Continuous Electrical Stimulation Affects Initial Growth and Proliferation of Adipose-Derived Stem Cells
Abstract
1. Introduction
2. Experimental Section
2.1. Cell Culture
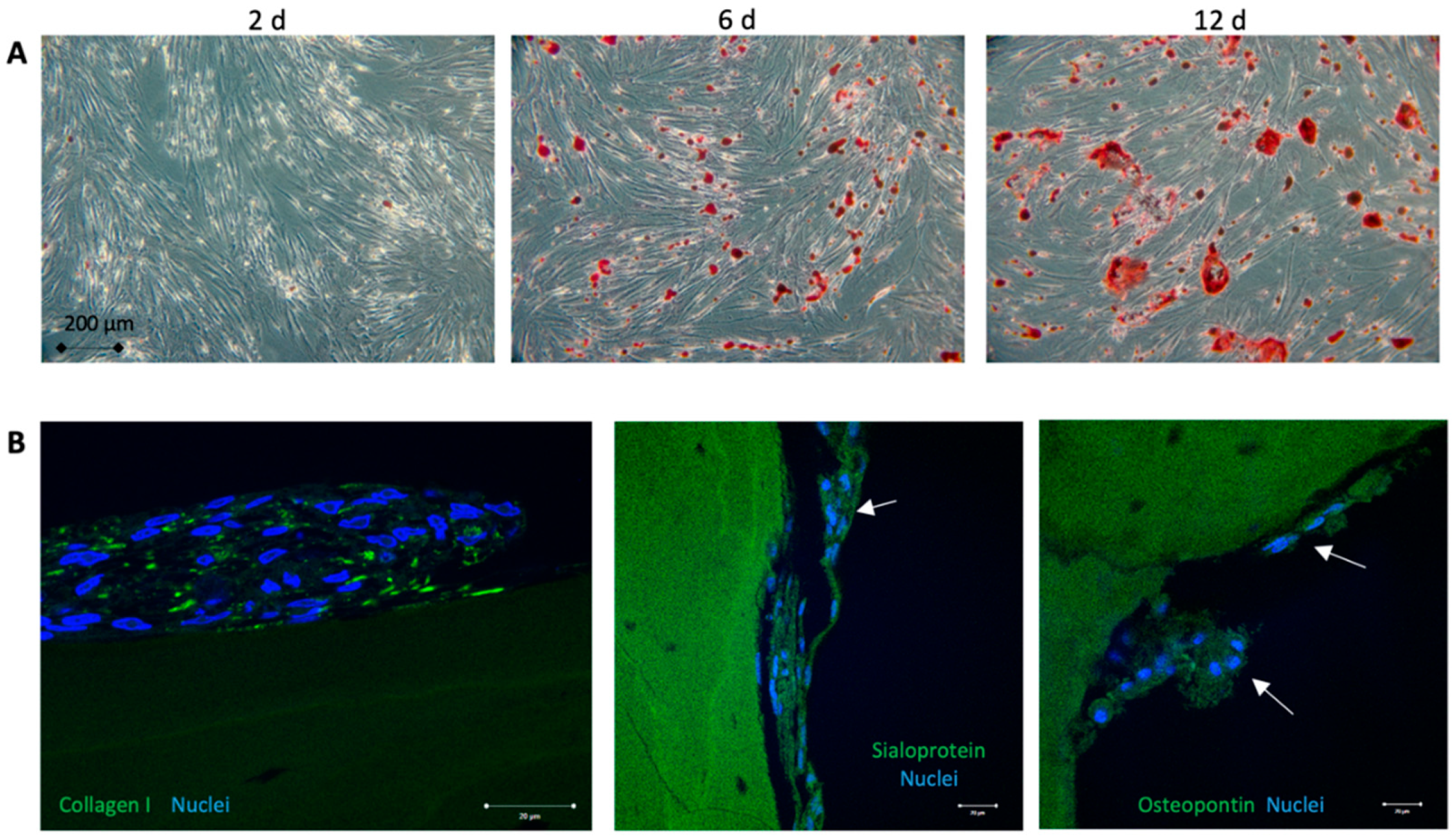
2.2. Osteogenic Differentiation of Adipose-Derived Stem Cells
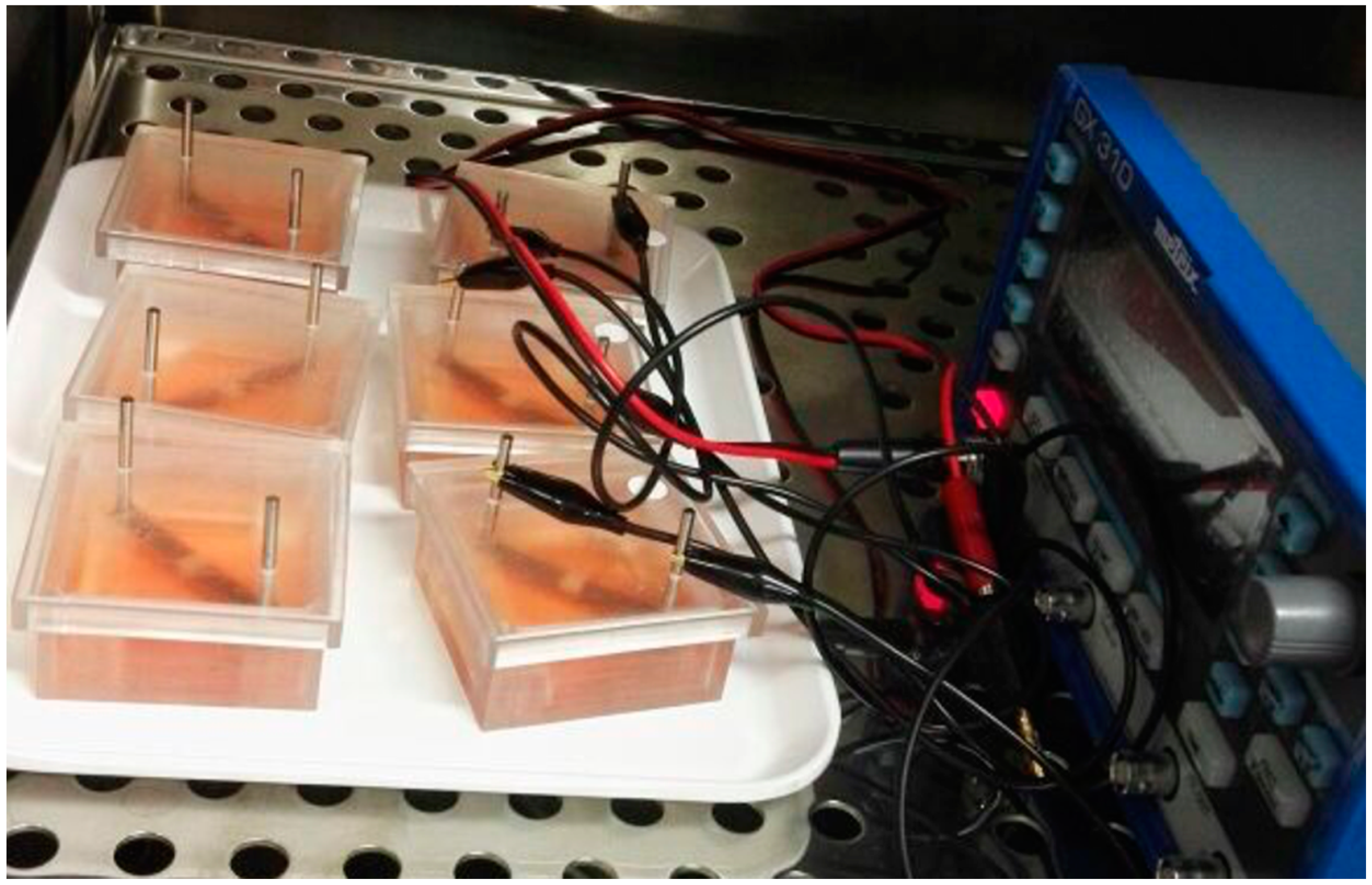
2.3. Electrical Stimulation System and Cell Preparation
2.4. Electrical Stimulation Pattern
2.5. Cell Number and Cellular Surface Coverage
2.6. Cell Proliferation
2.7. Cell Cycle Analysis
2.8. Statistics
3. Results
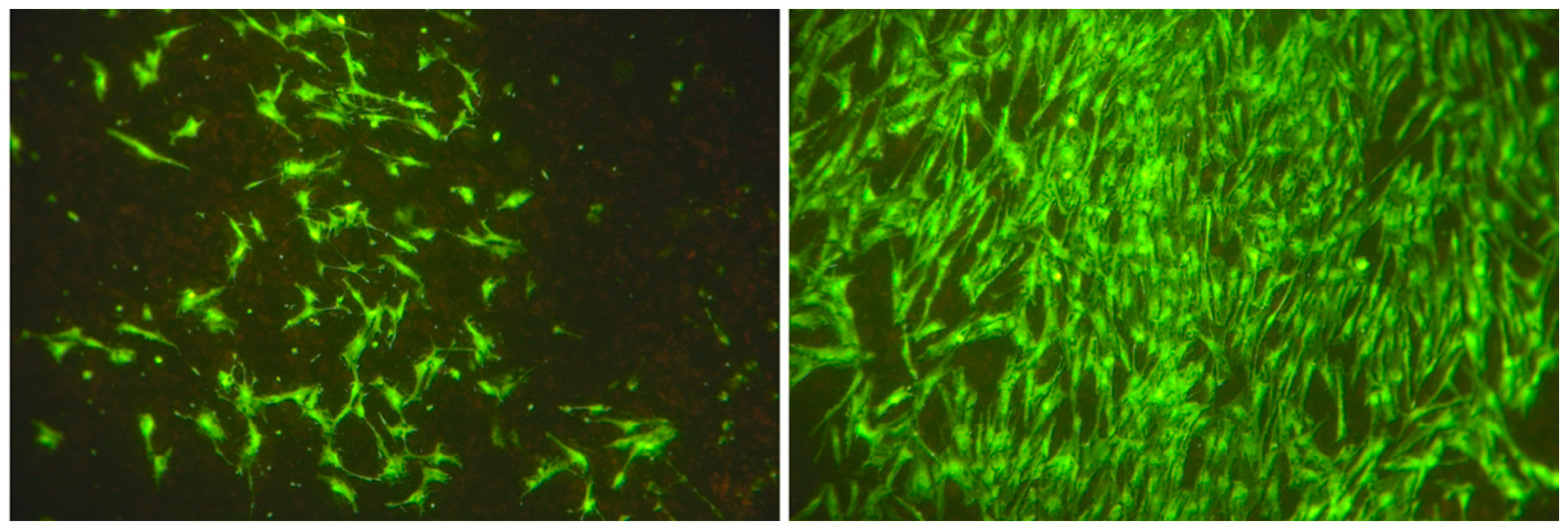
3.1. Cell Characterization
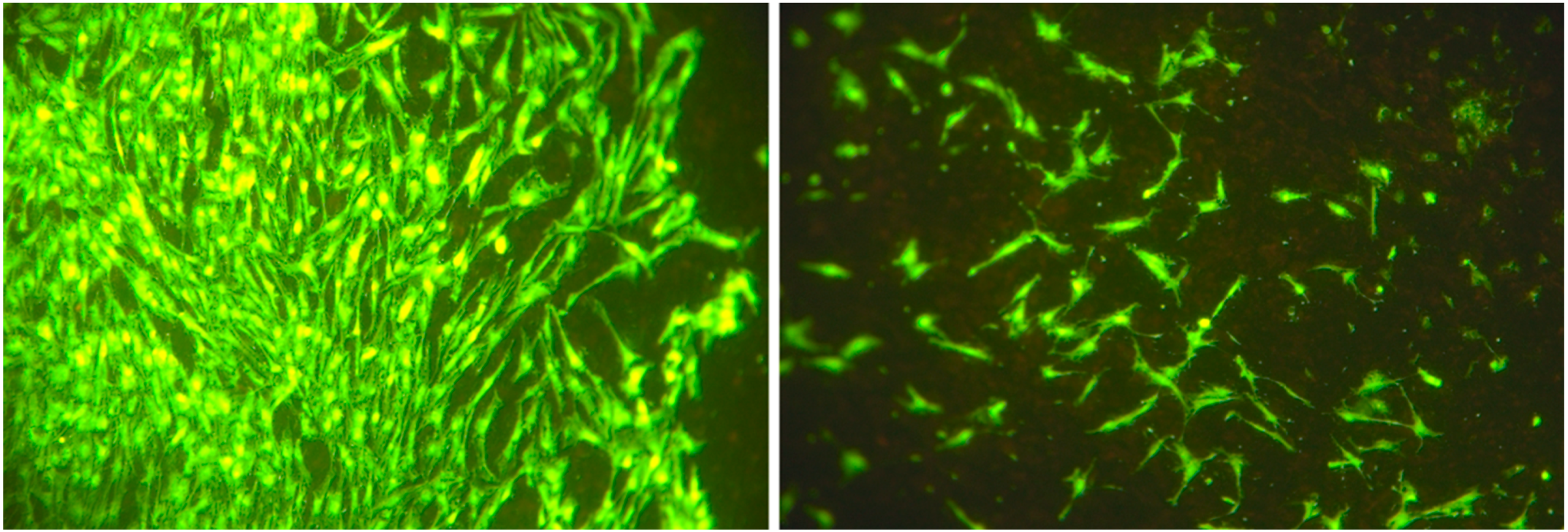
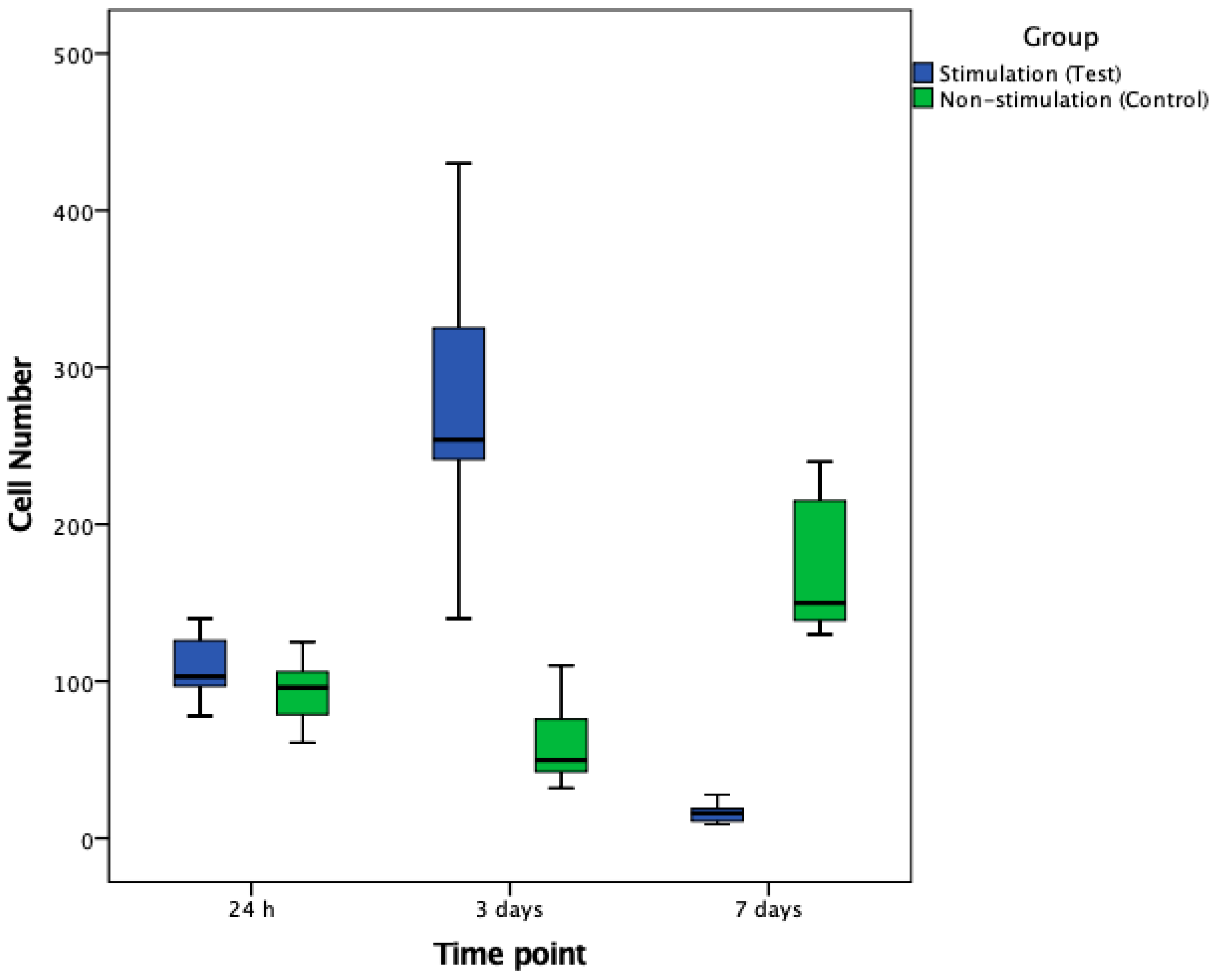
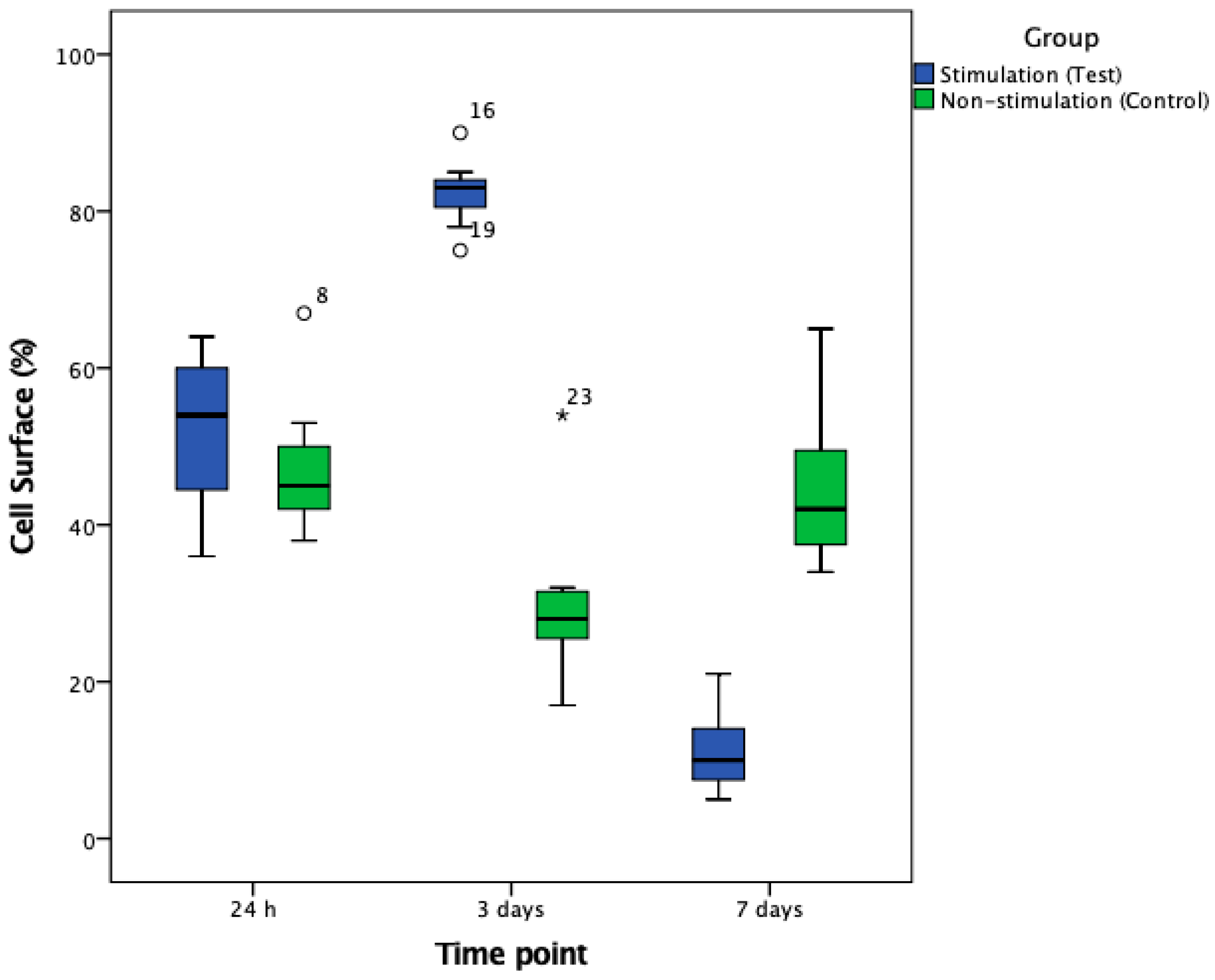
3.2. Cell Number and Cellular Surface Coverage
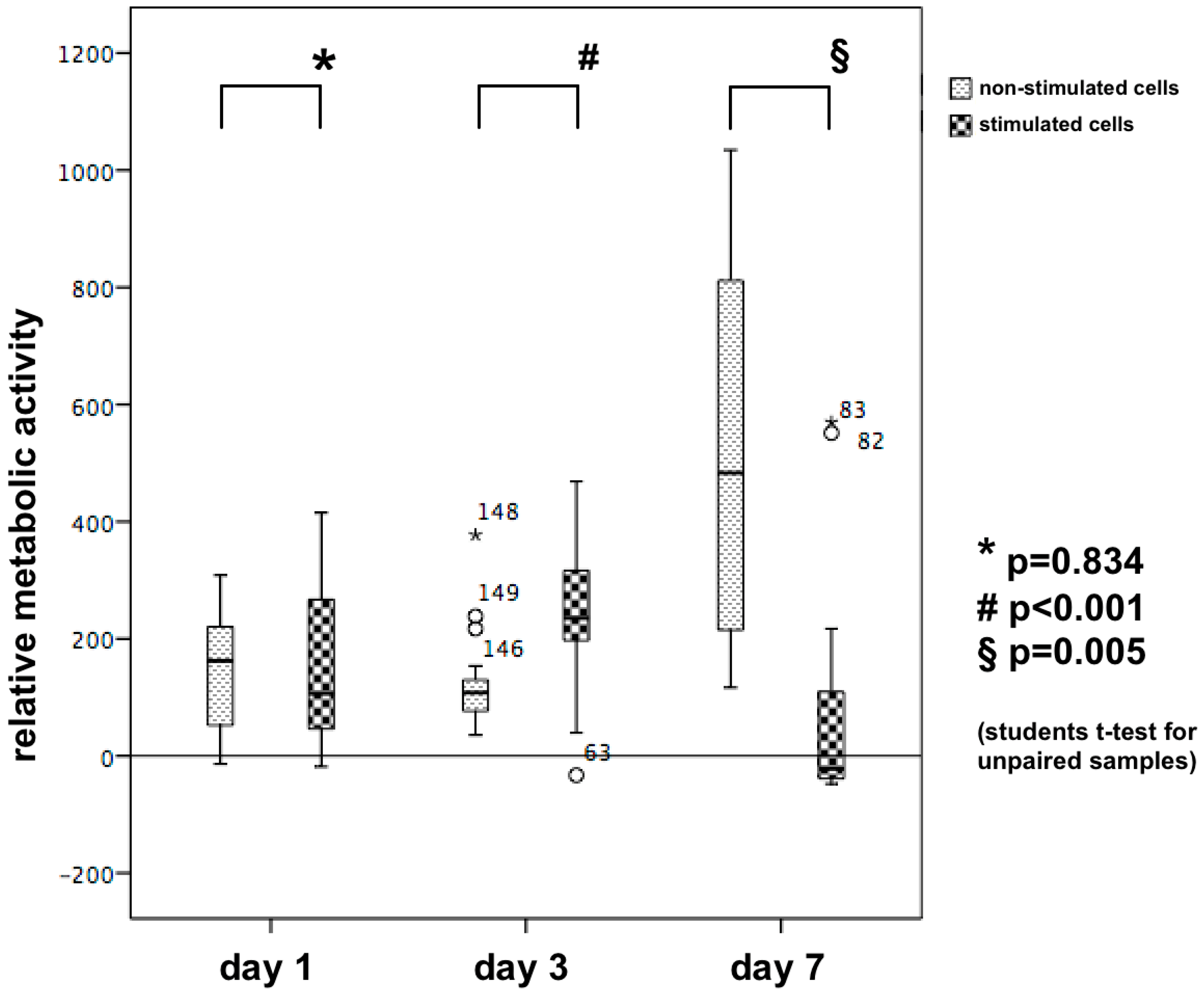
3.3. Cell Proliferation/Relative Metabolic Activity
XTT
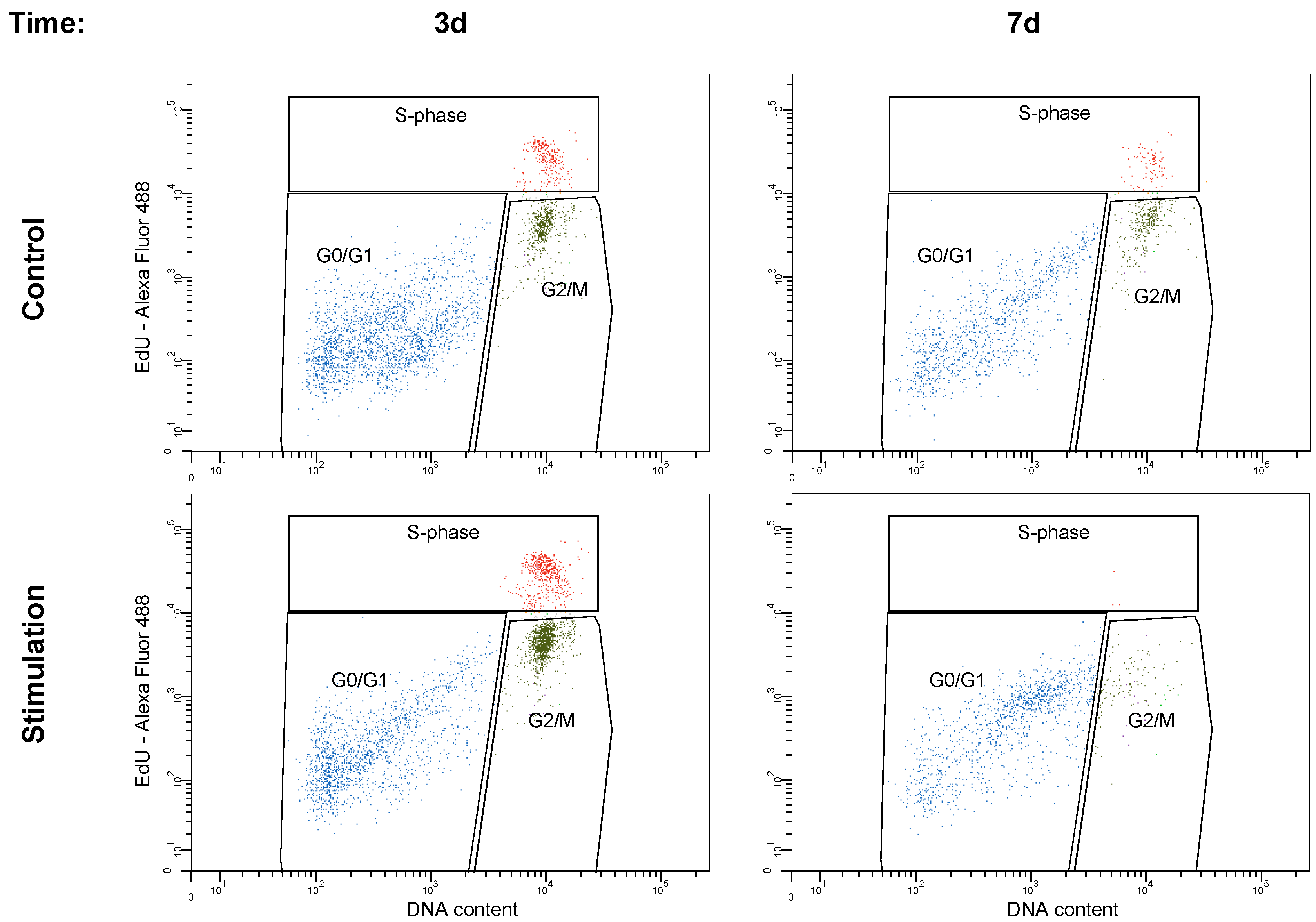
3.4. Cell Cycle Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hernández-Bule, M.L.; Paíno, C.L.; Trillo, M.Á.; Úbeda, A. Electric Stimulation at 448 kHz Promotes Proliferation of Human Mesenchymal Stem Cells. Cell. Physiol. Biochem. 2014, 34, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.-S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Why are MSCs therapeutic? New data: New insight. J. Pathol. 2009, 217, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Krueger, E.; Magri, L.M.S.; Botelho, A.S.; Bach, F.S.; Rebellato, C.L.K.; Fracaro, L.; Fragoso, F.Y.I.; Villanova, J.A., Jr.; Brofman, P.R.S.; Popovic-Maneski, L. Effects of low-intensity electrical stimulation and adipose derived stem cells transplantation on the time-domain analysis-based electromyographic signals in dogs with SCI. Neurosci. Lett. 2019, 696, 38–45. [Google Scholar] [CrossRef]
- Vissers, C.; Ming, G.-l.; Song, H. Nanoparticle technology and stem cell therapy team up against neurodegenerative disorders. Adv. Drug Deliv. Rev. 2019, 148, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.R.; Frisen, J.; Fritsche-Danielson, R.; Melton, D.A.; Murry, C.E.; Weissman, I.L. Regenerating the field of cardiovascular cell therapy. Nat. Biotechnol. 2019, 37, 232–237. [Google Scholar] [CrossRef]
- Rothrauff, B.B.; Pirosa, A.; Lin, H.; Sohn, J.; Langhans, M.T.; Tuan, R.S. Stem Cell Therapy for Musculoskeletal Diseases. In Principles of Regenerative Medicine; Elsevier: Cambridge MA, USA, 2019; pp. 953–970. [Google Scholar] [CrossRef]
- Law, L.; Hunt, C.L.; van Wijnen, A.J.; Nassr, A.; Larson, A.N.; Eldrige, J.S.; Mauck, W.D.; Pingree, M.J.; Yang, J.; Muir, C.W.; et al. Office-Based Mesenchymal Stem Cell Therapy for the Treatment of Musculoskeletal Disease: A Systematic Review of Recent Human Studies. Pain Med. 2019, 20, 1570–1583. [Google Scholar] [CrossRef]
- Kawasaki, H.; Mizuseki, K.; Nishikawa, S.; Kaneko, S.; Kuwana, Y.; Nakanishi, S.; Nishikawa, S.I.; Sasai, Y. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron 2000, 28, 31–40. [Google Scholar] [CrossRef]
- Wichterle, H.; Lieberam, I.; Porter, J.A.; Jessell, T.M. Directed Differentiation of Embryonic Stem Cells into Motor Neurons. Cell 2002, 110, 385–397. [Google Scholar] [CrossRef]
- Hronik-Tupaj, M.; Rice, W.L.; Cronin-Golomb, M.; Kaplan, D.L.; Georgakoudi, I. Osteoblastic differentiation and stress response of human mesenchymal stem cells exposed to alternating current electric fields. Biomed. Eng. Online 2011, 10, 9. [Google Scholar] [CrossRef]
- Dubey, A.K.; Gupta, S.D.; Basu, B. Optimization of electrical stimulation parameters for enhanced cell proliferation on biomaterial surfaces. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98B, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Wang, Z.; Cui, C.; Zhu, Y.; Shi, J.; Wang, J.; Chen, M. Rapid Electrical Stimulation Increased Cardiac Apoptosis Through Disturbance of Calcium Homeostasis and Mitochondrial Dysfunction in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Cell. Physiol. Biochem. 2018, 47, 1167–1180. [Google Scholar] [CrossRef]
- Hardy, J.G.; Villancio-Wolter, M.K.; Sukhavasi, R.C.; Mouser, D.J.; Aguilar, D.; Geissler, S.A.; Kaplan, D.L.; Schmidt, C.E. Electrical Stimulation of Human Mesenchymal Stem Cells on Conductive Nanofibers Enhances their Differentiation toward Osteogenic Outcomes. Macromol. Rapid Commun. 2015, 36, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- O’Hearn, S.F.; Ackerman, B.J.; Mower, M.M. Paced monophasic and biphasic waveforms alter transmembrane potentials and metabolism of human fibroblasts. Biochem. Biophys. Rep. 2016, 8, 249–253. [Google Scholar] [CrossRef]
- Ross, C.L. The use of electric, magnetic, and electromagnetic field for directed cell migration and adhesion in regenerative medicine. Biotechnol. Prog. 2017, 33, 5–16. [Google Scholar] [CrossRef]
- Rohde, M.; Ziebart, J.; Kirschstein, T.; Sellmann, T.; Porath, K.; Kuhl, F.; Delenda, B.; Bahls, C.; van Rienen, U.; Bader, R.; et al. Human Osteoblast Migration in DC Electrical Fields Depends on Store Operated Ca(2+)-Release and Is Correlated to Upregulation of Stretch-Activated TRPM7 Channels. Front. Bioeng. Biotechnol. 2019, 7, 422. [Google Scholar] [CrossRef]
- Love, M.R.; Sripetchwandee, J.; Palee, S.; Chattipakorn, S.C.; Mower, M.M.; Chattipakorn, N. Effects of biphasic and monophasic electrical stimulation on mitochondrial dynamics, cell apoptosis, and cell proliferation. J. Cell. Physiol. 2019, 234, 816–824. [Google Scholar] [CrossRef]
- Kumar, A.; Nune, K.C.; Misra, R.D. Electric field-mediated growth of osteoblasts—The significant impact of dynamic flow of medium. Biomater. Sci. 2016, 4, 136–144. [Google Scholar] [CrossRef]
- Zhao, Z.; Qin, L.; Reid, B.; Pu, J.; Hara, T.; Zhao, M. Directing migration of endothelial progenitor cells with applied DC electric fields. Stem Cell Res. 2012, 8, 38–48. [Google Scholar] [CrossRef]
- Kim, I.S.; Song, J.K.; Zhang, Y.L.; Lee, T.H.; Cho, T.H.; Song, Y.M.; Kim, D.K.; Kim, S.J.; Hwang, S.J. Biphasic electric current stimulates proliferation and induces VEGF production in osteoblasts. Biochim. Biophys. Acta 2006, 1763, 907–916. [Google Scholar] [CrossRef]
- Leppik, L.; Oliveira, K.M.C.; Bhavsar, M.B.; Barker, J.H. Electrical stimulation in bone tissue engineering treatments. Eur. J. Trauma Emerg. Surg. 2020, 46, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Yadollahpour, A.; Rashidi, S.; Ghotbeddin, Z.; Jalilifar, M.; Rezaee, Z. Electromagnetic Fields for the Treatments of Wastewater: A Review of Applications and Future Opportunities. J. Pure Appl. Microbiol. 2014, 8, 3711–3719. [Google Scholar]
- Thompson, W.R.; Rubin, C.T.; Rubin, J. Mechanical regulation of signaling pathways in bone. Gene 2012, 503, 179–193. [Google Scholar] [CrossRef]
- Mittelmeier, W.; Lehner, S.; Kraus, W.; Matter, H.P.; Gerdesmeyer, L.; Steinhauser, E. BISS: Concept and biomechanical investigations of a new screw system for electromagnetically induced internal osteostimulation. Arch. Orthop. Trauma Surg. 2004, 124, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Dauben, T.J.; Ziebart, J.; Bender, T.; Zaatreh, S.; Kreikemeyer, B.; Bader, R. A Novel In Vitro System for Comparative Analyses of Bone Cells and Bacteria under Electrical Stimulation. Biomed. Res. Int. 2016, 2016, 5178640. [Google Scholar] [CrossRef]
- Mardani, M.; Roshankhah, S.; Hashemibeni, B.; Salahshoor, M.; Naghsh, E.; Esfandiari, E. Induction of chondrogenic differentiation of human adipose-derived stem cells by low frequency electric field. Adv. Biomed. Res. 2016, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, H.; Wu, Z.; Wu, N.; Wang, Z.; Chen, X.; Wei, Y.; Zhang, P. Modulation of Osteogenesis in MC3T3-E1 Cells by Different Frequency Electrical Stimulation. PLoS ONE 2016, 11, e0154924. [Google Scholar] [CrossRef]
- Raben, H.; Kämmerer, P.W.; Bader, R.; van Rienen, U. Establishment of a Numerical Model to Design an Electro-Stimulating System for a Porcine Mandibular Critical Size Defect. Appl. Sci. 2019, 9, 2160. [Google Scholar] [CrossRef]
- Kuterbekov, M.; Machillot, P.; Baillet, F.; Jonas, A.M.; Glinel, K.; Picart, C. Design of experiments to assess the effect of culture parameters on the osteogenic differentiation of human adipose stromal cells. Stem Cell Res. Ther. 2019, 10, 256. [Google Scholar] [CrossRef]
- Frerich, B.; Winter, K.; Scheller, K.; Braumann, U.D. Comparison of different fabrication techniques for human adipose tissue engineering in severe combined immunodeficient mice. Artif. Organs 2012, 36, 227–237. [Google Scholar] [CrossRef]
- Zimmerlin, L.; Donnenberg, V.S.; Pfeifer, M.E.; Meyer, E.M.; Peault, B.; Rubin, J.P.; Donnenberg, A.D. Stromal vascular progenitors in adult human adipose tissue. Cytom. Part A 2010, 77, 22–30. [Google Scholar] [CrossRef]
- Heller, M.; Kumar, V.V.; Pabst, A.; Brieger, J.; Al-Nawas, B.; Kämmerer, P.W. Osseous response on linear and cyclic RGD-peptides immobilized on titanium surfaces in vitro and in vivo. J. Biomed. Mater. Res. A 2018, 106, 419–427. [Google Scholar] [CrossRef]
- Kämmerer, P.W.; Pabst, A.M.; Dau, M.; Staedt, H.; Al-Nawas, B.; Heller, M. Immobilization of BMP-2, BMP-7 and alendronic acid on titanium surfaces: Adhesion, proliferation and differentiation of bone marrow-derived stem cells. J. Biomed. Mater. Res. A 2020, 108, 212–220. [Google Scholar] [CrossRef]
- Heymann, P.G.; Ziebart, T.; Kämmerer, P.W.; Mandic, R.; Saydali, A.; Braun, A.; Neff, A.; Draenert, G.F. The enhancing effect of a laser photochemotherapy with cisplatin or zolendronic acid in primary human osteoblasts and osteosarcoma cells in vitro. J. Oral Pathol. Med. 2016, 45, 803–809. [Google Scholar] [CrossRef]
- Thiem, D.G.E.; Frick, R.W.; Goetze, E.; Gielisch, M.; Al-Nawas, B.; Kämmerer, P.W. Hyperspectral analysis for perioperative perfusion monitoring-a clinical feasibility study on free and pedicled flaps. Clin. Oral Investig. 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Hildebrandt, H.; Schmohl, J.U.; Kämmerer, P.W. Evaluation of Risk Parameters in Bone Regeneration Using a Customized Titanium Mesh: Results of a Clinical Study. Implant Dent. 2019, 28, 543–550. [Google Scholar] [CrossRef]
- Schiegnitz, E.; Kämmerer, P.W.; Sagheb, K.; Wendt, A.J.; Pabst, A.; Al-Nawas, B.; Klein, M.O. Impact of maxillary sinus augmentation on oral health-related quality of life. Int. J. Implant Dent. 2017, 3, 10. [Google Scholar] [CrossRef]
- Willenbacher, M.; Al-Nawas, B.; Berres, M.; Kämmerer, P.W.; Schiegnitz, E. The Effects of Alveolar Ridge Preservation: A Meta-Analysis. Clin. Implant Dent. Relat. Res. 2016, 18, 1248–1268. [Google Scholar] [CrossRef]
- Kim, C.; Yang, H.J.; Cho, T.H.; Lee, B.S.; Gwon, T.M.; Shin, S.; Kim, I.S.; Kim, S.J.; Hwang, S.J. Implantable electrical stimulation bioreactor with liquid crystal polymer-based electrodes for enhanced bone regeneration at mandibular large defects in rabbit. Med. Biol. Eng. Comput. 2020, 58, 383–399. [Google Scholar] [CrossRef]
- Silva, C.; Olate, S.; Pozzer, L.; Munoz, M.; Cantin, M.; Uribe, F.; de Albergaria-Barbosa, J.R. Electrical Stimulation in the Bone Repair of Defects Created in Rabbit Skulls. Int. J. Morphol. 2015, 33, 1146–1150. [Google Scholar] [CrossRef]
- Dergin, G.; Akta, M.; Gursoy, B.; Devecioglu, Y.; Kurkcu, M.; Benlidayi, E. Direct current electric stimulation in implant osseointegration: An experimental animal study with sheep. J. Oral Implantol. 2013, 39, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Vakhshori, V.; Bougioukli, S.; Sugiyama, O.; Kang, H.P.; Tang, A.H.; Park, S.H.; Lieberman, J.R. Ex vivo regional gene therapy with human adipose-derived stem cells for bone repair. Bone 2020, 138, 115524. [Google Scholar] [CrossRef]
- Winkler, S.; Mutschall, H.; Biggemann, J.; Fey, T.; Greil, P.; Korner, C.; Weisbach, V.; Meyer-Lindenberg, A.; Arkudas, A.; Horch, R.E.; et al. HUVEC support bone formation of ADSC-loaded and 3D-printed osteogenic matrices in the AV loop model. Tissue Eng. Part A 2020. [Google Scholar] [CrossRef] [PubMed]
- Roskies, M.G.; Fang, D.; Abdallah, M.N.; Charbonneau, A.M.; Cohen, N.; Jordan, J.O.; Hier, M.P.; Mlynarek, A.; Tamimi, F.; Tran, S.D. Three-dimensionally printed polyetherketoneketone scaffolds with mesenchymal stem cells for the reconstruction of critical-sized mandibular defects. Laryngoscope 2017, 127, E392–E398. [Google Scholar] [CrossRef]
- Wang, Z.; Han, L.; Sun, T.; Wang, W.; Li, X.; Wu, B. Construction of tissue-engineered bone with differentiated osteoblasts from adipose-derived stem cell and coral scaffolds at an ectopic site. Br. J. Oral Maxillofac. Surg. 2020. [Google Scholar] [CrossRef]
- Martinac, B. Mechanosensitive ion channels: Molecules of mechanotransduction. J. Cell Sci. 2004, 117, 2449–2460. [Google Scholar] [CrossRef]
- Wolf-Goldberg, T.; Barbul, A.; Ben-Dov, N.; Korenstein, R. Low electric fields induce ligand-independent activation of EGF receptor and ERK via electrochemical elevation of H(+) and ROS concentrations. Biochim. Biophys. Acta 2013, 1833, 1396–1408. [Google Scholar] [CrossRef]
- Cheng, N.; Van Hoof, H.; Bockx, E.; Hoogmartens, M.J.; Mulier, J.C.; De Dijcker, F.J.; Sansen, W.M.; De Loecker, W. The effects of electric currents on ATP generation, protein synthesis, and membrane transport of rat skin. Clin. Orthop. Relat. Res. 1982, 171, 264–272. [Google Scholar] [CrossRef]
- Delle Monache, S.; Angelucci, A.; Sanita, P.; Iorio, R.; Bennato, F.; Mancini, F.; Gualtieri, G.; Colonna, R.C. Inhibition of angiogenesis mediated by extremely low-frequency magnetic fields (ELF-MFs). PLoS ONE 2013, 8, e79309. [Google Scholar] [CrossRef]
- Kim, H.B.; Ahn, S.; Jang, H.J.; Sim, S.B.; Kim, K.W. Evaluation of corrosion behaviors and surface profiles of platinum-coated electrodes by electrochemistry and complementary microscopy: Biomedical implications for anticancer therapy. Micron 2007, 38, 747–753. [Google Scholar] [CrossRef]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef]
- Spadaro, J.A.; Becker, R.O. Function of implanted cathodes in electrode-induced bone growth. Med. Biol. Eng. Comput. 1979, 17, 769–775. [Google Scholar] [CrossRef]
- Brighton, C.T.; Adler, S.; Black, J.; Itada, N.; Friedenberg, Z.B. Cathodic oxygen consumption and electrically induced osteogenesis. Clin. Orthop. Relat. Res. 1975, 107, 277–282. [Google Scholar] [CrossRef]
- Leppik, L.; Bhavsar, M.B.; Oliveira, K.M.C.; Eischen-Loges, M.; Mobini, S.; Barker, J.H. Construction and Use of an Electrical Stimulation Chamber for Enhancing Osteogenic Differentiation in Mesenchymal Stem/Stromal Cells In Vitro. J. Vis. Exp. 2019. [Google Scholar] [CrossRef]
- Leppik, L.; Zhihua, H.; Mobini, S.; Thottakkattumana Parameswaran, V.; Eischen-Loges, M.; Slavici, A.; Helbing, J.; Pindur, L.; Oliveira, K.M.C.; Bhavsar, M.B.; et al. Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci. Rep. 2018, 8, 6307. [Google Scholar] [CrossRef]
- Miyamoto, H.; Sawaji, Y.; Iwaki, T.; Masaoka, T.; Fukada, E.; Date, M.; Yamamoto, K. Intermittent pulsed electromagnetic field stimulation activates the mTOR pathway and stimulates the proliferation of osteoblast-like cells. Bioelectromagnetics 2019, 40, 412–421. [Google Scholar] [CrossRef]
- Zhu, S.; Jing, W.; Hu, X.; Huang, Z.; Cai, Q.; Ao, Y.; Yang, X. Time-dependent effect of electrical stimulation on osteogenic differentiation of bone mesenchymal stromal cells cultured on conductive nanofibers. J. Biomed. Mater. Res. A 2017, 105, 3369–3383. [Google Scholar] [CrossRef]
- Oliveira, K.M.C.; Barker, J.H.; Berezikov, E.; Pindur, L.; Kynigopoulos, S.; Eischen-Loges, M.; Han, Z.; Bhavsar, M.B.; Henrich, D.; Leppik, L. Electrical stimulation shifts healing/scarring towards regeneration in a rat limb amputation model. Sci. Rep. 2019, 9, 11433. [Google Scholar] [CrossRef] [PubMed]
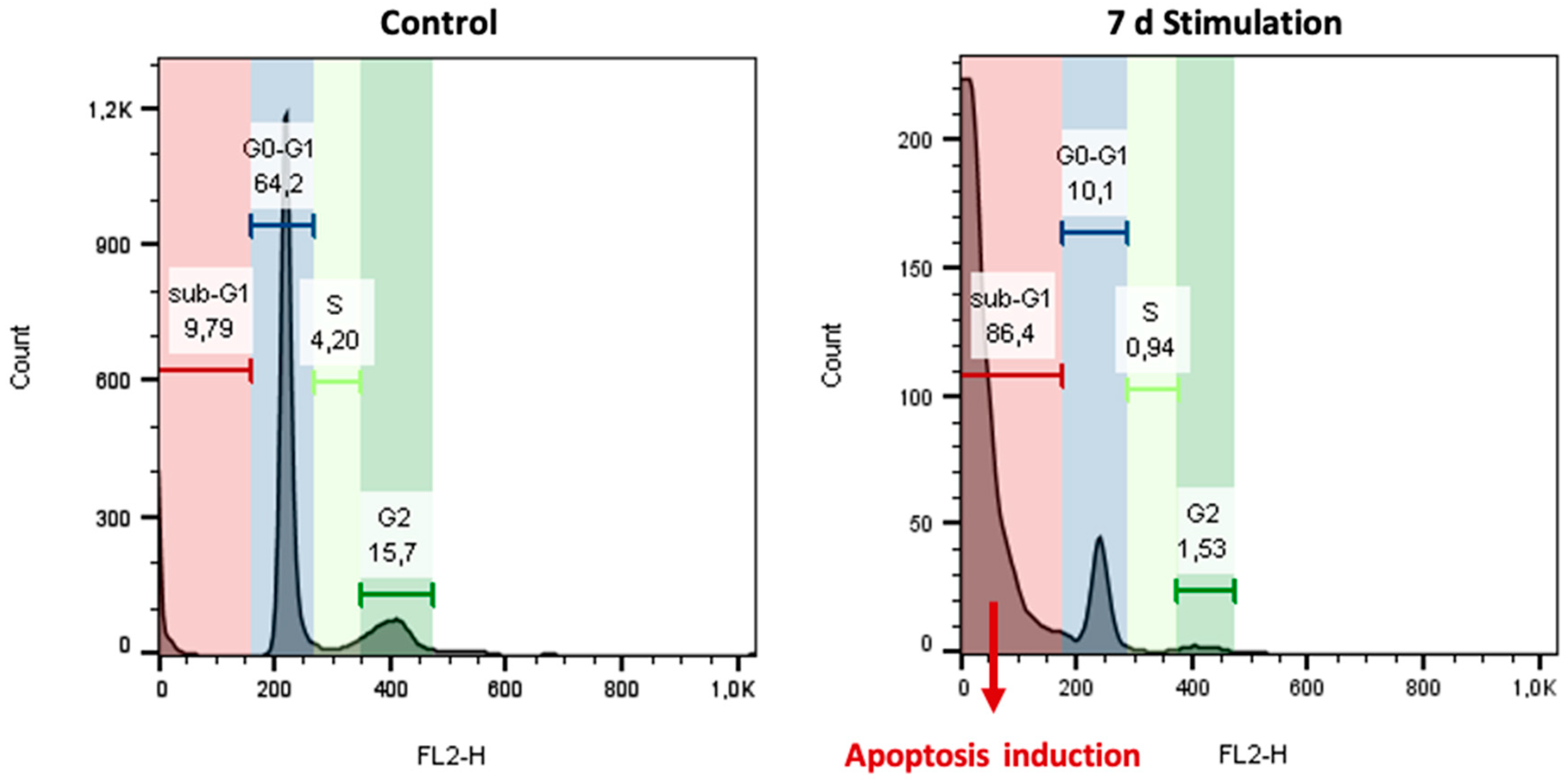
| Time | G0/G1 | S | G2/M | |
|---|---|---|---|---|
| 3 d | Control | 71.1 ± 6.8 | 8.4 ± 3.1 | 19.7 ± 5.7 |
| Stimulation | 48.6 ± 8.8 | 15.3 ± 6.7 | 35.2 ± 9.6 | |
| 7 d | Control | 70.1 ± 5.8 | 6.5 ± 4.8 | 22.0 ± 4.7 |
| Stimulation | 88.8 ± 7.2 | 0.3 ± 2.3 | 10.4 ± 5.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kämmerer, P.W.; Engel, V.; Plocksties, F.; Jonitz-Heincke, A.; Timmermann, D.; Engel, N.; Frerich, B.; Bader, R.; Thiem, D.G.E.; Skorska, A.; et al. Continuous Electrical Stimulation Affects Initial Growth and Proliferation of Adipose-Derived Stem Cells. Biomedicines 2020, 8, 482. https://doi.org/10.3390/biomedicines8110482
Kämmerer PW, Engel V, Plocksties F, Jonitz-Heincke A, Timmermann D, Engel N, Frerich B, Bader R, Thiem DGE, Skorska A, et al. Continuous Electrical Stimulation Affects Initial Growth and Proliferation of Adipose-Derived Stem Cells. Biomedicines. 2020; 8(11):482. https://doi.org/10.3390/biomedicines8110482
Chicago/Turabian StyleKämmerer, Peer W., Vivien Engel, Franz Plocksties, Anika Jonitz-Heincke, Dirk Timmermann, Nadja Engel, Bernhard Frerich, Rainer Bader, Daniel G. E. Thiem, Anna Skorska, and et al. 2020. "Continuous Electrical Stimulation Affects Initial Growth and Proliferation of Adipose-Derived Stem Cells" Biomedicines 8, no. 11: 482. https://doi.org/10.3390/biomedicines8110482
APA StyleKämmerer, P. W., Engel, V., Plocksties, F., Jonitz-Heincke, A., Timmermann, D., Engel, N., Frerich, B., Bader, R., Thiem, D. G. E., Skorska, A., David, R., Al-Nawas, B., & Dau, M. (2020). Continuous Electrical Stimulation Affects Initial Growth and Proliferation of Adipose-Derived Stem Cells. Biomedicines, 8(11), 482. https://doi.org/10.3390/biomedicines8110482







