Macrophage Stimulated by Low Ambient Temperature Hasten Tumor Growth via Glutamine Production
Abstract
1. Introduction
2. Experimental Section
2.1. In Vivo Exposure to Low Ambient Cold Temperature
2.2. In Vivo Macrophage Depletion
2.3. Cell Culture
2.4. Metabolite Measurements by CE-TOFMS
2.5. Statistical Analysis
3. Results
3.1. Low Ambient Temperature Accelerated Tumor Growth in an Allograft Model
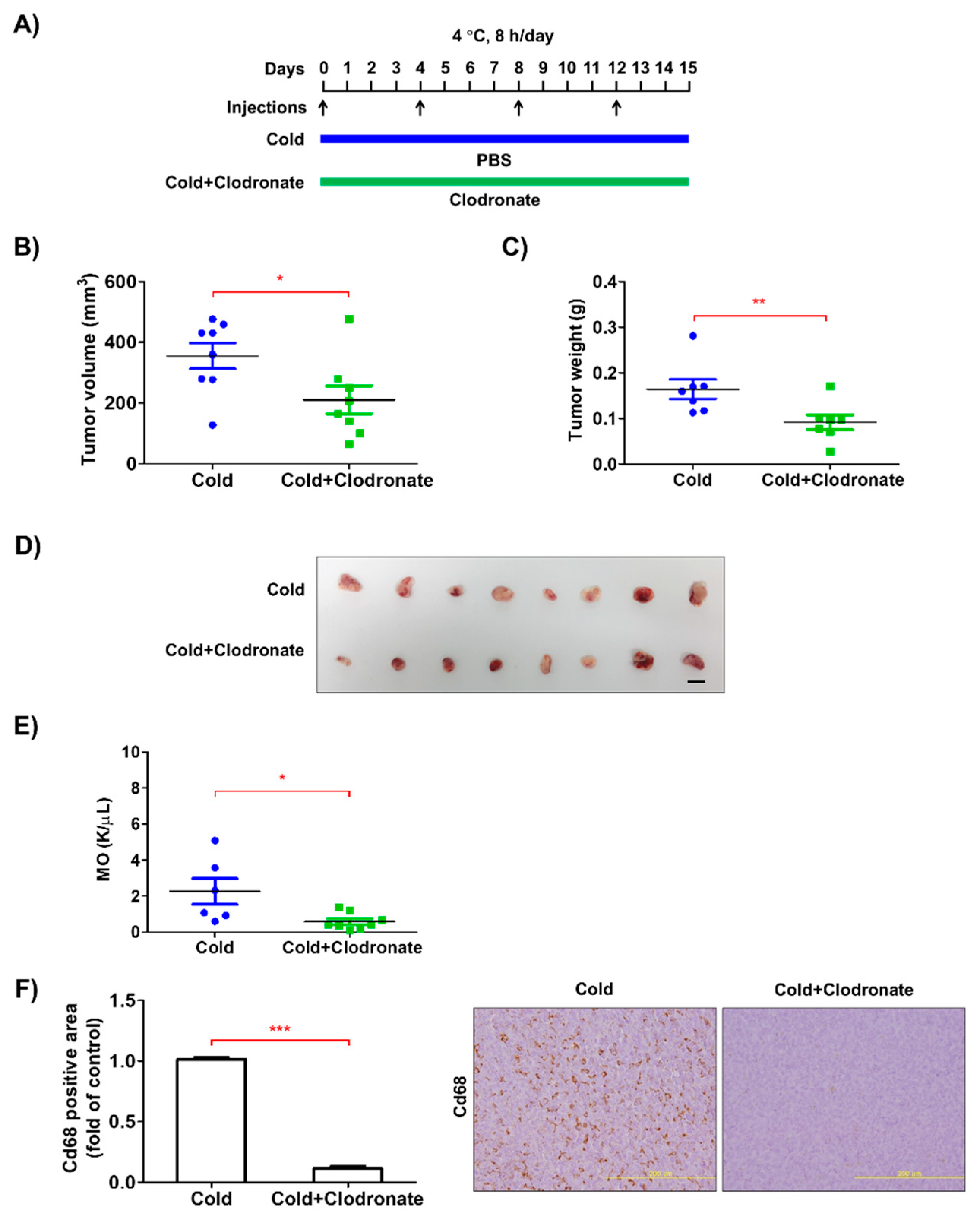
3.2. Depletion of Macrophages Reduced the Growth of Tumors in the Allograft Model
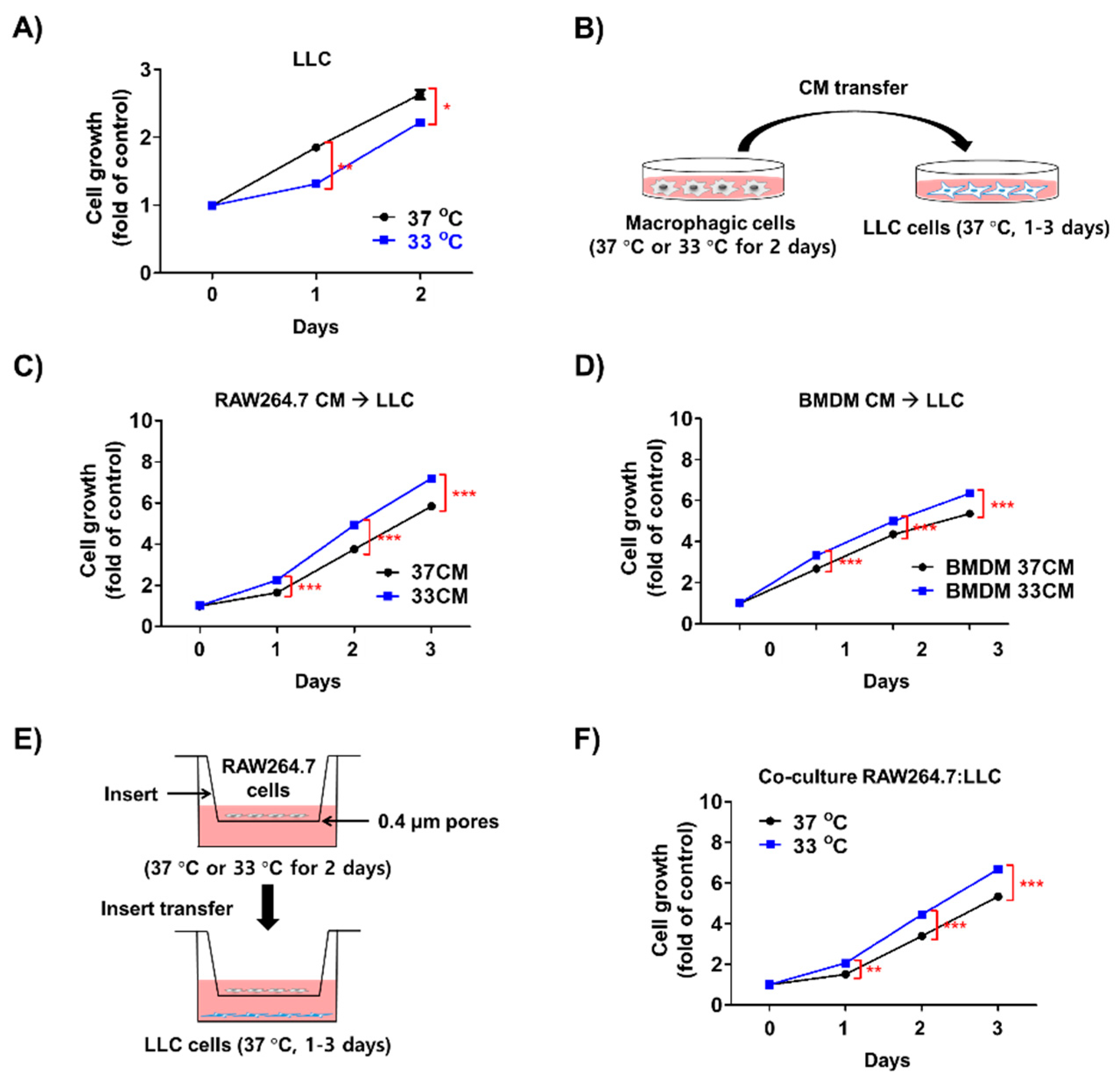
3.3. Low Temperature-Activated Macrophages Enhanced Cancer Cell Growth
3.4. Glutamine Levels Were Elevated in the Media of Macrophages Exposed to Low Temperature
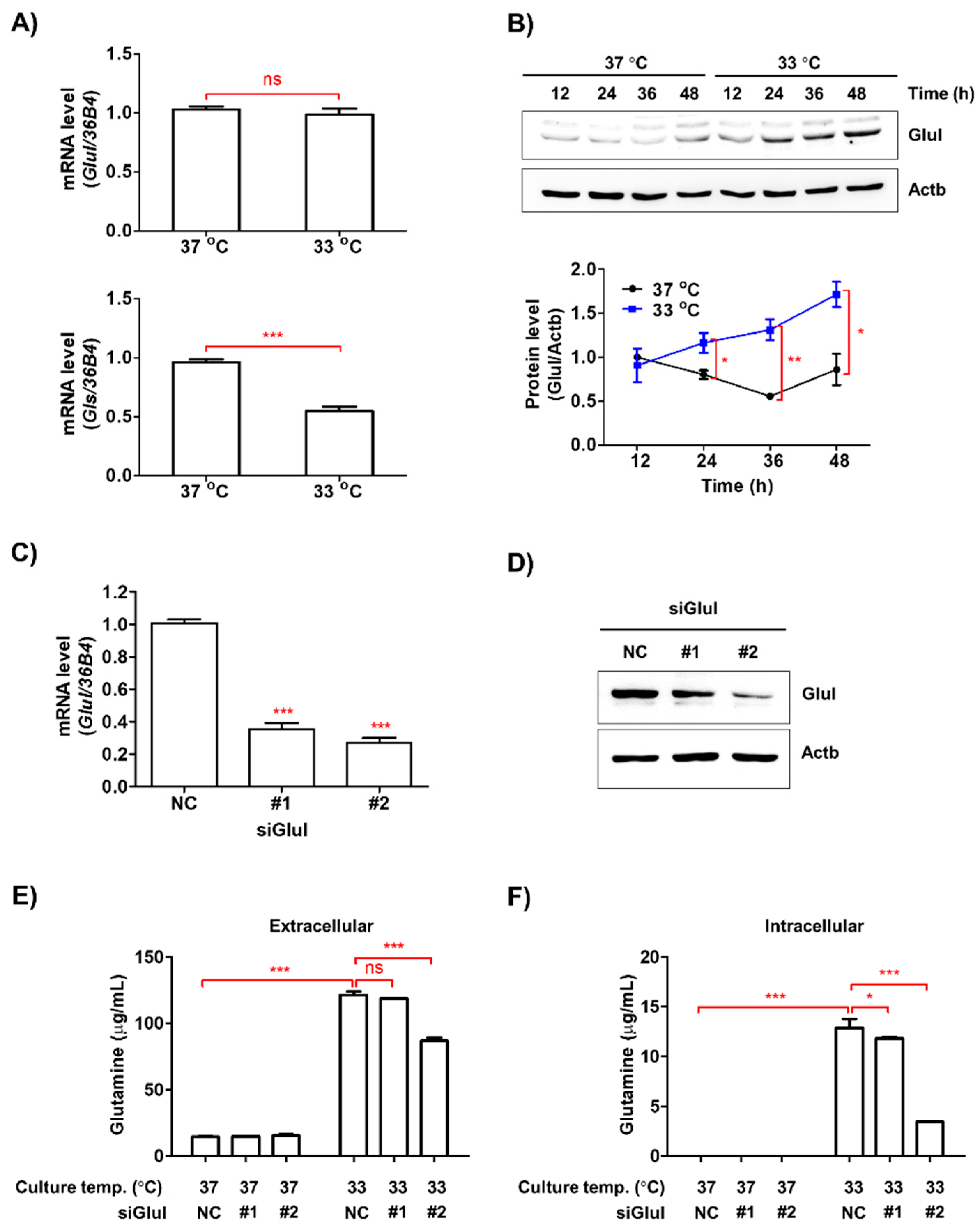
3.5. Low Ambient Temperature Stimulated Glutamine Secretion in Macrophages by Increasing Glutamine Synthetase (Glul) Protein Levels
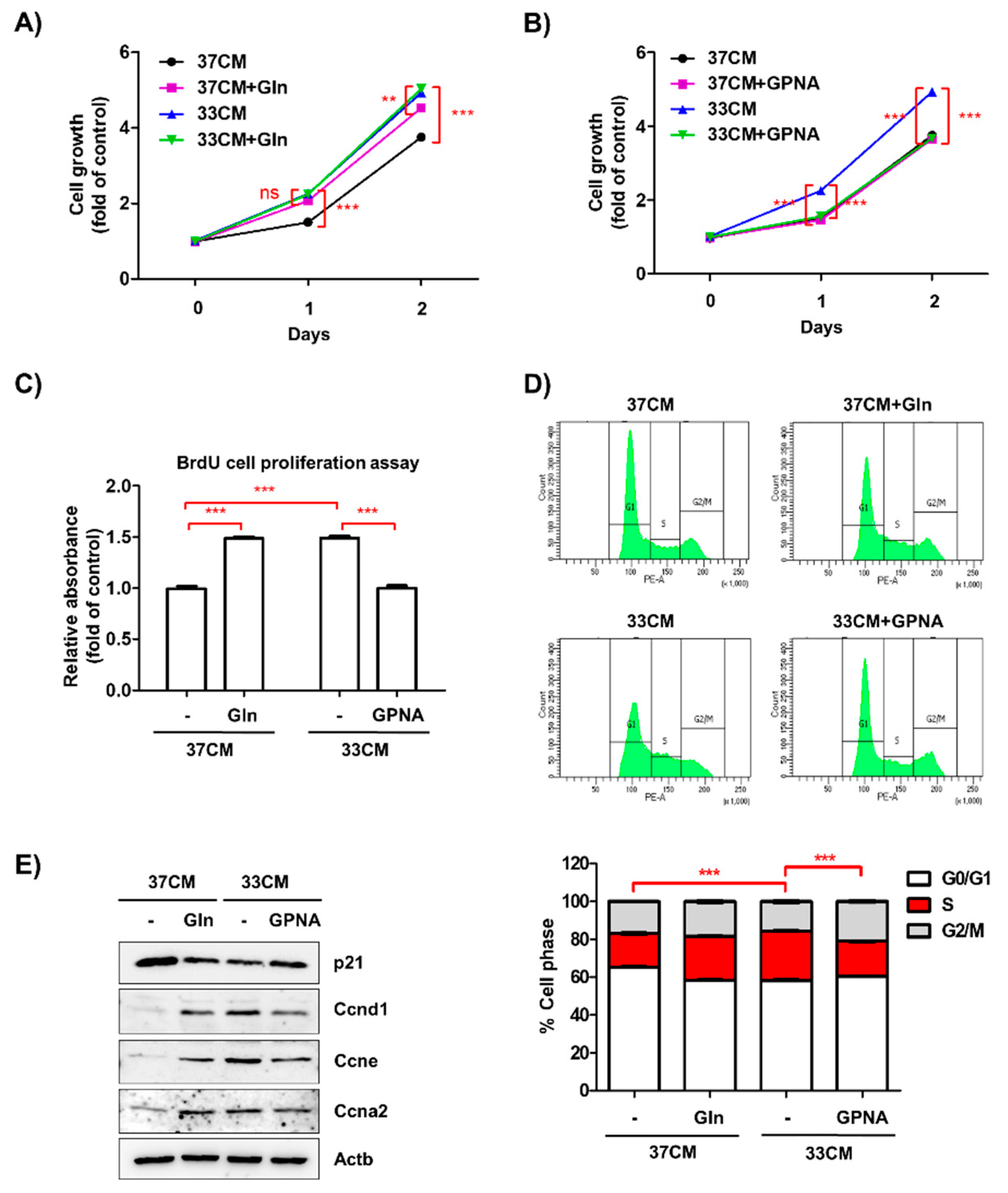
3.6. Extracellular Glutamine Derived from Low Temperature-Exposed Macrophages Enhanced Cancer Cell Growth
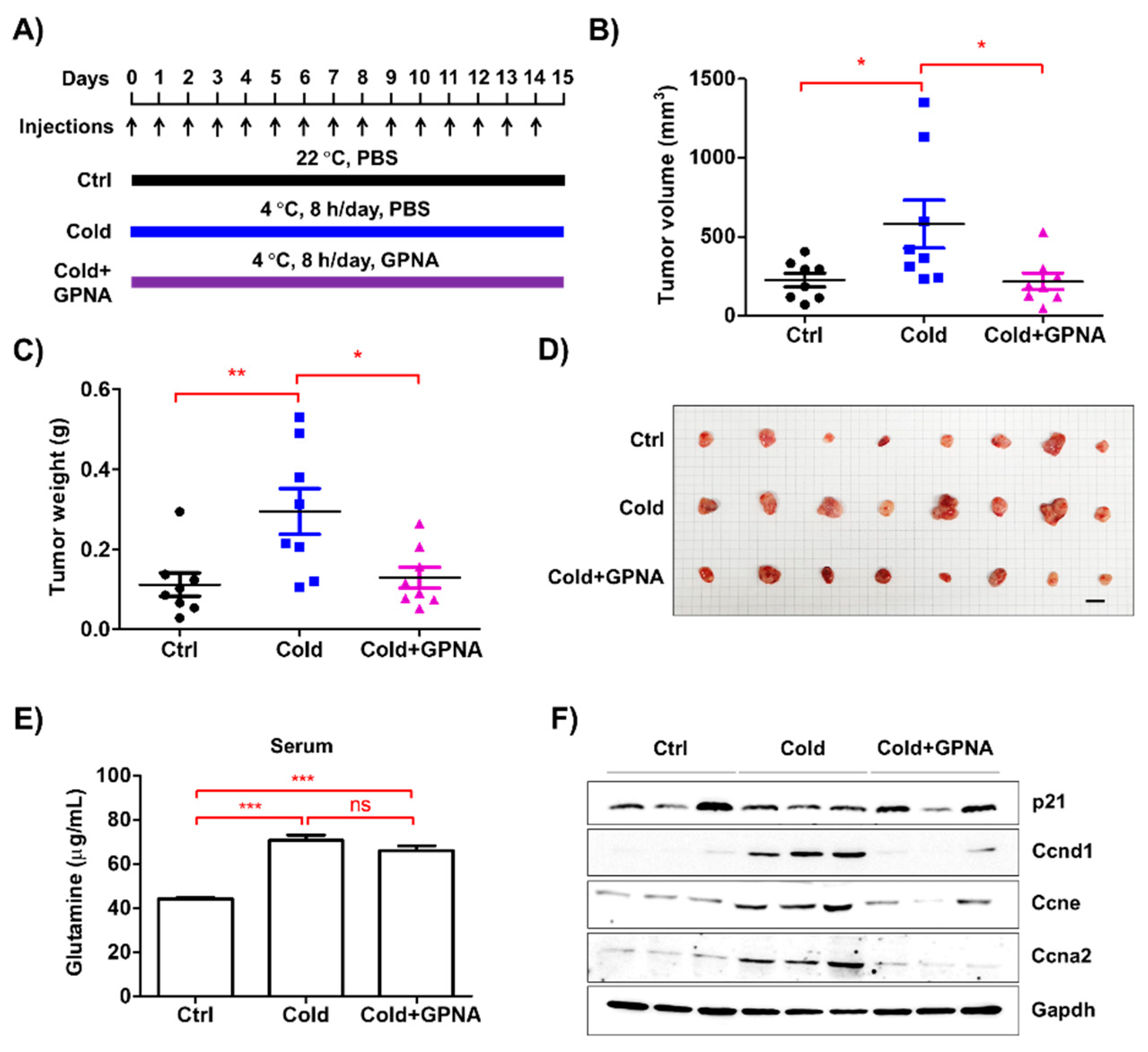
3.7. Inhibition of Glutamine Uptake Attenuated Low Temperature-Accelerated Allograft Tumor Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rabinowitz, P.; Conti, L. Links Among Human Health, Animal Health, and Ecosystem Health. Annu. Rev. Publ. Health 2013, 34, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A.; Guo, Y.; Hashizume, M.; Lavigne, E.; Zanobetti, A.; Schwartz, J.; Tobias, A.; Tong, S.; Rocklov, J.; Forsberg, B.; et al. Mortality risk attributable to high and low ambient temperature: A multicountry observational study. Lancet 2015, 386, 369–375. [Google Scholar] [CrossRef]
- Sharma, A.; Verma, H.K.; Joshi, S.; Panwar, M.S.; Mandal, C.C. A link between cold environment and cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 5953–5964. [Google Scholar] [CrossRef] [PubMed]
- Hart, J. Association Between Air Temperature and Cancer Death Rates in Florida: An Ecological Study. Dose-Response A Publ. Int. Hormesis Soc. 2015, 13. [Google Scholar] [CrossRef]
- Lehrer, S.; Rosenzweig, K.E. Cold Climate Is a Risk Factor for Thyroid Cancer. Clin. Thyroidol. 2014, 26, 273–276. [Google Scholar] [CrossRef]
- St-Hilaire, S.; Mannel, S.; Commendador, A.; Mandal, R.; Derryberry, D. Correlations between meteorological parameters and prostate cancer. Int. J. Health Geogr. 2010, 9, 19. [Google Scholar] [CrossRef]
- Kokolus, K.M.; Capitano, M.L.; Lee, C.T.; Eng, J.W.; Waight, J.D.; Hylander, B.L.; Sexton, S.; Hong, C.C.; Gordon, C.J.; Abrams, S.I.; et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc. Natl. Acad. Sci. USA 2013, 110, 20176–20181. [Google Scholar] [CrossRef]
- Du, G.; Zhao, B.; Zhang, Y.; Sun, T.; Liu, W.; Li, J.; Liu, Y.; Wang, Y.; Li, H.; Hou, X. Hypothermia activates adipose tissue to promote malignant lung cancer progression. PLoS ONE 2013, 8, e72044. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef]
- Gein, S.V.; Sharav’eva, I.L. Chronic cold stress modulates the function of peritoneal macrophages in vivo. Dokl. Biol. Sci. 2017, 474, 129–131. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Qiu, Y.; Cui, X.; Goh, Y.P.; Mwangi, J.; David, T.; Mukundan, L.; Brombacher, F.; Locksley, R.M.; Chawla, A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011, 480, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pavlova, N.N.; Thompson, C.B. Cancer cell metabolism: The essential role of the nonessential amino acid, glutamine. EMBO J. 2017, 36, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Renner, K.; Singer, K.; Koehl, G.E.; Geissler, E.K.; Peter, K.; Siska, P.J.; Kreutz, M. Metabolic Hallmarks of Tumor and Immune Cells in the Tumor Microenvironment. Front. Immunol. 2017, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Achreja, A.; Yeung, T.L.; Mangala, L.S.; Jiang, D.; Han, C.; Baddour, J.; Marini, J.C.; Ni, J.; Nakahara, R.; et al. Targeting Stromal Glutamine Synthetase in Tumors Disrupts Tumor Microenvironment-Regulated Cancer Cell Growth. Cell Metab. 2016, 24, 685–700. [Google Scholar] [CrossRef]
- Palmieri, E.M.; Menga, A.; Martin-Perez, R.; Quinto, A.; Riera-Domingo, C.; De Tullio, G.; Hooper, D.C.; Lamers, W.H.; Ghesquiere, B.; McVicar, D.W.; et al. Pharmacologic or Genetic Targeting of Glutamine Synthetase Skews Macrophages toward an M1-like Phenotype and Inhibits Tumor Metastasis. Cell Rep. 2017, 20, 1654–1666. [Google Scholar] [CrossRef]
- Moon, S.C.; Joo, S.Y.; Chung, T.W.; Choi, H.J.; Park, M.J.; Choi, H.J.; Bae, S.J.; Kim, K.J.; Kim, C.H.; Joo, M.; et al. Abiotic stress of ambient cold temperature regulates the host receptivity to pathogens by cell surfaced sialic acids. Biochem. Biophys. Res. Commun. 2016, 476, 159–166. [Google Scholar] [CrossRef]
- Soga, T.; Ohashi, Y.; Ueno, Y.; Naraoka, H.; Tomita, M.; Nishioka, T. Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J. Proteome Res. 2003, 2, 488–494. [Google Scholar] [CrossRef]
- Lim, S.; Honek, J.; Xue, Y.; Seki, T.; Cao, Z.; Andersson, P.; Yang, X.; Hosaka, K.; Cao, Y. Cold-induced activation of brown adipose tissue and adipose angiogenesis in mice. Nat. Protoc. 2012, 7, 606–615. [Google Scholar] [CrossRef]
- Ma, S.; Morilak, D.A. Chronic intermittent cold stress sensitises the hypothalamic-pituitary-adrenal response to a novel acute stress by enhancing noradrenergic influence in the rat paraventricular nucleus. J. Neuroendocr. 2005, 17, 761–769. [Google Scholar] [CrossRef]
- Blonska, M.; Agarwal, N.K.; Vega, F. Shaping of the tumor microenvironment: Stromal cells and vessels. Semin. Cancer Biol. 2015. [Google Scholar] [CrossRef]
- Vergara, M.; Becerra, S.; Berrios, J.; Osses, N.; Reyes, J.; Rodriguez-Moya, M.; Gonzalez, R.; Altamirano, C. Differential Effect of Culture Temperature and Specific Growth Rate on CHO Cell Behavior in Chemostat Culture. PLoS ONE 2014, 9, e093865. [Google Scholar] [CrossRef] [PubMed]
- White, M.D.; Bosio, C.M.; Duplantis, B.N.; Nano, F.E. Human body temperature and new approaches to constructing temperature-sensitive bacterial vaccines. Cell Mol. Life Sci. 2011, 68, 3019–3031. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Kutyavin, V.I.; Chawla, A. Tissue Immunometabolism: Development, Physiology, and Pathobiology. Cell Metab. 2017, 25, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Bott, A.J.; Peng, I.C.; Fan, Y.J.; Faubert, B.; Zhao, L.; Li, J.Y.; Neidler, S.; Sun, Y.; Jaber, N.; Krokowski, D.; et al. Oncogenic Myc Induces Expression of Glutamine Synthetase through Promoter Demethylation. Cell Metab. 2015, 22, 1068–1077. [Google Scholar] [CrossRef]
- Lopez-Soriano, F.J.; Alemany, M. Effect of cold-temperature exposure and acclimation on amino acid pool changes and enzyme activities of rat brown adipose tissue. Biochim. Biophys. Acta 1987, 925, 265–271. [Google Scholar] [CrossRef]
- Al-Badry, K.S.; Taha, H.M. Hibernation hypothermia and metabolism in hedgehogs--changes in free amino acids and related compounds. Comp. Biochem. Physiol. A Comp. Physiol. 1982, 72, 541–547. [Google Scholar] [CrossRef]
- Hall, J.R.; Short, C.E.; Rise, M.L.; Driedzic, W.R. Expression analysis of glycerol synthesis-related liver transcripts in rainbow smelt (Osmerus mordax) exposed to a controlled decrease in temperature. Physiol. Biochem. Zool. 2012, 85, 74–84. [Google Scholar] [CrossRef]
- Piperberg, J.B.; Reif-Lehrer, L. Glutamine synthetase in cultured whole retinas from the embryonic chick. Role of protein and RNA syntheses in 4 degrees C storage enhancement. Cell Biophys. 1984, 6, 131–148. [Google Scholar] [CrossRef]
- Cui, S.; Huang, F.; Wang, J.; Ma, X.; Cheng, Y.; Liu, J. A proteomic analysis of cold stress responses in rice seedlings. Proteomics 2005, 5, 3162–3172. [Google Scholar] [CrossRef]
- Rinalducci, S.; Egidi, M.G.; Karimzadeh, G.; Jazii, F.R.; Zolla, L. Proteomic analysis of a spring wheat cultivar in response to prolonged cold stress. Electrophoresis 2011, 32, 1807–1818. [Google Scholar] [CrossRef]
- Arad, G.; Freikopf, A.; Kulka, R.G. Glutamine-stimulated modification and degradation of glutamine synthetase in hepatoma tissue culture cells. Cell 1976, 8, 95–101. [Google Scholar] [CrossRef]
- Van Nguyen, T.; Lee, J.E.; Sweredoski, M.J.; Yang, S.-J.; Jeon, S.-J.; Harrison, J.S.; Yim, J.-H.; Lee, S.G.; Handa, H.; Kuhlman, B. Glutamine triggers acetylation-dependent degradation of glutamine synthetase via the thalidomide receptor cereblon. Mol. Cell 2016, 61, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Wang, Y.; Watford, M. Glutamine directly downregulates glutamine synthetase protein levels in mouse C2C12 skeletal muscle myotubes. J. Nutr. 2007, 137, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Huyghe, D.; Nakamura, Y.; Terunuma, M.; Faideau, M.; Haydon, P.; Pangalos, M.N.; Moss, S.J. Glutamine synthetase stability and subcellular distribution in astrocytes are regulated by gamma-aminobutyric type B receptors. J. Biol. Chem. 2014, 289, 28808–28815. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.; Seabra, A.; Melo, P.; Cullimore, J.; Carvalho, H. Post-translational regulation of cytosolic glutamine synthetase of Medicago truncatula. J. Exp. Bot. 2006, 57, 2751–2761. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Vanhove, K.; Derveaux, E.; Graulus, G.J.; Mesotten, L.; Thomeer, M.; Noben, J.P.; Guedens, W.; Adriaensens, P. Glutamine Addiction and Therapeutic Strategies in Lung Cancer. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, M.; Qian, J.; Hoeksema, M.D.; Wang, J.; Jacobovitz, M.; Ji, X.; Harris, F.T.; Harris, B.K.; Boyd, K.L.; Chen, H.; et al. Targeting SLC1a5-mediated glutamine dependence in non-small cell lung cancer. Int. J. Cancer 2015, 137, 1587–1597. [Google Scholar] [CrossRef]
- Hassanein, M.; Hoeksema, M.D.; Shiota, M.; Qian, J.; Harris, B.K.; Chen, H.; Clark, J.E.; Alborn, W.E.; Eisenberg, R.; Massion, P.P. SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin. Cancer Res. 2013, 19, 560–570. [Google Scholar] [CrossRef]
- Weiner, I.D.; Mitch, W.E.; Sands, J.M. Urea and Ammonia Metabolism and the Control of Renal Nitrogen Excretion. Clin. J. Am. Soc. Nephrol. 2015, 10, 1444–1458. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.-J.; Chung, T.-W.; Kim, K.-J.; Bae, B.; Kim, B.-S.; Kim, S.; Ryu, D.; Bae, S.-J.; Ha, K.-T. Macrophage Stimulated by Low Ambient Temperature Hasten Tumor Growth via Glutamine Production. Biomedicines 2020, 8, 381. https://doi.org/10.3390/biomedicines8100381
Lee E-J, Chung T-W, Kim K-J, Bae B, Kim B-S, Kim S, Ryu D, Bae S-J, Ha K-T. Macrophage Stimulated by Low Ambient Temperature Hasten Tumor Growth via Glutamine Production. Biomedicines. 2020; 8(10):381. https://doi.org/10.3390/biomedicines8100381
Chicago/Turabian StyleLee, Eun-Ji, Tae-Wook Chung, Keuk-Jun Kim, Boram Bae, Bo-Sung Kim, Suhkmann Kim, Dongryeol Ryu, Sung-Jin Bae, and Ki-Tae Ha. 2020. "Macrophage Stimulated by Low Ambient Temperature Hasten Tumor Growth via Glutamine Production" Biomedicines 8, no. 10: 381. https://doi.org/10.3390/biomedicines8100381
APA StyleLee, E.-J., Chung, T.-W., Kim, K.-J., Bae, B., Kim, B.-S., Kim, S., Ryu, D., Bae, S.-J., & Ha, K.-T. (2020). Macrophage Stimulated by Low Ambient Temperature Hasten Tumor Growth via Glutamine Production. Biomedicines, 8(10), 381. https://doi.org/10.3390/biomedicines8100381





