Abundance of Non-Polarized Lung Macrophages with Poor Phagocytic Function in Chronic Obstructive Pulmonary Disease (COPD)
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Broncho-Alveolar Lavage (BAL) Collection
2.3. Alveolar Macrophages
2.4. Inmunofluorescence Staining and Flow Cytometric Analysis
2.5. Measurement of Macrophage Phagocytosis
2.6. Gene Expression Measurement/Analysis
2.7. Statistical Analysis
2.8. Gene Expression Analysis
3. Results
3.1. Patient Characteristics
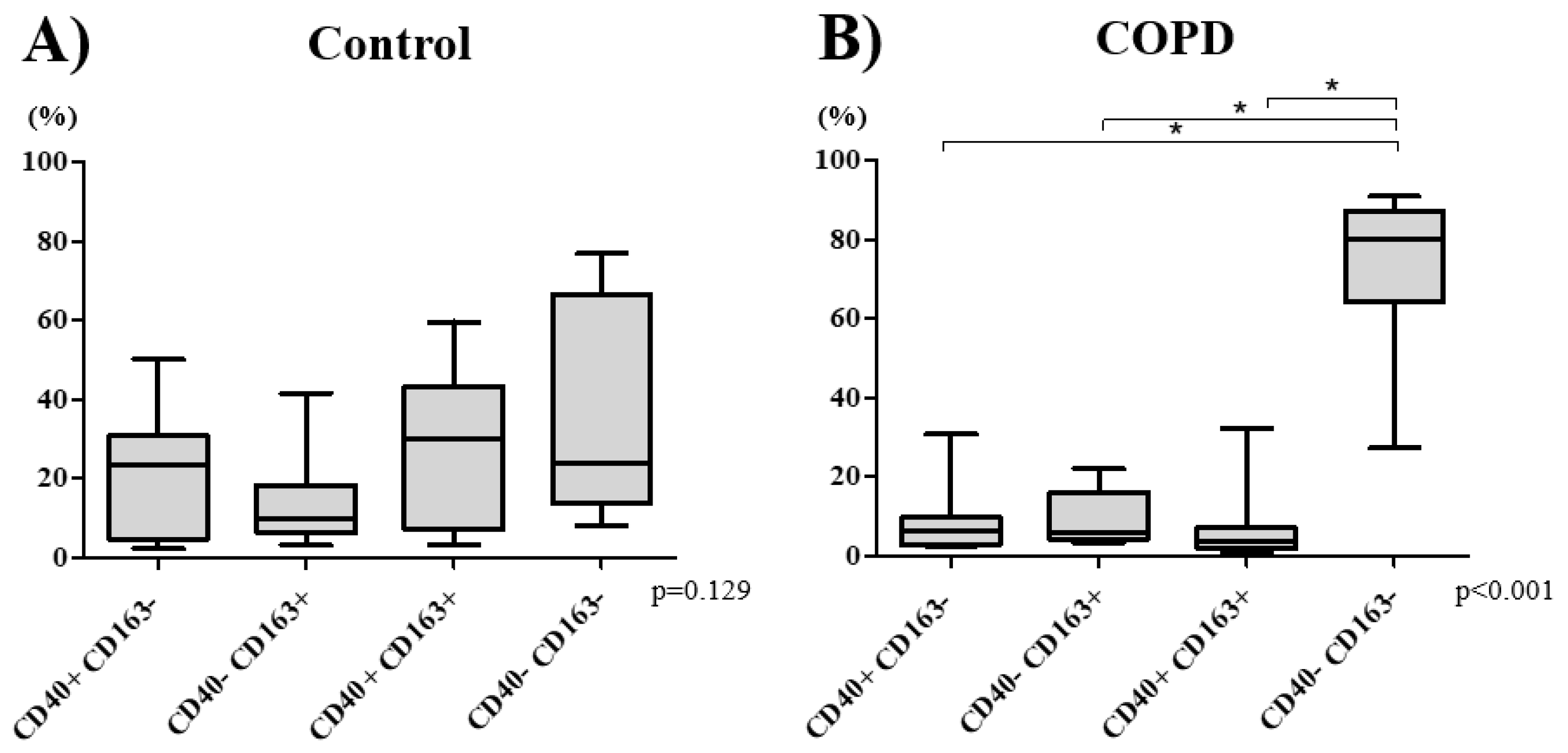
3.2. Macrophage Phenotype Distribution
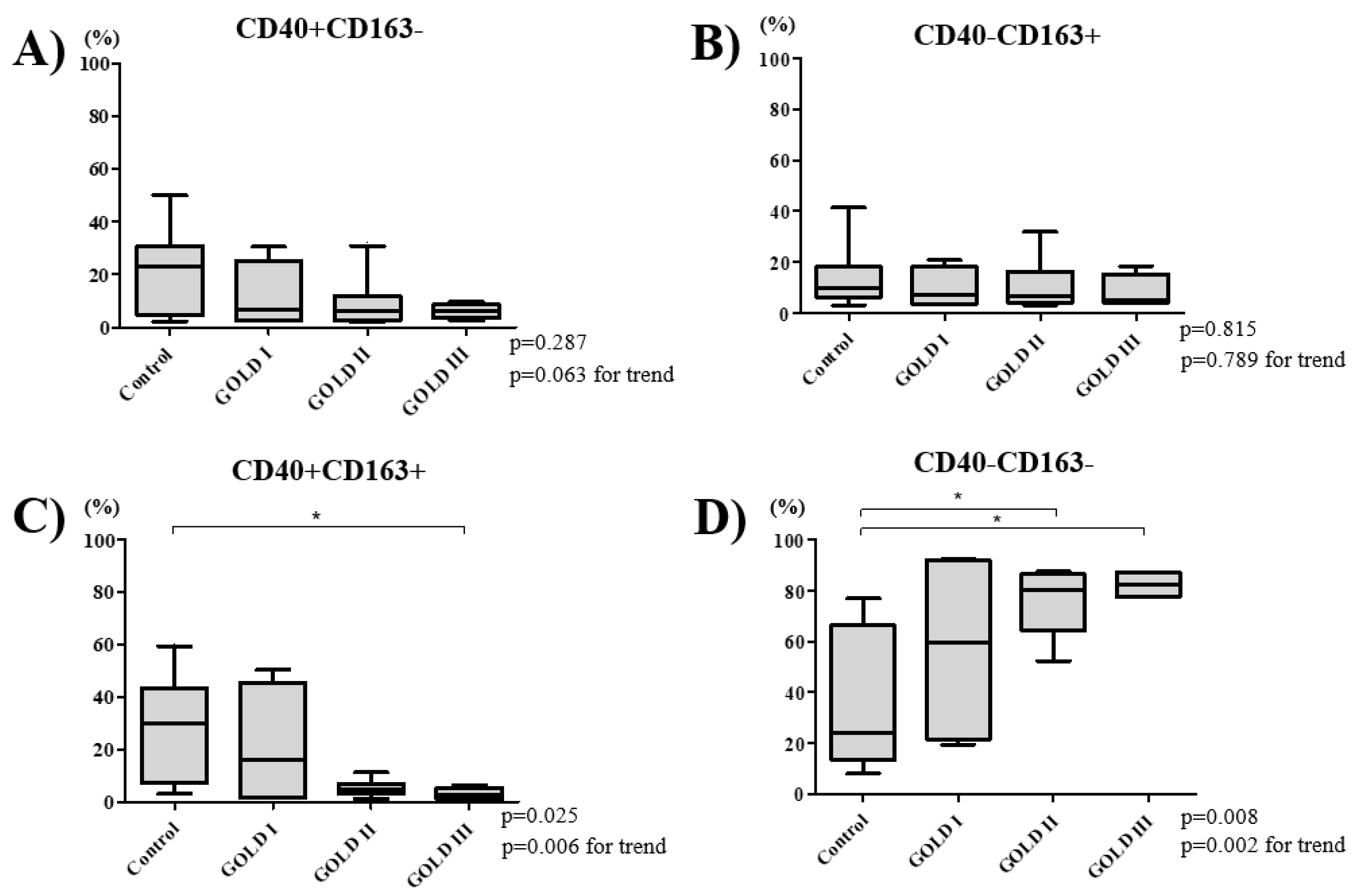
3.3. COPD Severity and Distribution of Macrophage Subtypes
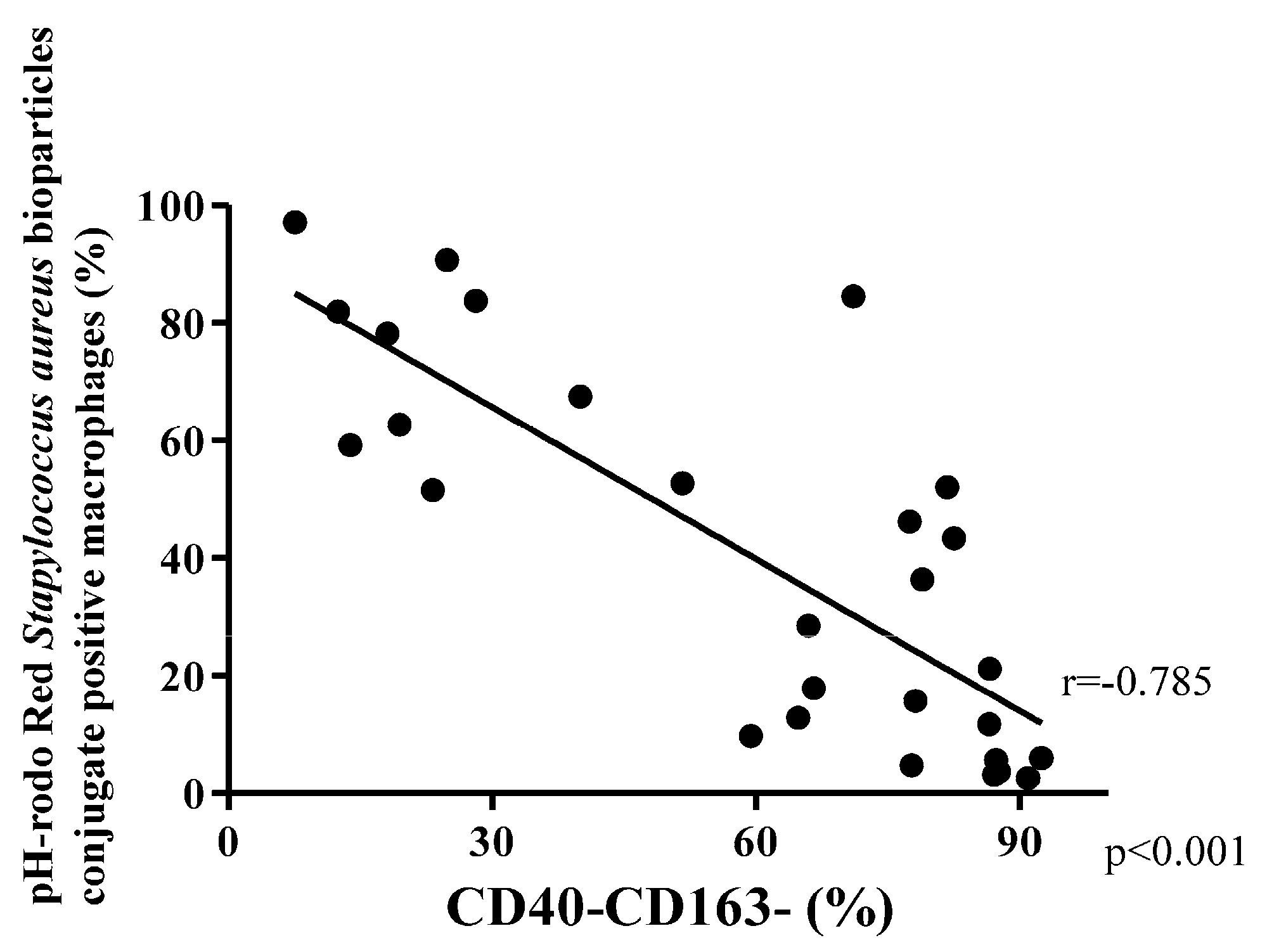
3.4. Phagocytosis Macrophage Subtypes
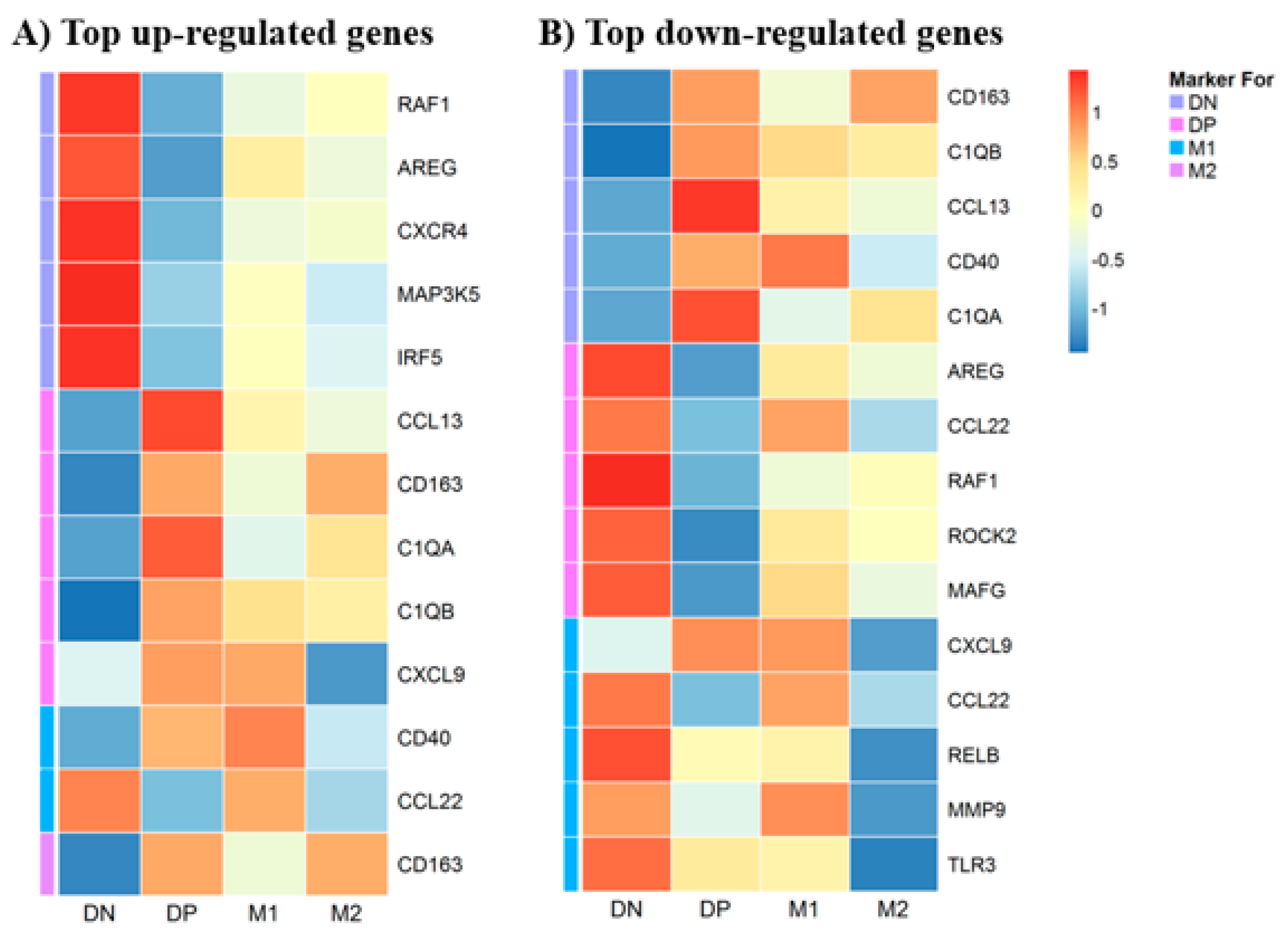
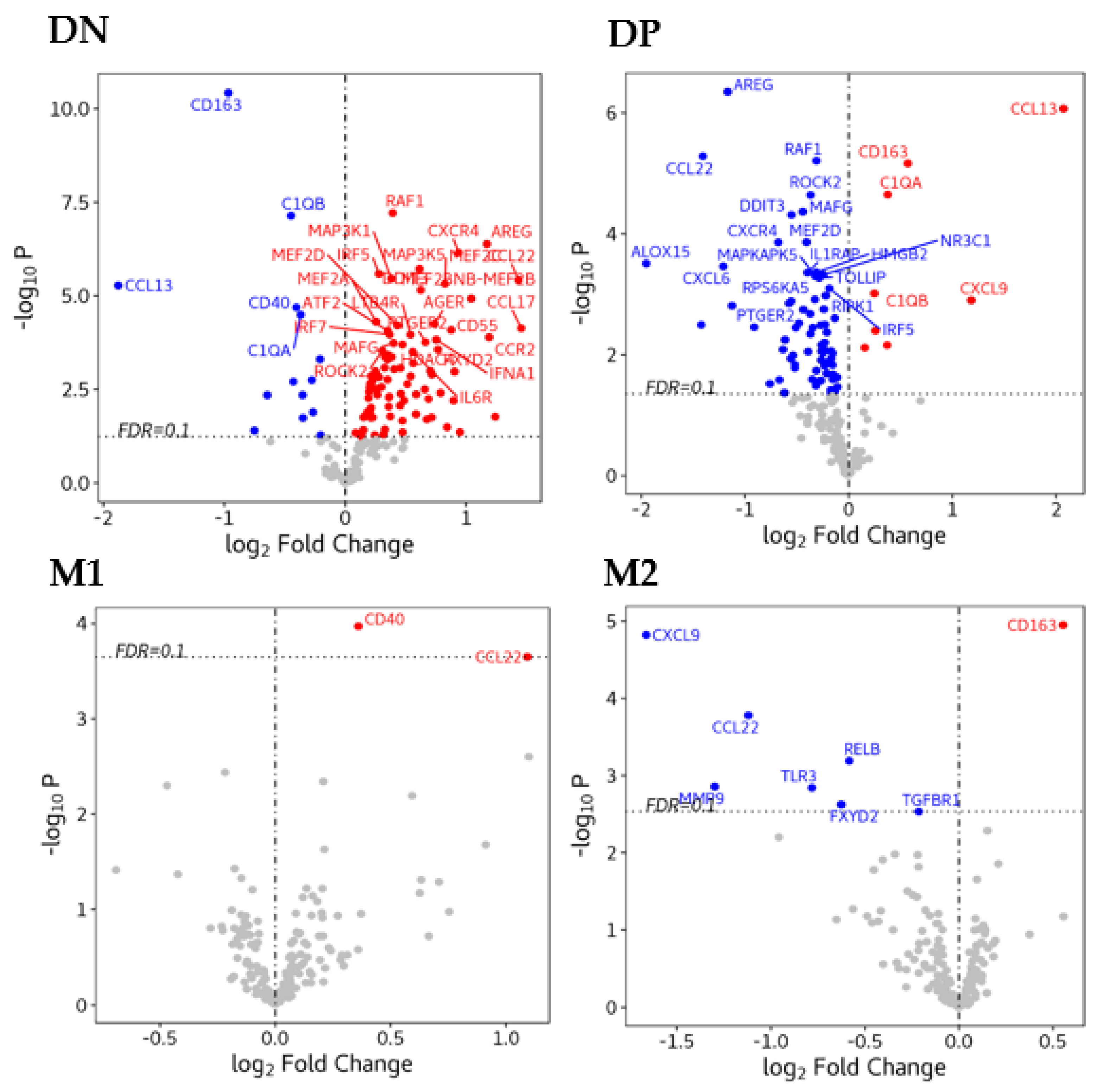
3.5. Gene Expression in Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data and Material Availability
References
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Miyata, R.; Van Eeden, S.F. The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicol. Appl. Pharmacol. 2011, 257, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Costabel, U.; Guzman, J. Effect of smoking on bronchoalveolar lavage constituents. Eur. Respir. J. 1992, 5, 776–779. [Google Scholar] [PubMed]
- Ando, M.; Sugimoto, M.; Nishi, R.; Suga, M.; Horio, S.; Kohrogi, H.; Shimazu, K.; Araki, S. Surface morphology and function of human pulmonary alveolar macrophages from smokers and non-smokers. Thorax 1984, 39, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Hogg, J.C.; Chu, F.; Utokaparch, S.; Woods, R.; Elliott, W.M.; Buzatu, L.; Cherniack, R.M.; Rogers, R.M.; Sciurba, F.C.; Coxson, H.; et al. The Nature of Small-Airway Obstruction in Chronic Obstructive Pulmonary Disease. New Engl. J. Med. 2004, 350, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.; Fraser, R.S.; Ghezzo, H.; Cosio, M.G. Alveolar inflammation and its relation to emphysema in smokers. Am. J. Respir. Crit. Care Med. 1995, 152, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Alveolar macrophages as orchestrators of COPD. COPD J. Chronic Obstr. Pulm. Dis. 2004, 1, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Byers, D.; Holtzman, M.J. Alternatively activated macrophages and airway disease. Chest 2011, 140, 768–774. [Google Scholar] [CrossRef]
- Bazzan, E.; Turato, G.; Tinè, M.; Radu, C.M.; Balestro, E.; Rigobello, C.; Biondini, D.; Schiavon, M.; Lunardi, F.; Baraldo, S.; et al. Dual polarization of human alveolar macrophages progressively increases with smoking and COPD severity. Respir. Res. 2017, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Eapen, M.S.; Hansbro, P.M.; McAlinden, K.; Kim, R.Y.; Ward, C.; Hackett, T.L.; Walters, E.H.; Sohal, S.S. Abnormal M1/M2 macrophage phenotype profiles in the small airway wall and lumen in smokers and chronic obstructive pulmonary disease (COPD). Sci. Rep. 2017, 7, 13392. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S.; Matthews, G.; Mukaro, V.; Ahern, J.; Shivam, A.; Hodge, G.; Holmes, M.; Jersmann, H.; Reynolds, P.N. Cigarette Smoke-Induced Changes to Alveolar Macrophage Phenotype and Function Are Improved by Treatment with Procysteine. Am. J. Respir. Cell Mol. Boil. 2011, 44, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Shaykhiev, R.; Krause, A.; Salit, J.; Strulovici-Barel, Y.; Harvey, B.-G.; O’Connor, T.P.; Crystal, R.G. Smoking-dependent reprogramming of alveolar macrophage polarization: Implication for pathogenesis of chronic obstructive pulmonary disease. J. Immunol. 2009, 183, 2867–2883. [Google Scholar] [CrossRef] [PubMed]
- Kunz, L.I.Z.; Lapperre, T.S.; Snoeck-Stroband, J.B.; Budulac, S.; Timens, W.; Van Wijngaarden, S.; Schrumpf, J.A.; Rabe, K.F.; Postma, D.S.; Sterk, P.J.; et al. Smoking status and anti-inflammatory macrophages in bronchoalveolar lavage and induced sputum in COPD. Respir. Res. 2011, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Van Eeden, S.F. Lung Macrophage Phenotypes and Functional Responses: Role in the Pathogenesis of COPD. Int. J. Mol. Sci. 2018, 19, 582. [Google Scholar] [CrossRef]
- Akata, K.; Van Eeden, S.F. Lung Macrophage Functional Properties in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2020, 21, 853. [Google Scholar] [CrossRef]
- Rabe, K.F.; Hurd, S.; Anzueto, A.; Barnes, P.J.; Buist, S.A.; Calverley, P.; Zielinski, J. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2007, 176, 532–555. [Google Scholar] [CrossRef]
- Hodge, S.; Hodge, G.; Ahern, J.; Jersmann, H.; Holmes, M.; Rey, P.N. Smoking Alters Alveolar Macrophage Recognition and Phagocytic Ability Implications in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2007, 37, 748–755. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Taylor, L.; Lee, H.; Cowley, S.A.; James, W.S.; Iqbal, A.J.; Greaves, D.R. A novel real time imaging platform to quantify macrophage phagocytosis. Biochem. Pharmacol. 2016, 116, 107–119. [Google Scholar] [CrossRef]
- Berenson, C.S.; Kruzel, R.L.; Wrona, C.T.; Mammen, M.J.; Sethi, S. Impaired Innate COPD Alveolar Macrophage Responses and Toll-Like Receptor-9 Polymorphisms. PLoS ONE 2015, 10, e0134209. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.E.; Finney-Hayward, T.K.; Quint, J.K.; Thomas, C.M.R.; Tudhope, S.J.; Wedzicha, J.A.; Barnes, P.J.; Donnelly, L.E. Defective macrophage phagocytosis of bacteria in COPD. Eur. Respir. J. 2009, 35, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Löfdahl, M.; Wahlström, J.; Sköld, C.M. Different inflammatory cell pattern and macrophage phenotype in chronic obstructive pulmonary disease patients, smokers and non-smokers. Clin. Exp. Immunol. 2006, 145, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Thomas, A.; Lenz, L.L.; Munder, M. Macrophage: SHIP of Immunity. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Landsman, L.; Jung, S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J. Immunol. 2007, 179, 3488–3494. [Google Scholar] [CrossRef] [PubMed]
- Phipps, J.C.; Aronoff, D.M.; Curtis, J.L.; Goel, D.; O’Brien, E.; Mancuso, P. Cigarette Smoke Exposure Impairs Pulmonary Bacterial Clearance and Alveolar Macrophage Complement-Mediated Phagocytosis of Streptococcus pneumoniae. Infect. Immun. 2009, 78, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Berenson, C.S.; Kruzel, R.L.; Eberhardt, E.; Sethi, S. Phagocytic Dysfunction of Human Alveolar Macrophages and Severity of Chronic Obstructive Pulmonary Disease. J. Infect. Dis. 2013, 208, 2036–2045. [Google Scholar] [CrossRef] [PubMed]
- Martí-Lliteras, P.; Regueiro, V.; Morey, P.; Hood, D.W.; Saus, C.; Sauleda, J.; Agustí, A.G.N.; Bengoechea, J.A.; Garmendia, J. Nontypeable Haemophilus influenzae Clearance by Alveolar Macrophages Is Impaired by Exposure to Cigarette Smoke. Infect. Immun. 2009, 77, 4232–4242. [Google Scholar] [CrossRef]
- Berenson, C.S.; Garlipp, M.A.; Grove, L.J.; Maloney, J.; Sethi, S. Impaired Phagocytosis of NontypeableHaemophilus influenzaeby Human Alveolar Macrophages in Chronic Obstructive Pulmonary Disease. J. Infect. Dis. 2006, 194, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; D’Adda, D.; Falchi, M.; Dall’Asta, L. The macrophagic activity of patients affected by pneumonia or chronic obstructive pulmonary disease. Int. J. Tissue React. 1996, 18, 109–114. [Google Scholar] [PubMed]
- Vecchiarelli, A.; Dottorini, M.; Puliti, M.; Todisco, T.; Cenci, E.; Bistoni, F. Defective Candidacidal Activity of Alveolar Macrophages and Peripheral Blood Monocytes from Patients with Chronic Obstructive Pulmonary Disease. Am. Rev. Respir. Dis. 1991, 143, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Seemungal, T.A.R.; Donaldson, G.C.; Paul, E.A.; Bestall, J.; Jeffries, D.J.; Wedzicha, J.A. Effect of Exacerbation on Quality of Life in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1998, 157, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Gerrick, K.Y.; Gerrick, E.R.; Gupta, A.; Wheelan, S.J.; Yegnasubramanian, S.; Jaffee, E.M. Transcriptional profiling identifies novel regulators of macrophage polarization. PLoS ONE 2018, 13, e0208602. [Google Scholar] [CrossRef] [PubMed]
- Beyer, M.; Mallmann, M.R.; Xue, J.; Staratschek-Jox, A.; Vorholt, D.; Krebs, W.; Sommer, D.; Sander, J.; Mertens, C.; Nino-Castro, A.C.; et al. High-Resolution Transcriptome of Human Macrophages. PLoS ONE 2012, 7, e45466. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional Profiling of the Human Monocyte-to-Macrophage Differentiation and Polarization: New Molecules and Patterns of Gene Expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef]
- Van Kooten, C.; Banchereau, J. CD40-CD40 ligand. J. Leukoc. Boil. 2000, 67, 2–17. [Google Scholar] [CrossRef]
- Hogger, P.; Dreier, J.; Droste, A.; Buck, F.; Sorg, C. Identification of the integral membrane protein RM3/1 on human monocytes as a glucocorticoid-inducible member of the scavenger receptor cysteine-rich family (CD163). J. Immunol. 1998, 161, 1883–1890. [Google Scholar]
- Fraser, D.A.; Tenner, A.J. Directing an appropriate immune response: The role of defense collagens and other soluble pattern recognition molecules. Curr. Drug Targets 2008, 9, 113–122. [Google Scholar] [CrossRef]
- Bobak, D.A.; Frank, M.M.; Tenner, A.J. C1q acts synergistically with phorbol dibutyrate to activate CR1-mediated phagocytosis by human mononuclear phagocytes. Eur. J. Immunol. 1988, 18, 2001–2007. [Google Scholar] [CrossRef]
- Bobak, D.A.; Gaither, T.A.; Frank, M.M.; Tenner, A.J. Modulation of FcR function by complement: Subcomponent C1q enhances the phagocytosis of IgG-opsonized targets by human monocytes and culture-derived macrophages. J. Immunol. 1987, 138, 1150–1156. [Google Scholar] [PubMed]
- Benoit, M.E.; Clarke, E.; Morgado, P.; Fraser, D.A.; Tenner, A.J. Complement protein C1q directs macrophage polarization and limits inflammasome activity during the uptake of apoptotic cells. J. Immunol. 2012, 188, 5682–5693. [Google Scholar] [CrossRef] [PubMed]
- Spivia, W.; Magno, P.S.; Le, P.; Fraser, D.A. Complement protein C1q promotes macrophage anti-inflammatory M2-like polarization during the clearance of atherogenic lipoproteins. Inflamm. Res. 2014, 63, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, C.; Rapp, U.R. Isotype-Specific Functions of Raf Kinases. Exp. Cell Res. 1999, 253, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wood, K.; Mamon, H.; Haeer, W.; Roberts, T. Raf-l: A Kinase Currently without a Cause but Not Lacking In Effects. Cell 1991, 64, 479–482. [Google Scholar] [CrossRef]
- Zaiss, D.M.W.; Gause, W.C.; Osborne, L.C.; Artis, D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity 2015, 42, 216–226. [Google Scholar] [CrossRef]
- Berasain, C.; Avila, M.A. Amphiregulin. Semin. Cell Dev. Biol. 2014, 28, 31–41. [Google Scholar] [CrossRef]
- Mograbi, B.; Rochet, N.; Imbert, V.; Bourget, I.; Bocciardi, R.; Emiliozzi, C.; Rossi, B. Human monocytes express amphiregulin and heregulin growth factors upon activation. Eur. Cytokine Netw. 1997, 8, 73–81. [Google Scholar]
- Ko, J.H.; Kim, H.J.; Jeong, H.J.; Lee, H.J.; Oh, J. Mesenchymal Stem and Stromal Cells Harness Macrophage-Derived Amphiregulin to Maintain Tissue Homeostasis. Cell Rep. 2020, 30, 3806–3820. [Google Scholar] [CrossRef]
- Yamane, S.; Ishida, S.; Hanamoto, Y.; Kumagai, K.-I.; Masuda, R.; Tanaka, K.; Shiobara, N.; Yamane, N.; Mori, T.; Juji, T.; et al. Proinflammatory role of amphiregulin, an epidermal growth factor family member whose expression is augmented in rheumatoid arthritis patients. J. Inflamm. 2008, 5, 5. [Google Scholar] [CrossRef]
- Carter, A.B.; Monick, M.M.; Hunninghake, G.W. Both Erk and p38 Kinases Are Necessary for Cytokine Gene Transcription. Am. J. Respir. Cell Mol. Boil. 1999, 20, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.-Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.-F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Shih, C.-H.; Lin, Y.-C.; Yang, Y.-L.; Chen, B.-C. MEKK1, JNK, and SMAD3 mediate CXCL12-stimulated connective tissue growth factor expression in human lung fibroblasts. J. Biomed. Sci. 2018, 25, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Naguro, I.; Runchel, C.; Ichijo, H. The roles of ASK family proteins in stress responses and diseases. Cell Commun. Signal. 2009, 7, 9. [Google Scholar] [CrossRef]
- Nygaard, G.; Di Paolo, J.A.; Hammaker, D.; Boyle, D.L.; Budas, G.; Notte, G.T.; Mikaelian, I.; Barry, V.; Firestein, G.S. Regulation and function of apoptosis signal-regulating kinase 1 in rheumatoid arthritis. Biochem. Pharmacol. 2018, 151, 282–290. [Google Scholar] [CrossRef]
- Xu, D.; Li, R.; Wu, J.; Jiang, L.; Zhong, H.A. Drug Design Targeting the CXCR4/CXCR7/CXCL12 Pathway. Curr. Top. Med. Chem. 2016, 16, 1441–1451. [Google Scholar] [CrossRef]
- Khorasanizadeh, M.; Eskian, M.; Gelfand, E.W.; Rezaei, N. Mitogen-activated protein kinases as therapeutic targets for asthma. Pharmacol. Ther. 2017, 174, 112–126. [Google Scholar] [CrossRef]
- Li, L.; Lv, G.; Wang, B.; Kuang, L. The role of lncRNA XIST/miR-211 axis in modulating the proliferation and apoptosis of osteoarthritis chondrocytes through CXCR4 and MAPK signaling. Biochem. Biophys. Res. Commun. 2018, 503, 2555–2562. [Google Scholar] [CrossRef]
- Husebø, G.R.; Bakke, P.S.; Grønseth, R.; Hardie, J.A.; Ueland, T.; Aukrust, P.; Eagan, T.M.L. Macrophage migration inhibitory factor, a role in COPD. Am. J. Physiol. Cell. Mol. Physiol. 2016, 311, L1–L7. [Google Scholar] [CrossRef]
- Goto, Y.; Hogg, J.C.; Whalen, B.; Shih, C.-H.; Ishii, H.; Van Eeden, S.F. Monocyte Recruitment into the Lungs in Pneumococcal Pneumonia. Am. J. Respir. Cell Mol. Boil. 2004, 30, 620–626. [Google Scholar] [CrossRef]
- Goto, Y.; Hogg, J.C.; Shih, C.-H.; Ishii, H.; Vincent, R.; Van Eeden, S.F. Exposure to ambient particles accelerates monocyte release from bone marrow in atherosclerotic rabbits. Am. J. Physiol. Cell. Mol. Physiol. 2004, 287, L79–L85. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Baßler, K.; Fujii, W.; Kapellos, T.S.; Horne, A.; Reiz, B.; Dudkin, E.; Luecken, M.; Reusch, N.; Osei-Sarpong, C.; Warnat-Herresthal, S.; et al. Alterations of multiple alveolar macrophage states in chronic obstructive pulmonary disease. bioRxiv 2020. [Google Scholar] [CrossRef]



| Characteristics | Control (n = 10) | COPD (n = 18) | p Value |
|---|---|---|---|
| Age, years | 65.0 (60.8–68.8) | 65.0 (60.5–70.8) | 0.665 |
| Sex, male | 3 (30) | 14 (78) | 0.020 |
| Smoking status | |||
| Current | 0 (0) | 8 (44) | 0.025 |
| Ex | 3 (30) | 9 (50) | 0.434 |
| Never | 7 (70) | 1 (6) | <0.001 |
| Pack-year smoked | 0.0 (0.0–1.5) | 39.3 (19.3–48.8) | <0.001 |
| Pulmonary function | |||
| FVC, % predicted | 94.6 (85.5–109.3) | 85.0 (72.8–95.7) | 0.144 |
| FEV1, % predicted | 95.0 (81.6–104.2) | 68.5 (53.2–73.8) | 0.001 |
| FEV1/FVC | 77.8 (74.9–82.3) | 60.8 (50.9–68.4) | <0.001 |
| GOLD stage I/II/III/IV | - | 4/10/4/0 | |
| BAL findings | |||
| Total cell count (X105/mL) | 0.8 (0.3–1.3) | 1.3 (0.5–2.3) | 0.352 |
| Macrophages (%) | 96.0 (95.0–96.5) | 94.0 (92.0–97.0) | 0.445 |
| Lymphocytes (%) | 1.5 (0.8–2.3) | 2.0 (1.0–5.0) | 0.369 |
| Neutrophils (%) | 2.0 (1.0–2.3) | 1.0 (1.0–3.0) | 1.000 |
| Eosinophils (%) | 0.0 (0.0–1.0) | 0.0 (0.0–0.8) | 0.646 |
| Inhalation therapy | |||
| Formoterol (%) | 0 (0) | 1 (5.6) | 0.643 |
| Budesonide/Formoterol (%) | 0 (0) | 2 (11.1) | 0.405 |
| Fluticasone/Salmeterol (%) | 0 (0) | 4 (22.2) | 0.149 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akata, K.; Yamasaki, K.; Leitao Filho, F.S.; Yang, C.X.; Takiguchi, H.; Sahin, B.; Whalen, B.A.; Yang, C.W.T.; Leung, J.M.; Sin, D.D.; et al. Abundance of Non-Polarized Lung Macrophages with Poor Phagocytic Function in Chronic Obstructive Pulmonary Disease (COPD). Biomedicines 2020, 8, 398. https://doi.org/10.3390/biomedicines8100398
Akata K, Yamasaki K, Leitao Filho FS, Yang CX, Takiguchi H, Sahin B, Whalen BA, Yang CWT, Leung JM, Sin DD, et al. Abundance of Non-Polarized Lung Macrophages with Poor Phagocytic Function in Chronic Obstructive Pulmonary Disease (COPD). Biomedicines. 2020; 8(10):398. https://doi.org/10.3390/biomedicines8100398
Chicago/Turabian StyleAkata, Kentaro, Kei Yamasaki, Fernando Sergio Leitao Filho, Chen Xi Yang, Hiroto Takiguchi, Basak Sahin, Beth A. Whalen, Cheng Wei Tony Yang, Janice M. Leung, Don D. Sin, and et al. 2020. "Abundance of Non-Polarized Lung Macrophages with Poor Phagocytic Function in Chronic Obstructive Pulmonary Disease (COPD)" Biomedicines 8, no. 10: 398. https://doi.org/10.3390/biomedicines8100398
APA StyleAkata, K., Yamasaki, K., Leitao Filho, F. S., Yang, C. X., Takiguchi, H., Sahin, B., Whalen, B. A., Yang, C. W. T., Leung, J. M., Sin, D. D., & van Eeden, S. F. (2020). Abundance of Non-Polarized Lung Macrophages with Poor Phagocytic Function in Chronic Obstructive Pulmonary Disease (COPD). Biomedicines, 8(10), 398. https://doi.org/10.3390/biomedicines8100398







