The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship
Abstract
:1. Introduction
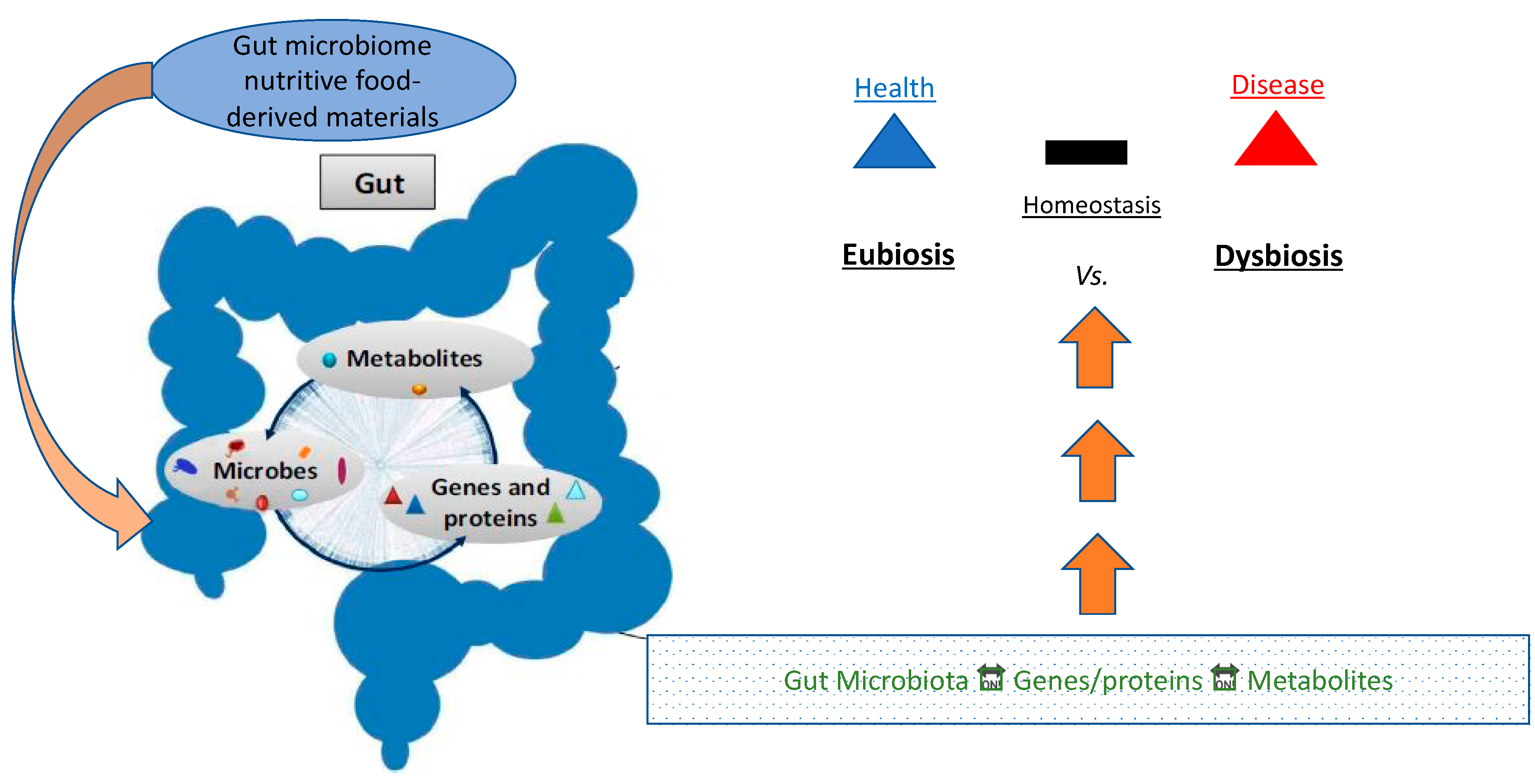
2. The Microbiome
Eubiosis vs. Dysbiosis
3. Major Metabolic Contributors to Microbiome Profile Identity (SCFA, BCAA, LPS)
3.1. LPS
3.2. Short Chain Fatty Acids (SCFAs)
3.3. Branched Chain Amino Acids (BCAAs)
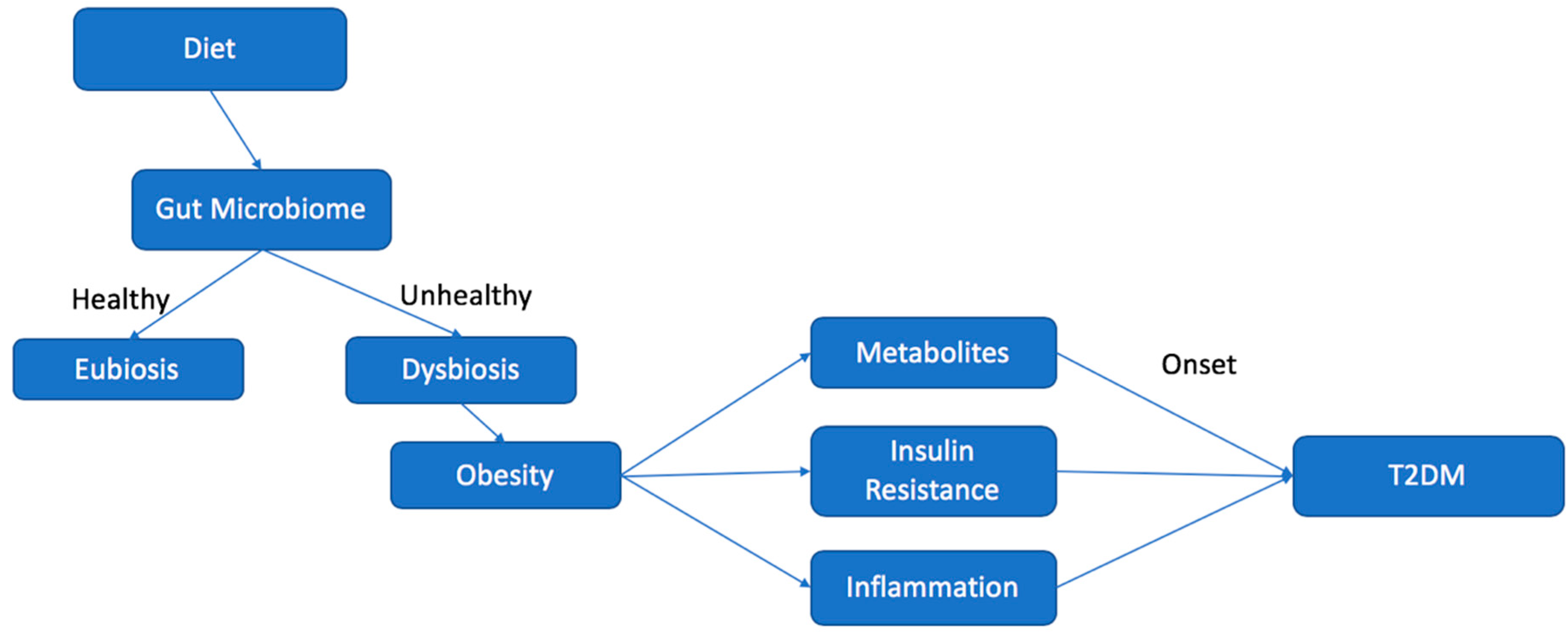
4. Dysbiosis and the Development of T2DM
4.1. Inflammation
4.2. Insulin Resistance
4.3. Oxidative Stress
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Defining Adult Overweight and Obesity Overweight & Obesity CDC. Available online: https://www.cdc.gov/obesity/adult/defining.html (accessed on 24 October 2019).
- CDC. Adult Obesity Facts Overweight & Obesity CDC. Centers for Disease Control and Prevention, Centers for Disease Control and Prevention. Available online: www.cdc.gov/obesity/data/adult.html (accessed on 13 August 2018).
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Vaiserman, A. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [Green Version]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.; Jones, W.; Affourtit, J.; Gordon, J.I. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8836–8847. [Google Scholar] [CrossRef]
- Thomas, F.; Hehemann, J.H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and gut Bacteroidetes: The food connection. Front. Microbiol. 2011, 2, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Kein, C.L. 1.3.4 Digestible and Indigestible Carbohydrates. In Pediatric Nutrition in Practice; Karger: Basel, Switzerland, 2008; pp. 42–46. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, S.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Vital, M.; Karch, A.; Pieper, D.H. Colonic Butyrate-Producing Communities in Humans: An Overview Using Omics Data. MSystems 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Sutcliffe, I.C. A phylum level perspective on bacterial cell envelope architecture. Trends Microbiol. 2010, 18, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Semova, I.; Carten, J.D.; Stombaugh, J.; MacKey, L.C.; Knight, R.; Farber, S.A.; Rawls, J.F. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 2012. [Google Scholar] [CrossRef] [Green Version]
- Definition: Fatty Acids (for Parents)-Kids Health. Available online: https://kidshealth.org/en/parents/fatty-acids.html (accessed on 17 October 2019).
- Jazayeri, S.; Tehrani-Doost, M.; Keshavarz, S.A.; Hosseini, M.; Djazayery, A.; Amini, H.; Jalali, M.; Peet, M. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust. N. Z. J. Psychiatry 2008, 42, 192–198. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’orazio, N. Omega-3 polyunsaturated fatty acids: Benefits and endpoints in sport. Nutrients 2019, 11, 46. [Google Scholar] [CrossRef] [Green Version]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Liou, A.P.; Paziuk, M.; Luevano, J.-M.; Machineni, S.; Turnbaugh, P.J.; Kaplan, L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013, 5, 178ra41. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Chi, M.M.; Scull, B.P.; Rigby, R.; Schwerbrock, N.M.J.; Magness, S.; Jobin, C.; Lund, P.K. High-fat diet: Bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE 2010, 5, e12191. [Google Scholar] [CrossRef] [Green Version]
- Saad, M.J.A.; Santos, A.; Prada, P.O. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef]
- Graham, C.; Mullen, A.; Whelan, K. Obesity and the gastrointestinal microbiota: A review of associations and mechanisms. Nutr. Rev. 2015, 73, 376–385. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Crittenden, A.N.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Sikalidis, A.K. Amino Acids and Immune Response: A Role for Cysteine, Glutamine, Phenylalanine, Tryptophan and Arginine in T-cell Function and Cancer? Pathol. Oncol. Res. 2015, 21, 9–17. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; MacKay, C.R.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Kristo, A.; Klimis-Zacas, D.; Sikalidis, A. Protective Role of Dietary Berries in Cancer. Antioxidants 2016, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Sowers, S. A Primer on Branched Chain Amino Acids. 2009. Available online: www.hchs.edu (accessed on 24 November 2019).
- Chen, X.; Yang, W. Branched-chain amino acids and the association with type 2 diabetes. J. Diabetes Investig. 2015, 6, 369–370. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Svetkey, L.P.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [Green Version]
- Yoon, M.S. mTOR as a key regulator in maintaining skeletal muscle mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannan, K.M.; Thomas, G.; Pearson, R.B. Activation of S6K1 (p70 ribosomal protein S6 kinase 1) requires an initial calcium-dependent priming event involving formation of a high-molecular-mass signalling complex. Biochem. J. 2003, 370, 469–477. [Google Scholar] [CrossRef]
- Um, S.H.; D’Alessio, D.; Thomas, G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006, 3, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Gerszten, R.E.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- BCAA Benefits: A Review of Branched-Chain Amino Acids. Available online: https://www.healthline.com/nutrition/bcaa#section1 (accessed on 11 November 2019).
- Sikalidis, A.K.; Stipanuk, M.H. Growing Rats Respond to a Sulfur Amino Acid–Deficient Diet by Phosphorylation of the α Subunit of Eukaryotic Initiation Factor 2 Heterotrimeric Complex and Induction of Adaptive Components of the Integrated Stress Response. J. Nutr. 2010, 140, 1080–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikalidis, A.K.; Mazor, K.M.; Kang, M.; Liu, H.; Stipanuk, M.H. Total 4EBP1 Is Elevated in Liver of Rats in Response to Low Sulfur Amino Acid Intake. J. Amino Acids 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BCAAs: Do Branched Chain Amino Acids Build Muscle? Men’s Health. Available online: https://www.menshealth.com/nutrition/a19545329/branched-chain-amino-acids/ (accessed on 12 October 2019).
- Diabetes Mellitus: Type 1 vs 2, Symptoms, Causes, & Treatments. Available online: https://www.webmd.com/diabetes/guide/types-of-diabetes-mellitus#3 (accessed on 12 October 2019).
- Type 2 Diabetes-Symptoms and Causes-Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-conditions/type-2-diabetes/symptoms-causes/syc-20351193 (accessed on 8 October 2019).
- National Institutes of Health. NIH Study Shows How Insulin Stimulates Fat Cells to Take in Glucose. 2010. Available online: https://www.nih.gov/news-events/news-releases/nih-study-shows-how-insulin-stimulates-fat-cells-take-glucose (accessed on 11 November 2019).
- How Insulin Works with Glucose. Kaiser Permanente Washington. Available online: https://wa.kaiserpermanente.org/healthAndWellness/index.jhtml?item=%2Fcommon%2FhealthAndWellness%2Fconditions%2Fdiabetes%2FinsulinProcess.htm (accessed on 8 October 2019).
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Wang, J.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The role of inflammation in diabetes: Current concepts and future perspectives. Eur. Cardiol. Rev. 2019, 14, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Nordmann, T.M.; Dror, E.; Schulze, F.; Traub, S.; Berishvili, E.; Barbieux, C.; Boni-Schnetzler, M.; Donath, M.Y. The Role of Inflammation in β-cell Dedifferentiation. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Zwickey, H.; Horgan, A.; Hanes, D.; Schiffke, H.; Moore, A.; Wahbeh, A.; Jordan, J.; Ojeda, L.; Wahbeh, H.; Purnell, J.Q.; et al. Effect of the Anti-Inflammatory Diet in People with Diabetes and Pre-Diabetes: A Randomized Controlled Feeding Study. J. Restor. Med. 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Pessin, J.E.; Kwon, H. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. 2013, 4, 71. [Google Scholar] [CrossRef] [Green Version]
- Graessler, J.; Qin, Y.; Zhong, H.; Zhang, J.; Licinio, J.; Wong, M.L.; Xu, A.; Chavikas, T.; Bornstein, A.B.; Bornstein, S.R.; et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: Correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013, 13, 514–522. [Google Scholar] [CrossRef] [Green Version]
- C-Reactive Protein Test-Mayo Clinic. Available online: https://www.mayoclinic.org/tests-procedures/c-reactive-protein-test/about/pac-20385228 (accessed on 17 October 2019).
- Metabolic Syndrome-Symptoms and Causes-Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-conditions/metabolic-syndrome/symptoms-causes/syc-20351916 (accessed on 12 October 2019).
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga–Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota from Lean Donors Increases Insulin Sensitivity in Individuals with Metabolic Syndrome. Gastroenterology 2012, 143, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ko, G. Effect of metformin on metabolic improvement and gut microbiota. Appl. Environ. Microbiol. 2014, 80, 5935–5943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- What Are Free Radicals? Live Science. Available online: https://www.livescience.com/54901-free-radicals.html (accessed on 17 October 2019).
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betteridge, D.J. What is oxidative stress? Metab. Clin. Exp. 2000, 49 (Suppl. 1), 3–8. [Google Scholar] [CrossRef]
- Qiao, Y.; Sun, J.; Ding, Y.; Le, G.; Shi, Y. Alterations of the gut microbiota in high-fat diet mice is strongly linked to oxidative stress. Appl. Microbiol. Biotechnol. 2013, 97, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Sound, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Type 2 Diabetes Diet Guidelines: Foods to Eat, Foods to Avoid. Available online: https://www.medicinenet.com/diabetic_diet_for_type_2_diabetes/article.htm (accessed on 31 October 2019).
- Sikalidis, A.K. From Food for Survival to Food for Personalized Optimal Health: A Historical Perspective of How Food and Nutrition Gave Rise to Nutrigenomics. J. Am. Coll. Nutr. 2019, 38, 84–95. [Google Scholar] [CrossRef]
- The Microbiome Diet Review: Food Lists, Benefits, and Meal Plan. 2019. Available online: https://www.healthline.com/nutrition/microbiome-diet#downsides (accessed on 28 December 2019).
- Hemoglobin A1c. Understand the Test. 2019. Available online: labtestsonline.org/tests/hemoglobin-a1c (accessed on 2 January 2020).
- Probiotics May Help Reduce Blood Sugar Levels Live Science. 2016. Available online: https://www.livescience.com/56861-probiotics-blood-sugar-dash-diet.html (accessed on 28 December 2019).
- Sikalidis, A.K.; Fitch, M.D.; Fleming, S.E. Diet induced obesity increases the risk of colonic tumorigenesis in mice. Pathol. Oncol. Res. 2013, 19, 657–666. [Google Scholar] [CrossRef]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The effects of vegetarian and vegan diets on gut microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Carmody, R.N.; Bisanz, J.E.; Bowen, B.P.; Maurice, C.F.; Lyalina, S.; Louie, K.B.; Treen, D.; Chadaideh, K.S.; Rekdal, V.M.; Turnbaugh, P.J.; et al. Cooking shapes the structure and function of the gut microbiome. Nat. Microbiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kristo, A.S.; Tzanidaki., G.; Lygeros, A.; Sikalidis, A.K. Bile sequestration potential of an edible mineral (clinoptilolite) under simulated digestion of a high-fat meal; An in vitro investigation. Food Funct. 2015, 6, 3818–3827. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship. Biomedicines 2020, 8, 8. https://doi.org/10.3390/biomedicines8010008
Sikalidis AK, Maykish A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship. Biomedicines. 2020; 8(1):8. https://doi.org/10.3390/biomedicines8010008
Chicago/Turabian StyleSikalidis, Angelos K., and Adeline Maykish. 2020. "The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship" Biomedicines 8, no. 1: 8. https://doi.org/10.3390/biomedicines8010008
APA StyleSikalidis, A. K., & Maykish, A. (2020). The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship. Biomedicines, 8(1), 8. https://doi.org/10.3390/biomedicines8010008





