylinositol 3′-kinase-independent p70 S6 kinase activation by fibroblast growth factor receptor-1 is important for proliferation but not differentiation of endothelial cells. J. Biol. Chem. 1997, 272, 23347–23353. [Google Scholar] [CrossRef]
- Abid, M.R.; Guo, S.; Minami, T.; Spokes, K.C.; Ueki, K.; Skurk, C.; Walsh, K.; Aird, W.C. Vascular endothelial growth factor activates PI3K/Akt/forkhead signaling in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 294–300. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, W.; Yang, J.H.; Chang, P.Y.; Walterscheid, J.P.; Chen, H.H.; Marcelli, M.; Tang, D.; Lee, Y.T.; Liao, W.S.; et al. Electronegative LDL impairs vascular endothelial cell integrity in diabetes by disrupting fibroblast growth factor 2 (FGF2) autoregulation. Diabetes 2008, 57, 158–166. [Google Scholar] [CrossRef]
- Sawamura, T.; Kakino, A.; Fujita, Y. LOX-1: A multiligand receptor at the crossroads of response to danger signals. Curr. Opin. Lipidol. 2012, 23, 439–445. [Google Scholar] [CrossRef]
- Inoue, T.; Ishida, T.; Inoue, T.; Saito, A.; Ezura, M.; Uenohara, H.; Fujimura, M.; Sato, K.; Endo, T.; Omodaka, S.; et al. Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 Levels as a Biomarker of Acute Intracerebral Hemorrhage. J. Stroke Cerebrovasc. Dis. 2019, 28, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, V.; Pingitore, A.; Traghella, I.; Storti, S.; Parri, S.; Berti, S.; Ndreu, R.; Andrenelli, A.; Palmieri, C.; Iervasi, G.; et al. Emerging Biomarkers of Oxidative Stress in Acute and Stable Coronary Artery Disease: Levels and Determinants. Antioxidants 2019, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Wang, Y.C.; Chang, S.S.; Lo, P.H.; Chang, C.M.; Lu, J.; Burns, A.R.; Chen, C.H.; Kakino, A.; Sawamura, T.; et al. Detection of a High Ratio of Soluble to Membrane-Bound LOX-1 in Aspirated Coronary Thrombi From Patients With ST-Segment-Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e014008. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Ito, H.; Akiyoshi, M.; Kume, N.; Yoshitomi, H.; Mitsuoka, H.; Tanida, S.; Murata, K.; Shibuya, H.; Kasahara, T.; et al. Lectin-like oxidized low-density lipoprotein receptor 1 signal is a potent biomarker and therapeutic target for human rheumatoid arthritis. Arthritis Rheum. 2012, 64, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Brunssen, C.; Wolk, S.; Reeps, C.; Morawietz, H. Soluble LOX-1: A Novel Biomarker in Patients With Coronary Artery Disease, Stroke, and Acute Aortic Dissection? J. Am. Heart Assoc. 2020, 9, e013803. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Arai, Y.; Kurihara, H.; Kita, T.; Sawamura, T. Overexpression of lectin-like oxidized low-density lipoprotein receptor-1 induces intramyocardial vasculopathy in apolipoprotein E-null mice. Circ. Res. 2005, 97, 176–184. [Google Scholar] [CrossRef]
- Mehta, J.L.; Sanada, N.; Hu, C.P.; Chen, J.; Dandapat, A.; Sugawara, F.; Satoh, H.; Inoue, K.; Kawase, Y.; Jishage, K.; et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ. Res. 2007, 100, 1634–1642. [Google Scholar] [CrossRef]
- Kataoka, H.; Kume, N.; Miyamoto, S.; Minami, M.; Moriwaki, H.; Murase, T.; Sawamura, T.; Masaki, T.; Hashimoto, N.; Kita, T. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation 1999, 99, 3110–3117. [Google Scholar] [CrossRef]
- Mattaliano, M.D.; Huard, C.; Cao, W.; Hill, A.A.; Zhong, W.; Martinez, R.V.; Harnish, D.C.; Paulsen, J.E.; Shih, H.H. LOX-1-dependent transcriptional regulation in response to oxidized LDL treatment of human aortic endothelial cells. Am. J. Physiol. Cell Physiol. 2009, 296, C1329–C1337. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Goel, A.; Mehta, J.L. LOX-1: Regulation, Signaling and Its Role in Atherosclerosis. Antioxidants 2019, 8, 218. [Google Scholar] [CrossRef]
- Sagar, D.; Gaddipati, R.; Ongstad, E.L.; Bhagroo, N.; An, L.L.; Wang, J.; Belkhodja, M.; Rahman, S.; Manna, Z.; Davis, M.A.; et al. LOX-1: A potential driver of cardiovascular risk in SLE patients. PLoS ONE 2020, 15, e0229184. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ogura, S.; Chen, J.; Little, P.J.; Moss, J.; Liu, P. LOX-1 in atherosclerosis: Biological functions and pharmacological modifiers. Cell Mol. Life Sci. 2013, 70, 2859–2872. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Singh, R.M.; Liu, L.; Chen, H.; Singh, B.M.; Kazzaz, N.; Mehta, J.L. Oxidized-LDL through LOX-1 increases the expression of angiotensin converting enzyme in human coronary artery endothelial cells. Cardiovasc. Res. 2003, 57, 238–243. [Google Scholar] [CrossRef][Green Version]
- Li, D.; Mehta, J.L. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of human coronary artery endothelial cells: Evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Ishibashi, T.; Sawamura, T.; Inoue, N.; Kamioka, M.; Uekita, H.; Ohkawara, H.; Sakamoto, T.; Sakamoto, N.; Okamoto, Y.; et al. LOX-1-MT1-MMP axis is crucial for RhoA and Rac1 activation induced by oxidized low-density lipoprotein in endothelial cells. Cardiovasc. Res. 2009, 84, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.G.; Zhang, Q.J.; Li, B.W.; Li, L.H.; Song, X.H.; Xiong, C.M.; Zou, Y.B.; Liu, B.Y.; Han, J.Q.; Xiu, R.J. The circulating level of miR-122 is a potential risk factor for endothelial dysfunction in young patients with essential hypertension. Hypertens. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Cosentino, F.; Camici, G.G.; Akhmedov, A.; Vanhoutte, P.M.; Tanner, F.C.; Luscher, T.F. Oxidized low-density lipoprotein activates p66Shc via lectin-like oxidized low-density lipoprotein receptor-1, protein kinase C-beta, and c-Jun N-terminal kinase kinase in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2090–2097. [Google Scholar] [CrossRef]
- Ma, S.C.; Hao, Y.J.; Jiao, Y.; Wang, Y.H.; Xu, L.B.; Mao, C.Y.; Yang, X.L.; Yang, A.N.; Tian, J.; Zhang, M.H.; et al. Homocysteineinduced oxidative stress through TLR4/NFkappaB/DNMT1mediated LOX1 DNA methylation in endothelial cells. Mol. Med. Rep. 2017, 16, 9181–9188. [Google Scholar] [CrossRef]
- Yu, E.P.; Bennett, M.R. The role of mitochondrial DNA damage in the development of atherosclerosis. Free Radic. Biol. Med. 2016, 100, 223–230. [Google Scholar] [CrossRef]
- Hu, C.; Dandapat, A.; Sun, L.; Chen, J.; Marwali, M.R.; Romeo, F.; Sawamura, T.; Mehta, J.L. LOX-1 deletion decreases collagen accumulation in atherosclerotic plaque in low-density lipoprotein receptor knockout mice fed a high-cholesterol diet. Cardiovasc. Res. 2008, 79, 287–293. [Google Scholar] [CrossRef]
- Lu, J.; Mitra, S.; Wang, X.; Khaidakov, M.; Mehta, J.L. Oxidative stress and lectin-like ox-LDL-receptor LOX-1 in atherogenesis and tumorigenesis. Antioxid. Redox. Signal. 2011, 15, 2301–2333. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Koziel, R.; Pircher, H.; Kratochwil, M.; Lener, B.; Hermann, M.; Dencher, N.A.; Jansen-Durr, P. Mitochondrial respiratory chain complex I is inactivated by NADPH oxidase Nox4. Biochem. J. 2013, 452, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, M.; Migliaccio, E.; Orsini, F.; Paolucci, D.; Moroni, M.; Contursi, C.; Pelliccia, G.; Luzi, L.; Minucci, S.; Marcaccio, M.; et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 2005, 122, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Trinei, M.; Berniakovich, I.; Beltrami, E.; Migliaccio, E.; Fassina, A.; Pelicci, P.; Giorgio, M. P66Shc signals to age. Aging (Albany N.Y.) 2009, 1, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.T.; Wang, G.J.; Kuo, C.C.; Hsieh, J.Y.; Lee, A.S.; Chang, C.M.; Wang, C.C.; Shen, M.Y.; Huang, C.C.; Sawamura, T.; et al. Electronegative Low-density Lipoprotein Increases Coronary Artery Disease Risk in Uremia Patients on Maintenance Hemodialysis. Medicine 2016, 95, e2265. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Sanson, M.; Auge, N.; Vindis, C.; Muller, C.; Bando, Y.; Thiers, J.C.; Marachet, M.A.; Zarkovic, K.; Sawa, Y.; Salvayre, R.; et al. Oxidized low-density lipoproteins trigger endoplasmic reticulum stress in vascular cells: Prevention by oxygen-regulated protein 150 expression. Circ. Res. 2009, 104, 328–336. [Google Scholar] [CrossRef]
- Gora, S.; Maouche, S.; Atout, R.; Wanherdrick, K.; Lambeau, G.; Cambien, F.; Ninio, E.; Karabina, S.A. Phospholipolyzed LDL induces an inflammatory response in endothelial cells through endoplasmic reticulum stress signaling. FASEB J. 2010, 24, 3284–3297. [Google Scholar] [CrossRef]
- Gargalovic, P.S.; Gharavi, N.M.; Clark, M.J.; Pagnon, J.; Yang, W.P.; He, A.; Truong, A.; Baruch-Oren, T.; Berliner, J.A.; Kirchgessner, T.G.; et al. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2490–2496. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, R.; Shen, G.X. Impact of cyanidin-3-glucoside on glycated LDL-induced NADPH oxidase activation, mitochondrial dysfunction and cell viability in cultured vascular endothelial cells. Int. J. Mol. Sci. 2012, 13, 15867–15880. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Xie, X.; Le, K.; Li, W.; Moghadasian, M.H.; Beta, T.; Shen, G.X. Endoplasmic reticulum stress in diabetic mouse or glycated LDL-treated endothelial cells: Protective effect of Saskatoon berry powder and cyanidin glycans. J. Nutr. Biochem. 2015, 26, 1248–1253. [Google Scholar] [CrossRef]
- Benitez, S.; Bancells, C.; Ordonez-Llanos, J.; Sanchez-Quesada, J.L. Pro-inflammatory action of LDL(−) on mononuclear cells is counteracted by increased IL10 production. Biochim. Biophys. Acta 2007, 1771, 613–622. [Google Scholar] [CrossRef]
- Benitez, S.; Camacho, M.; Bancells, C.; Vila, L.; Sanchez-Quesada, J.L.; Ordonez-Llanos, J. Wide proinflammatory effect of electronegative low-density lipoprotein on human endothelial cells assayed by a protein array. Biochim. Biophys. Acta 2006, 1761, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- De Castellarnau, C.; Sanchez-Quesada, J.L.; Benitez, S.; Rosa, R.; Caveda, L.; Vila, L.; Ordonez-Llanos, J. Electronegative LDL from normolipemic subjects induces IL-8 and monocyte chemotactic protein secretion by human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2281–2287. [Google Scholar] [CrossRef] [PubMed]
- Demuth, K.; Myara, I.; Chappey, B.; Vedie, B.; Pech-Amsellem, M.A.; Haberland, M.E.; Moatti, N. A cytotoxic electronegative LDL subfraction is present in human plasma. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Hodis, H.N.; Kramsch, D.M.; Avogaro, P.; Bittolo-Bon, G.; Cazzolato, G.; Hwang, J.; Peterson, H.; Sevanian, A. Biochemical and cytotoxic characteristics of an in vivo circulating oxidized low density lipoprotein (LDL-). J. Lipid Res. 1994, 35, 669–677. [Google Scholar]
- Estruch, M.; Rajamaki, K.; Sanchez-Quesada, J.L.; Kovanen, P.T.; Oorni, K.; Benitez, S.; Ordonez-Llanos, J. Electronegative LDL induces priming and inflammasome activation leading to IL-1beta release in human monocytes and macrophages. Biochim. Biophys. Acta 2015, 1851, 1442–1449. [Google Scholar] [CrossRef]
- Estruch, M.; Sanchez-Quesada, J.L.; Ordonez-Llanos, J.; Benitez, S. Inflammatory intracellular pathways activated by electronegative LDL in monocytes. Biochim. Biophys. Acta 2016, 1861 Pt A, 963–969. [Google Scholar] [CrossRef]
- Glass, C.K.; Witztum, J.L. Atherosclerosis. the road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.; Chang, P.Y.; Lu, S.C. L5-LDL from ST-elevation myocardial infarction patients induces IL-1beta production via LOX-1 and NLRP3 inflammasome activation in macrophages. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H265–H274. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.; Chang, P.Y.; Kuo, T.L.; Lu, S.C. Electronegative L5-LDL induces the production of G-CSF and GM-CSF in human macrophages through LOX-1 involving NF-kappaB and ERK2 activation. Atherosclerosis 2017, 267, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.K.; Chen, P.K.; Lan, J.L.; Chang, S.H.; Hsieh, T.Y.; Liao, P.J.; Chen, C.H.; Chen, D.Y. Association of Electronegative LDL with Macrophage Foam Cell Formation and CD11c Expression in Rheumatoid Arthritis Patients. Int. J. Mol. Sci. 2020, 21, 5883. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, A.M.; Faine, L.A.; Grosso, D.M.; de Las Heras, B.; Bosca, L.; Abdalla, D.S. Electronegative LDL induction of apoptosis in macrophages: Involvement of Nrf2. Biochim. Biophys. Acta 2010, 1801, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Parguina, A.F.; Grigorian-Shamagian, L.; Agra, R.M.; Lopez-Otero, D.; Rosa, I.; Alonso, J.; Teijeira-Fernandez, E.; Gonzalez-Juanatey, J.R.; Garcia, A. Variations in platelet proteins associated with ST-elevation myocardial infarction: Novel clues on pathways underlying platelet activation in acute coronary syndromes. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2957–2964. [Google Scholar] [CrossRef]
- Podrez, E.A.; Byzova, T.V. Prothrombotic lipoprotein patterns in stroke. Blood 2016, 127, 1221–1222. [Google Scholar] [CrossRef]
- Hou, X.; Summer, R.; Chen, Z.; Tian, Y.; Ma, J.; Cui, J.; Hao, X.; Guo, L.; Xu, H.; Wang, H.; et al. Lipid Uptake by Alveolar Macrophages Drives Fibrotic Responses to Silica Dust. Sci. Rep. 2019, 9, 399. [Google Scholar] [CrossRef]
- Yoshida, H.; Quehenberger, O.; Kondratenko, N.; Green, S.; Steinberg, D. Minimally oxidized low-density lipoprotein increases expression of scavenger receptor A, CD36, and macrosialin in resident mouse peritoneal macrophages. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 794–802. [Google Scholar] [CrossRef]
- Nagy, L.; Tontonoz, P.; Alvarez, J.G.; Chen, H.; Evans, R.M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 1998, 93, 229–240. [Google Scholar] [CrossRef]
- Rahalkar, A.R.; Hegele, R.A. Monogenic pediatric dyslipidemias: Classification, genetics and clinical spectrum. Mol. Genet. Metab. 2008, 93, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R. Protease variants, LDL, and coronary heart disease. N. Engl. J. Med. 2006, 354, 1310–1312. [Google Scholar] [CrossRef] [PubMed]
- Sisman, G.; Erzin, Y.; Hatemi, I.; Caglar, E.; Boga, S.; Singh, V.; Senturk, H. Familial chylomicronemia syndrome related chronic pancreatitis: A single-center study. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 209–214. [Google Scholar] [CrossRef]
- Steinberg, D. Atherogenesis in perspective: Hypercholesterolemia and inflammation as partners in crime. Nat. Med. 2002, 8, 1211–1217. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. A receptor-mediated pathway for cholesterol homeostasis. Science 1986, 232, 34–47. [Google Scholar] [CrossRef]
- Stalenhoef, A.F.; van ‘t Laar, A. Clinical significance of current perspectives in cholesterol metabolism. Ned. Tijdschr. Geneeskd. 1986, 130, 951–955. [Google Scholar]
- Goldstein, J.L.; Brown, M.S. Molecular medicine. The cholesterol quartet. Science 2001, 292, 1310–1312. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Steinberg, D. The LDL modification hypothesis of atherogenesis: An update. J. Lipid Res. 2009, 50, S376–S381. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Wieland, E.; Steinberg, D. A role for endothelial cell lipoxygenase in the oxidative modification of low density lipoprotein. Proc. Natl. Acad. Sci. USA 1989, 86, 1046–1050. [Google Scholar] [CrossRef]
- Benz, D.J.; Mol, M.; Ezaki, M.; Mori-Ito, N.; Zelan, I.; Miyanohara, A.; Friedmann, T.; Parthasarathy, S.; Steinberg, D.; Witztum, J.L. Enhanced levels of lipoperoxides in low density lipoprotein incubated with murine fibroblast expressing high levels of human 15-lipoxygenase. J. Biol. Chem. 1995, 270, 5191–5197. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D. Low density lipoprotein oxidation and its pathobiological significance. J. Biol. Chem. 1997, 272, 20963–20966. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Ishikawa, T.; Hosoai, H.; Suzukawa, M.; Ayaori, M.; Hisada, T.; Sawada, S.; Yonemura, A.; Higashi, K.; Ito, T.; et al. Inhibitory effect of tea flavonoids on the ability of cells to oxidize low density lipoprotein. Biochem. Pharmacol. 1999, 58, 1695–1703. [Google Scholar] [CrossRef]
- Yoshida, H.; Sasaki, K.; Namiki, Y.; Sato, N.; Tada, N. Edaravone, a novel radical scavenger, inhibits oxidative modification of low-density lipoprotein (LDL) and reverses oxidized LDL-mediated reduction in the expression of endothelial nitric oxide synthase. Atherosclerosis 2005, 179, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Lai, C.L.; Lee, C.T.; Lin, C.Y. Electronegative Low-Density Lipoprotein L5 Impairs Viability and NGF-Induced Neuronal Differentiation of PC12 Cells via LOX-1. Int. J. Mol. Sci. 2017, 18, 1744. [Google Scholar] [CrossRef]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 69, e71–e126. [Google Scholar] [CrossRef]
- Pollak, A.W.; Norton, P.T.; Kramer, C.M. Multimodality imaging of lower extremity peripheral arterial disease: Current role and future directions. Circ. Cardiovasc. Imaging 2012, 5, 797–807. [Google Scholar] [CrossRef]
- Shishehbor, M.H.; White, C.J.; Gray, B.H.; Menard, M.T.; Lookstein, R.; Rosenfield, K.; Jaff, M.R. Critical Limb Ischemia: An Expert Statement. J. Am. Coll. Cardiol. 2016, 68, 2002–2015. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef]
- Wadhera, R.K.; Steen, D.L.; Khan, I.; Giugliano, R.P.; Foody, J.M. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J. Clin. Lipidol. 2016, 10, 472–489. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Siontis, G.C.M.; Piccolo, R.; Mavridis, D.; Raber, L.; Mach, F.; Windecker, S. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: A meta-analysis of randomized trials. Eur. Heart J. 2018, 39, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.; Yang, H.; Zhang, P.; Wu, F.; Li, Y.; Zhou, Y.; Zhang, X.; Ma, H.; Zhang, W.; et al. alpha-Linolenic acid but not linolenic acid protects against hypertension: Critical role of SIRT3 and autophagic flux. Cell Death Dis. 2020, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Stafylas, P.C.; Sarafidis, P.A. Carvedilol in hypertension treatment. Vasc. Health Risk Manag. 2008, 4, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, G.; Egan, C.G. Use of carvedilol in hypertension: An update. Vasc. Health Risk Manag. 2012, 8, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Sy, R.G.; Nevado, J.B., Jr.; Llanes, E.J.B.; Magno, J.D.A.; Ona, D.I.D.; Punzalan, F.E.R.; Reganit, P.F.M.; Santos, L.E.G.; Tiongco, R.H.P., 2nd; Aherrera, J.A.M.; et al. The Klotho Variant rs36217263 Is Associated With Poor Response to Cardioselective Beta-Blocker Therapy Among Filipinos. Clin. Pharmacol. Ther. 2020, 107, 221–226. [Google Scholar] [CrossRef]
- Silva, I.V.G.; de Figueiredo, R.C.; Rios, D.R.A. Effect of Different Classes of Antihypertensive Drugs on Endothelial Function and Inflammation. Int. J. Mol. Sci. 2019, 20, 3548. [Google Scholar] [CrossRef] [PubMed]
- Erbs, S.; Beck, E.B.; Linke, A.; Adams, V.; Gielen, S.; Krankel, N.; Mobius-Winkler, S.; Hollriegel, R.; Thiele, H.; Hambrecht, R.; et al. High-dose rosuvastatin in chronic heart failure promotes vasculogenesis, corrects endothelial function, and improves cardiac remodeling—Results from a randomized, double-blind, and placebo-controlled study. Int. J. Cardiol. 2011, 146, 56–63. [Google Scholar] [CrossRef]
- Szygula-Jurkiewicz, B.; Szczurek, W.; Krol, B.; Zembala, M. The role of statins in chronic heart failure. Kardiochir Torakochirurgia Pol. 2014, 11, 301–305. [Google Scholar] [CrossRef]
- Cangiano, E.; Marchesini, J.; Campo, G.; Francolini, G.; Fortini, C.; Carra, G.; Miccoli, M.; Ceconi, C.; Tavazzi, L.; Ferrari, R. ACE inhibition modulates endothelial apoptosis and renewal via endothelial progenitor cells in patients with acute coronary syndromes. Am. J. Cardiovasc. Drugs 2011, 11, 189–198. [Google Scholar] [CrossRef]
- Brugts, J.J.; Bertrand, M.; Remme, W.; Ferrari, R.; Fox, K.; MacMahon, S.; Chalmers, J.; Simoons, M.L.; Boersma, E. The Treatment Effect of an ACE-Inhibitor Based Regimen with Perindopril in Relation to Beta-Blocker use in 29,463 Patients with Vascular Disease: A Combined Analysis of Individual Data of ADVANCE, EUROPA and PROGRESS Trials. Cardiovasc. Drugs Ther. 2017, 31, 391–400. [Google Scholar] [CrossRef]
- Rangaswami, J.; McCullough, P.A. Heart Failure in End-Stage Kidney Disease: Pathophysiology, Diagnosis, and Therapeutic Strategies. Semin. Nephrol. 2018, 38, 600–617. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.J.; Mitsnefes, M. Cardiovascular Disease in Children and Adolescents With Chronic Kidney Disease. Semin. Nephrol. 2018, 38, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Kern, A.; Gil, R.; Gorny, J.; Sienkiewicz, E.; Bojko, K.; Wasilewski, G. Patient with ST-elevation myocardial infarction, coronary artery embolism and no signs of coronary atherosclerosis in angiography. Postepy Kardiol. Interwencyjnej 2015, 11, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Vernon, S.T.; Coffey, S.; D’Souza, M.; Chow, C.K.; Kilian, J.; Hyun, K.; Shaw, J.A.; Adams, M.; Roberts-Thomson, P.; Brieger, D.; et al. ST-Segment-Elevation Myocardial Infarction (STEMI) Patients Without Standard Modifiable Cardiovascular Risk Factors-How Common Are They, and What Are Their Outcomes? J. Am. Heart Assoc. 2019, 8, e013296. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef]
- Chen, C.J.; Yang, T.C.; Chang, C.; Lu, S.C.; Chang, P.Y. Homocysteine is a bystander for ST-segment elevation myocardial infarction: A case-control study. BMC Cardiovasc. Disord. 2018, 18, 33. [Google Scholar] [CrossRef]
- Zou, C.; Hu, H. Use of pioglitazone in the treatment of diabetes: Effect on cardiovascular risk. Vasc. Health Risk Manag. 2013, 9, 429–433. [Google Scholar] [CrossRef]
- Yu, X.; Chen, P.; Wang, H.; Zhu, T. Pioglitazone ameliorates endothelial dysfunction in those with impaired glucose regulation among the first-degree relatives of type 2 diabetes mellitus patients. Med. Princ. Pract. 2013, 22, 156–160. [Google Scholar] [CrossRef]
- Abdullah, C.S.; Li, Z.; Wang, X.; Jin, Z.Q. Depletion of T lymphocytes ameliorates cardiac fibrosis in streptozotocin-induced diabetic cardiomyopathy. Int. Immunopharmacol. 2016, 39, 251–264. [Google Scholar] [CrossRef]
- Samuel, C.S.; Hewitson, T.D.; Zhang, Y.; Kelly, D.J. Relaxin ameliorates fibrosis in experimental diabetic cardiomyopathy. Endocrinology 2008, 149, 3286–3293. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, L.; Yang, K.; Fu, Y.; Liu, Y.; Chi, J.; Zhang, X.; Hong, S.; Ma, X.; Yin, X. H3 Relaxin Protects Against Myocardial Injury in Experimental Diabetic Cardiomyopathy by Inhibiting Myocardial Apoptosis, Fibrosis and Inflammation. Cell Physiol. Biochem. 2017, 43, 1311–1324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, C.M.; Li, J.; Meng, Z.J.; Wei, S.N.; Li, J.; Bucala, R.; Li, Y.L.; Chen, L. Berberine protects against palmitate-induced endothelial dysfunction: Involvements of upregulation of AMPK and eNOS and downregulation of NOX4. Mediators Inflamm. 2013, 2013, 260464. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.; Lam, K.S.; Li, Y.; Wong, W.T.; Ye, H.; Lau, C.W.; Vanhoutte, P.M.; Xu, A. Berberine prevents hyperglycemia-induced endothelial injury and enhances vasodilatation via adenosine monophosphate-activated protein kinase and endothelial nitric oxide synthase. Cardiovasc. Res. 2009, 82, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Suganya, N.; Bhakkiyalakshmi, E.; Sarada, D.V.; Ramkumar, K.M. Reversibility of endothelial dysfunction in diabetes: Role of polyphenols. Br. J. Nutr. 2016, 116, 223–246. [Google Scholar] [CrossRef]
- Forcheron, F.; Basset, A.; Abdallah, P.; Del Carmine, P.; Gadot, N.; Beylot, M. Diabetic cardiomyopathy: Effects of fenofibrate and metformin in an experimental model—The Zucker diabetic rat. Cardiovasc. Diabetol. 2009, 8, 16. [Google Scholar] [CrossRef]
- Baraka, A.; AbdelGawad, H. Targeting apoptosis in the heart of streptozotocin-induced diabetic rats. J. Cardiovasc. Pharmacol. Ther. 2010, 15, 175–181. [Google Scholar] [CrossRef]
- Arosio, E.; De Marchi, S.; Rigoni, A.; Prior, M.; Delva, P.; Lechi, A. Forearm haemodynamics, arterial stiffness and microcirculatory reactivity in rheumatoid arthritis. J. Hypertens. 2007, 25, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Limon, P.; Ortega, R.; Arias de la Rosa, I.; Abalos-Aguilera, M.D.C.; Perez-Sanchez, C.; Jimenez-Gomez, Y.; Peralbo-Santaella, E.; Font, P.; Ruiz-Vilches, D.; Ferrin, G.; et al. Tocilizumab improves the proatherothrombotic profile of rheumatoid arthritis patients modulating endothelial dysfunction, NETosis, and inflammation. Transl. Res. 2017, 183, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Fanouriakis, A.; Pamfil, C.; Rednic, S.; Sidiropoulos, P.; Bertsias, G.; Boumpas, D.T. Is it primary neuropsychiatric systemic lupus erythematosus? Performance of existing attribution models using physician judgment as the gold standard. Clin. Exp. Rheumatol. 2016, 34, 910–917. [Google Scholar] [PubMed]
- Nikolopoulos, D.; Fanouriakis, A.; Boumpas, D.T. Cerebrovascular Events in Systemic Lupus Erythematosus: Diagnosis and Management. Mediterr. J. Rheumatol. 2019, 30, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Aringer, M.; Leuchten, N.; Dorner, T. Biologicals and small molecules for systemic lupus erythematosus. Z. Rheumatol. 2020, 79, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Chang, H.J.; Sung, J.M.; Park, H.B.; Heo, R.; Rizvi, A.; Lin, F.Y.; Kumar, A.; Hadamitzky, M.; Kim, Y.J.; et al. Effects of Statins on Coronary Atherosclerotic Plaques: The PARADIGM Study. JACC Cardiovasc. Imaging 2018, 11, 1475–1484. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Nelson, A.J. Monitoring the Response to Statin Therapy: One Scan at a Time. JACC Cardiovasc. Imaging 2018, 11, 1485–1486. [Google Scholar] [CrossRef] [PubMed]
- Yla-Herttuala, S.; Bentzon, J.F.; Daemen, M.; Falk, E.; Garcia-Garcia, H.M.; Herrmann, J.; Hoefer, I.; Jauhiainen, S.; Jukema, J.W.; Krams, R.; et al. Stabilization of atherosclerotic plaques: An update. Eur. Heart J. 2013, 34, 3251–3258. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; Ke, L.Y.; Chan, H.C.; Chan, H.C.; Chen, C.C.; Cheng, K.H.; Lee, H.C.; Kuo, H.F.; Chang, C.T.; Chang, K.C.; et al. Four Statin Benefit Groups Defined by The 2013 ACC/AHA New Cholesterol Guideline are Characterized by Increased Plasma Level of Electronegative Low-Density Lipoprotein. Acta Cardiol. Sin. 2016, 32, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Miura, S.; Yanagi, D.; Noda, K.; Nishikawa, H.; Matsunaga, A.; Shirai, K.; Iwata, A.; Yoshinaga, K.; Adachi, H.; et al. Reduction of charge-modified LDL by statin therapy in patients with CHD or CHD risk factors and elevated LDL-C levels: The SPECIAL Study. Atherosclerosis 2008, 201, 353–359. [Google Scholar] [CrossRef]
- Zhang, B.; Matsunaga, A.; Rainwater, D.L.; Miura, S.; Noda, K.; Nishikawa, H.; Uehara, Y.; Shirai, K.; Ogawa, M.; Saku, K. Effects of rosuvastatin on electronegative LDL as characterized by capillary isotachophoresis: The ROSARY Study. J. Lipid Res. 2009, 50, 1832–1841. [Google Scholar] [CrossRef]
- Sena-Evangelista, K.C.; Pedrosa, L.F.; Paiva, M.S.; Dias, P.C.; Ferreira, D.Q.; Cozzolino, S.M.; Faulin, T.E.; Abdalla, D.S. The hypolipidemic and pleiotropic effects of rosuvastatin are not enhanced by its association with zinc and selenium supplementation in coronary artery disease patients: A double blind randomized controlled study. PLoS ONE 2015, 10, e0119830. [Google Scholar] [CrossRef]
- Vavlukis, M.; Vavlukis, A. Adding ezetimibe to statin therapy: Latest evidence and clinical implications. Drugs Context 2018, 7, 212534. [Google Scholar] [CrossRef]
- Page, M.M.; Watts, G.F. PCSK9 inhibitors—Mechanisms of action. Aust. Prescr. 2016, 39, 164–167. [Google Scholar] [CrossRef]
- Ikegami, Y.; Inoue, I.; Inoue, K.; Shinoda, Y.; Iida, S.; Goto, S.; Nakano, T.; Shimada, A.; Noda, M. The annual rate of coronary artery calcification with combination therapy with a PCSK9 inhibitor and a statin is lower than that with statin monotherapy. NPJ Aging Mech. Dis. 2018, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Mata, P.; Muniz, O.; Fuentes-Jimenez, F.; Diaz, J.L.; Zambon, D.; Tomas, M.; Martin, C.; Moyon, T.; Croyal, M.; et al. PCSK9 and lipoprotein (a) levels are two predictors of coronary artery calcification in asymptomatic patients with familial hypercholesterolemia. Atherosclerosis 2016, 254, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Kuhnast, S.; van der Hoorn, J.W.; Pieterman, E.J.; van den Hoek, A.M.; Sasiela, W.J.; Gusarova, V.; Peyman, A.; Schafer, H.L.; Schwahn, U.; Jukema, J.W.; et al. Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin. J. Lipid Res. 2014, 55, 2103–2112. [Google Scholar] [CrossRef]
- Pouwer, M.G.; Pieterman, E.J.; Worms, N.; Keijzer, N.; Jukema, J.W.; Gromada, J.; Gusarova, V.; Princen, H.M.G. Alirocumab, evinacumab, and atorvastatin triple therapy regresses plaque lesions and improves lesion composition in mice. J. Lipid Res. 2020, 61, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Berbee, J.F.; Wong, M.C.; Wang, Y.; van der Hoorn, J.W.; Khedoe, P.P.; van Klinken, J.B.; Mol, I.M.; Hiemstra, P.S.; Tsikas, D.; Romijn, J.A.; et al. Resveratrol protects against atherosclerosis, but does not add to the antiatherogenic effect of atorvastatin, in APOE*3-Leiden.CETP mice. J. Nutr. Biochem. 2013, 24, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.K.; Shih, H.H.; Parveen, F.; Lenzen, D.; Ito, E.; Chan, T.F.; Ke, L.Y. Identifying the Therapeutic Significance of Mesenchymal Stem Cells. Cells 2020, 9, 1145. [Google Scholar] [CrossRef]
- Back, M. Leukotriene signaling in atherosclerosis and ischemia. Cardiovasc. Drugs Ther. 2009, 23, 41–48. [Google Scholar] [CrossRef]
- Charo, I.F.; Taub, R. Anti-inflammatory therapeutics for the treatment of atherosclerosis. Nat. Rev. Drug Discov. 2011, 10, 365–376. [Google Scholar] [CrossRef]
- Bertrand, M.J.; Tardif, J.C. Inflammation and beyond: New directions and emerging drugs for treating atherosclerosis. Expert Opin. Emerg. Drugs 2017, 22, 1–26. [Google Scholar] [CrossRef]
- Tuttolomondo, A. Editorial: Treatment of atherosclerosis as an inflammatory disease. Curr. Pharm. Des. 2012, 18, 4265. [Google Scholar] [CrossRef]
- Raggi, P.; Genest, J.; Giles, J.T.; Rayner, K.J.; Dwivedi, G.; Beanlands, R.S.; Gupta, M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Riccioni, G.; Zanasi, A.; Vitulano, N.; Mancini, B.; D’Orazio, N. Leukotrienes in atherosclerosis: New target insights and future therapy perspectives. Mediators Inflamm. 2009, 2009, 737282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, J.; Kakkar, V.; Lu, X. Impact of MCP-1 in atherosclerosis. Curr. Pharm. Des. 2014, 20, 4580–4588. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Oikonomou, E.; Economou, E.K.; Crea, F.; Kaski, J.C. Inflammatory cytokines in atherosclerosis: Current therapeutic approaches. Eur. Heart J. 2016, 37, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Pieper, G.M. Enhanced, unaltered and impaired nitric oxide-mediated endothelium-dependent relaxation in experimental diabetes mellitus: Importance of disease duration. Diabetologia 1999, 42, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Forstermann, U. Nitric oxide in the pathogenesis of vascular disease. J. Pathol. 2000, 190, 244–254. [Google Scholar] [CrossRef]
- McLeod, D.S.; Lefer, D.J.; Merges, C.; Lutty, G.A. Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am. J. Pathol. 1995, 147, 642–653. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).