Aptamer-Conjugated Tb(III)-Doped Silica Nanoparticles for Luminescent Detection of Leukemia Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Oligonucleotides
2.3. Cell Lines
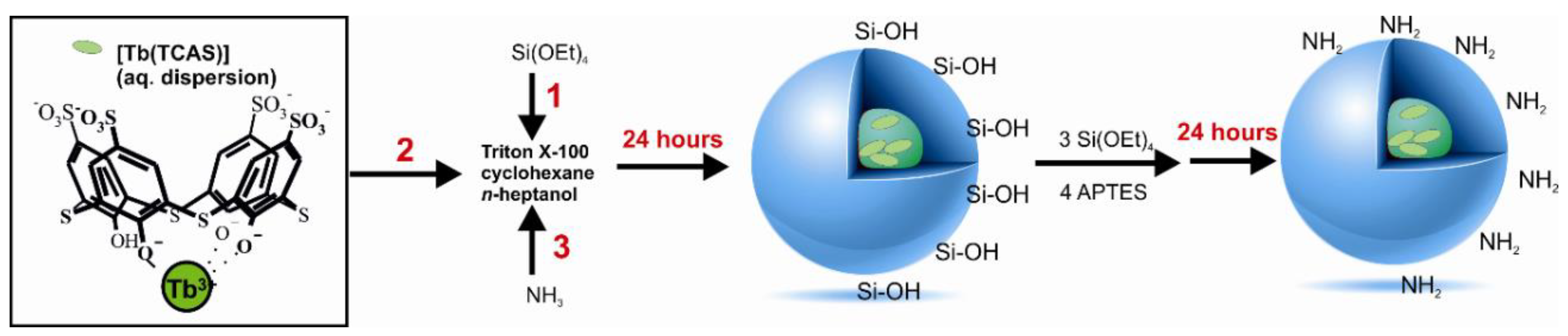
2.4. Synthesis of Nanoparticles
2.5. Methods
3. Results
3.1. Synthesis of silica nanoparticles and Their Modifications with Amino and Carboxyl Groups
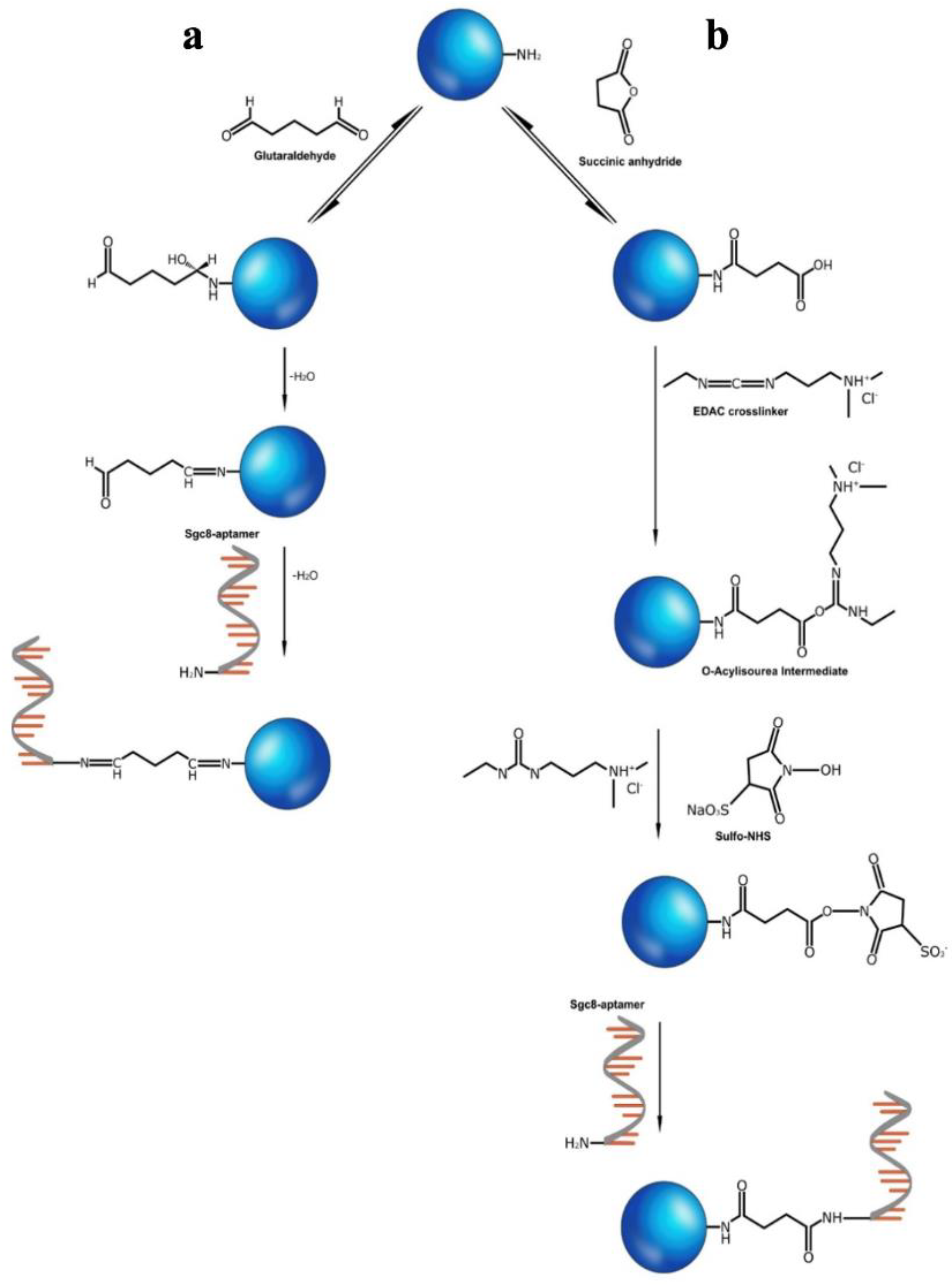
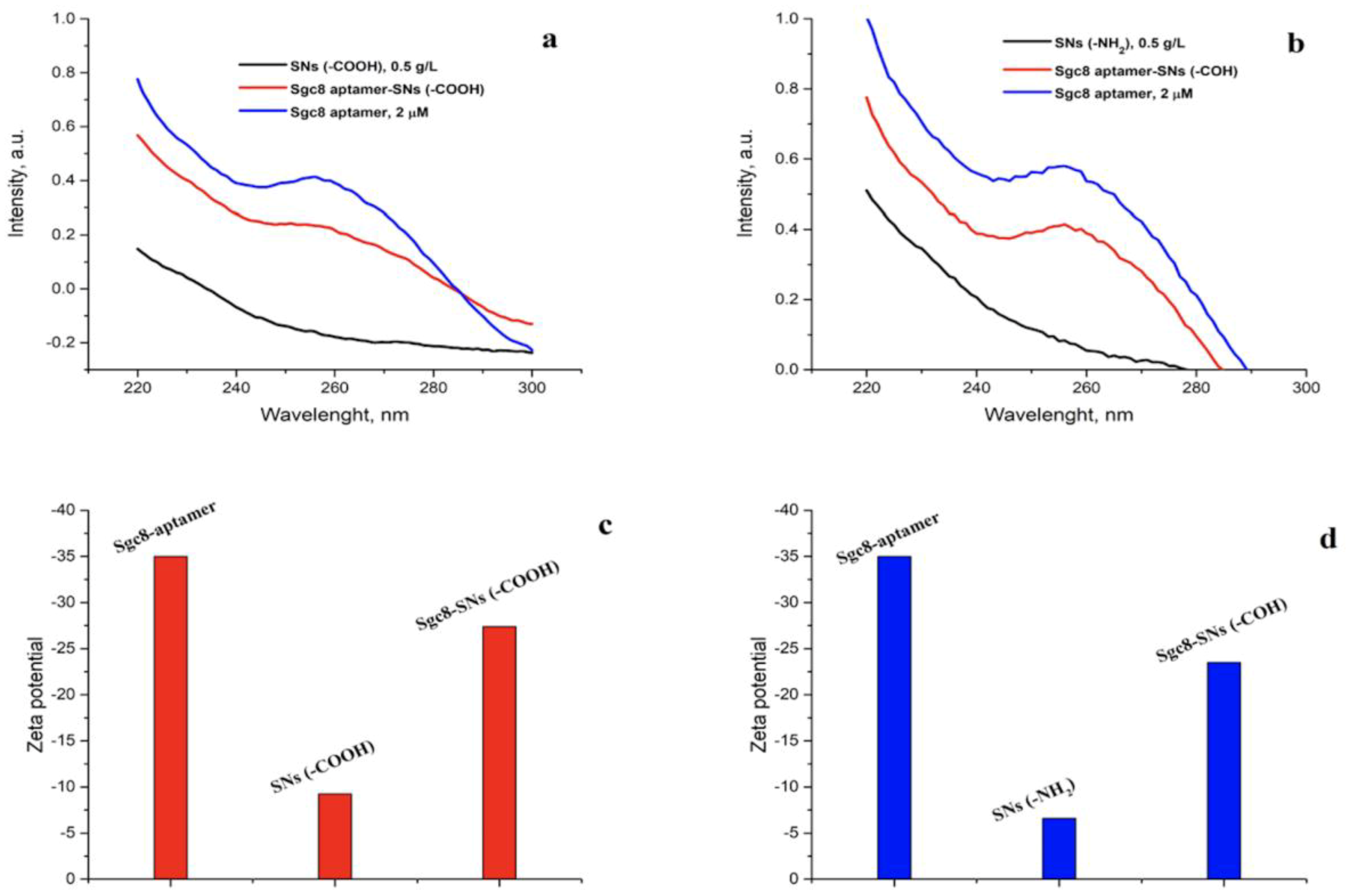
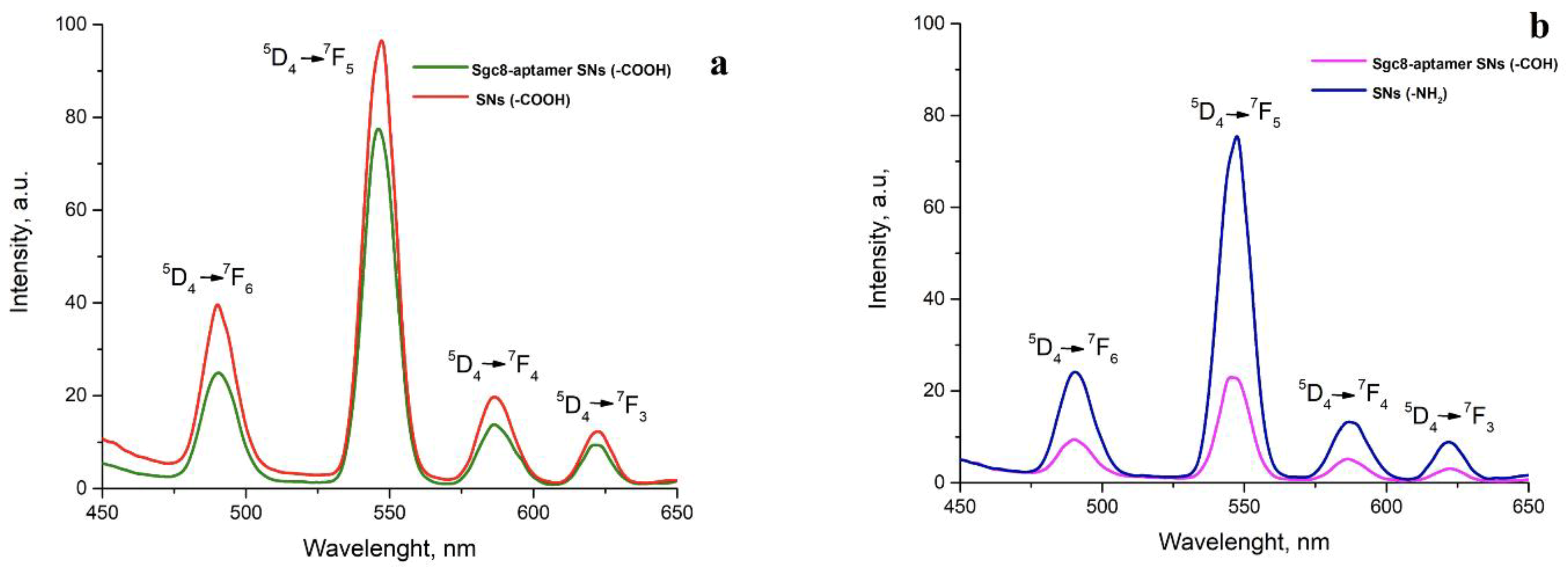
3.2. Conjugation of the Amino-Modified Aptamer with SNs
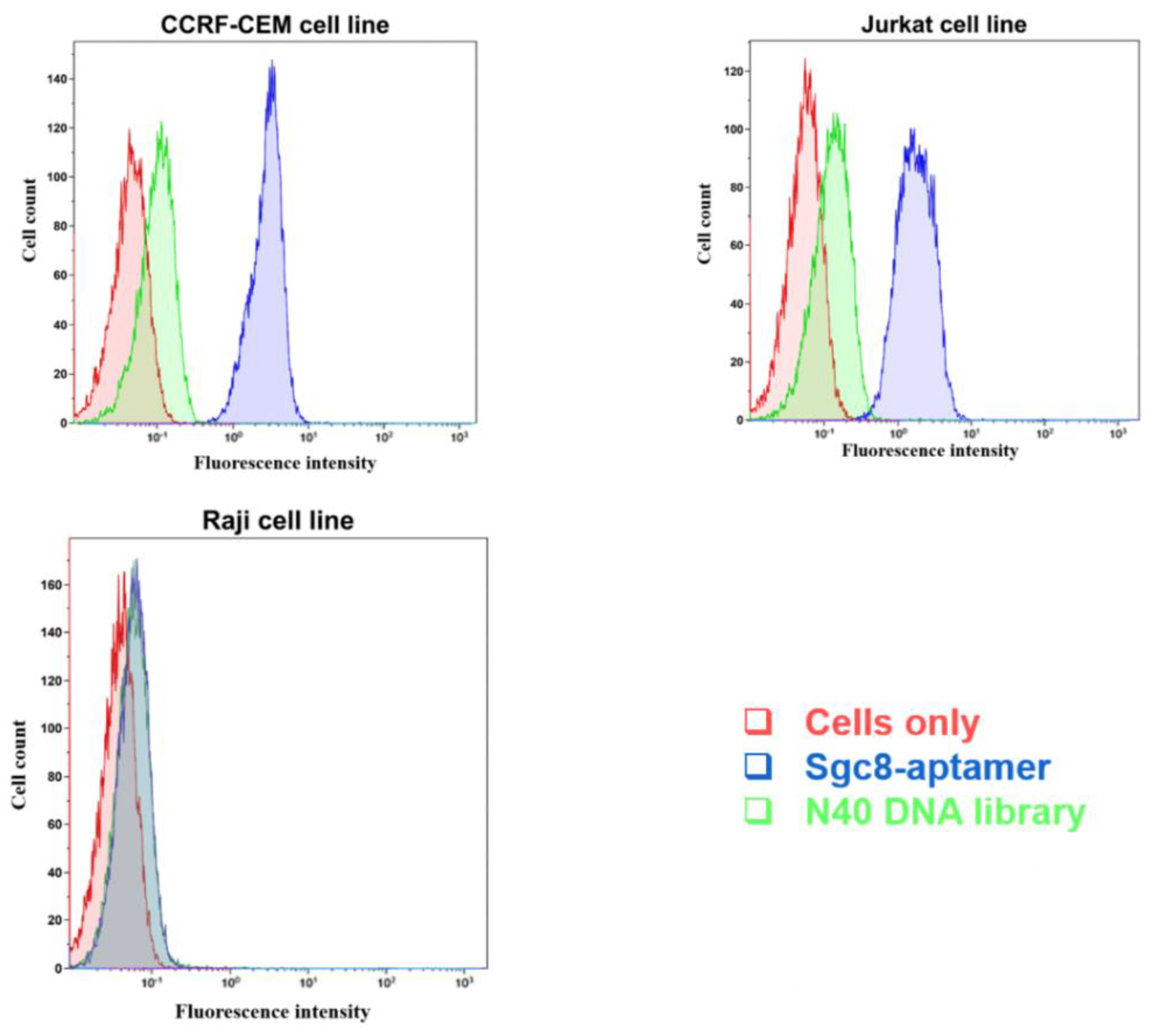
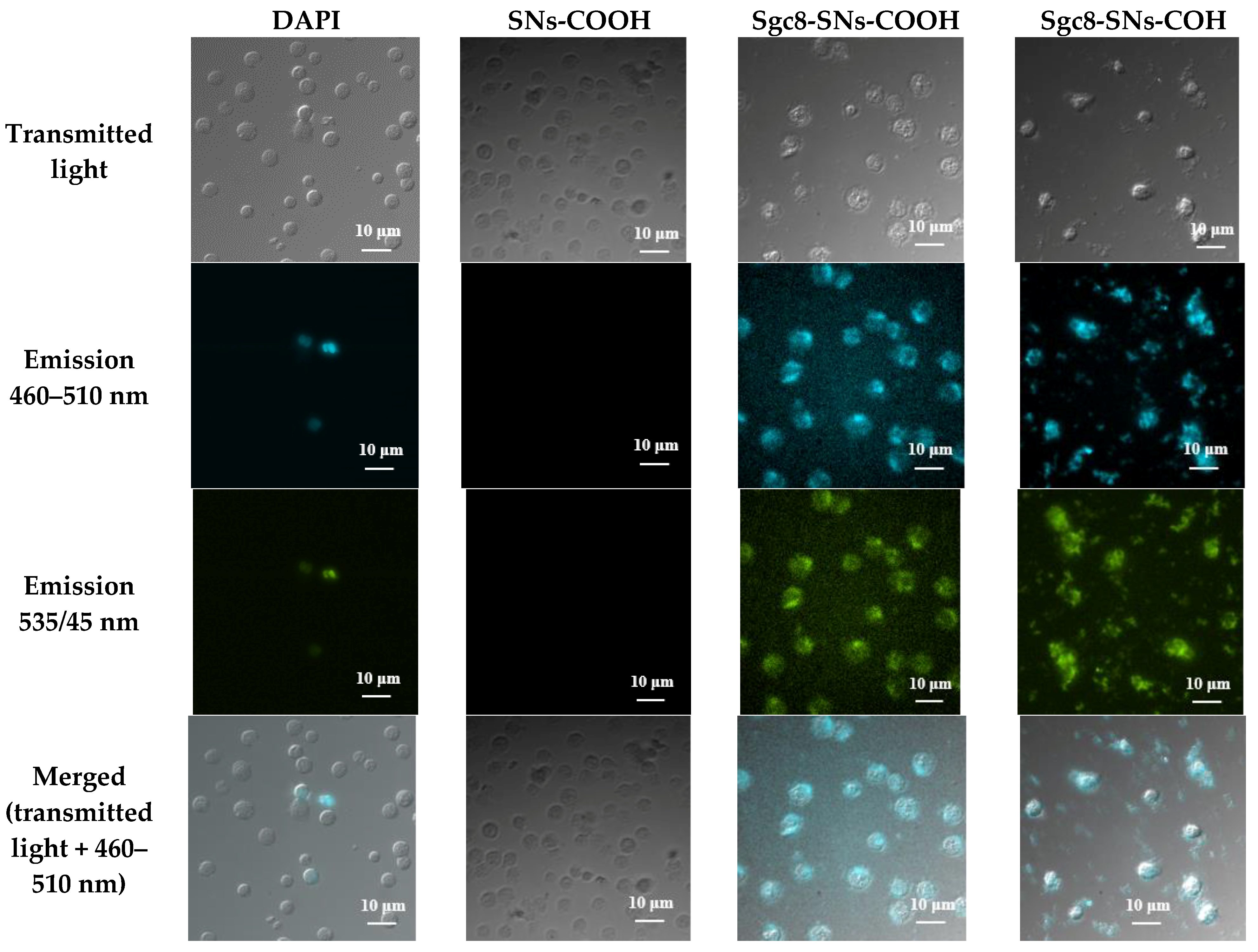
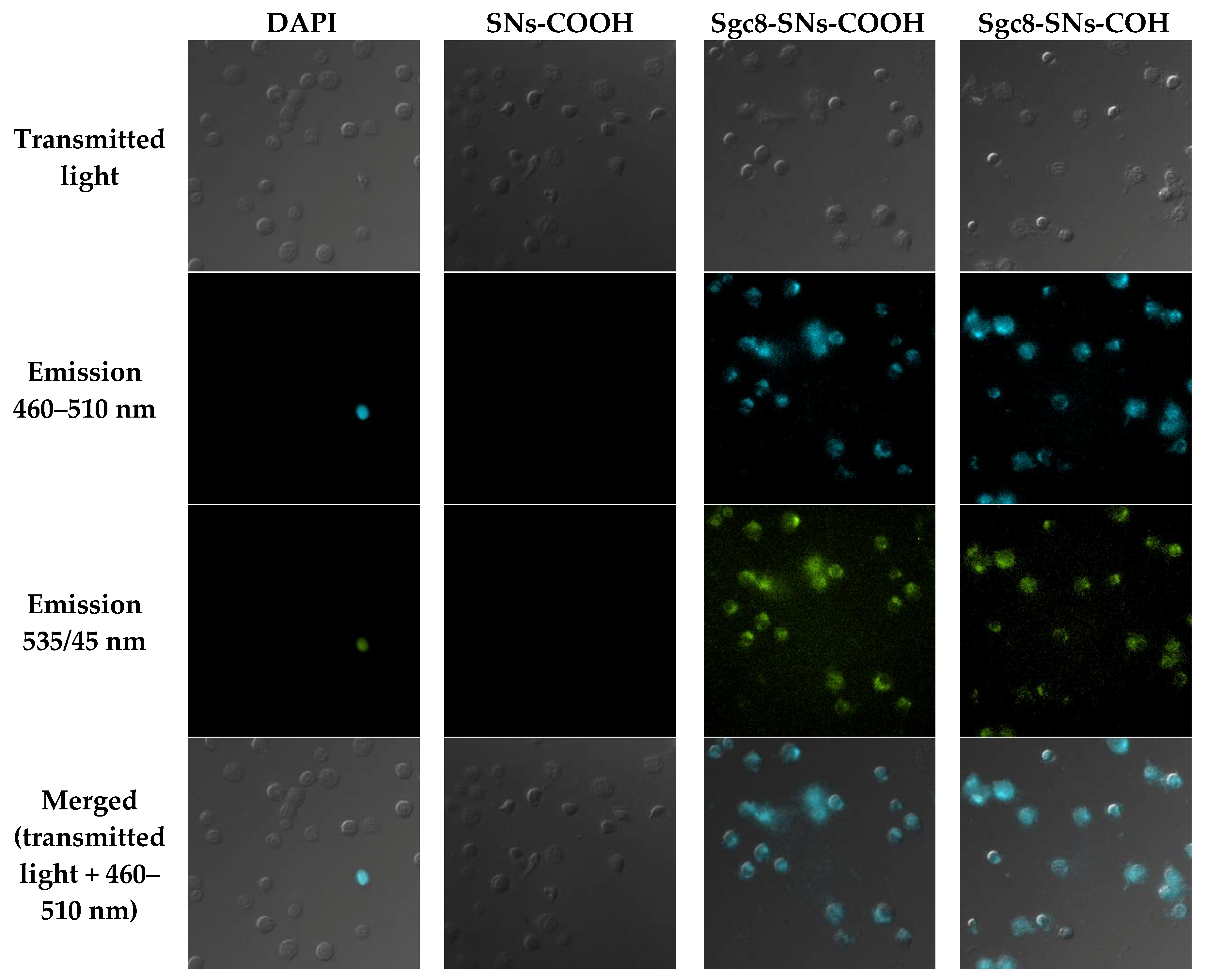
3.3. Detection of Leukemia Cells with Flow Cytometry and Fluorescent Microscopy
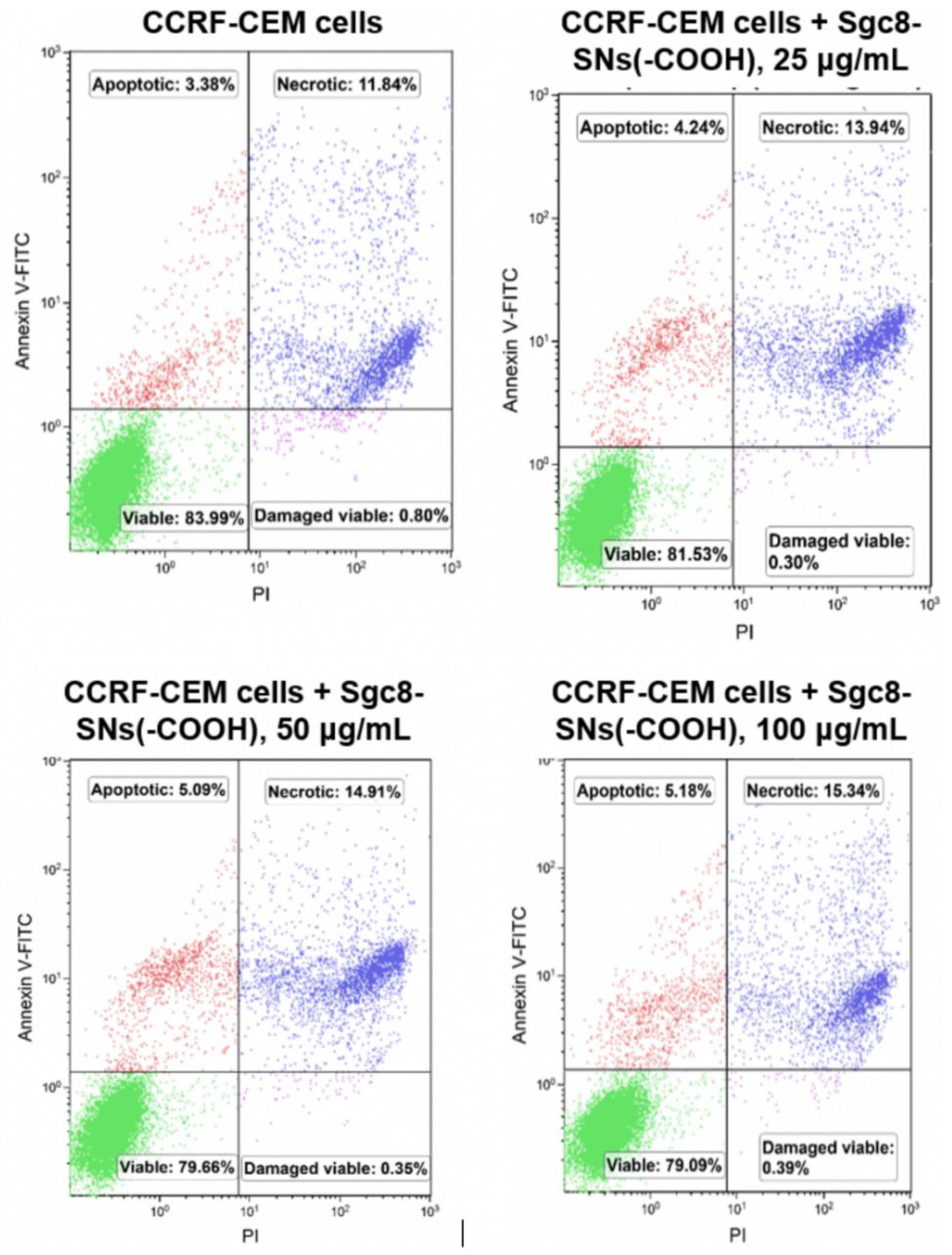
3.4. Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Suo, Z.; Liu, W.; Zhao, B.; Xing, F.; Zhang, Y.; Feng, L. DNA conformational polymorphism for biosensing applications. Biosens. Bioelectron. 2019, 131, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, D.; Platella, C.; Riccardi, C.; Moccia, F.; Montesarchio, D. Fluorescence Sensing Using DNA Aptamers in Cancer Research and Clinical Diagnostics. Cancers 2017, 9, 174. [Google Scholar] [CrossRef]
- Bauer, M.; Strom, M.; Hammond, D.S.; Shigdar, S. Anything You Can Do, I Can Do Better: Can Aptamers Replace Antibodies in Clinical Diagnostic Applications? Molecules 2019, 24, 4377. [Google Scholar] [CrossRef]
- Danesh, N.M.; Lavaee, P.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Targeted and controlled release delivery of daunorubicin to T-cell acute lymphoblastic leukemia by aptamer-modified gold nanoparticles. Int. J. Pharm. 2015, 489, 311–317. [Google Scholar] [CrossRef]
- Bagalkot, V.; Farokhzad, O.C.; Langer, R.; Jon, S. An aptamer-doxorubicin physical conjugate as a novel targeted drug-delivery platform. Angew. Chem. Int. Ed. Engl. 2006, 45, 8149–8152. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Khademhosseini, A.; Jon, S.; Hermmann, A.; Cheng, J.; Chin, C.; Kiselyuk, A.; Teply, B.; Eng, G.; Langer, R. Microfluidic system for studying the interaction of nanoparticles and microparticles with cells. Anal. Chem. 2005, 77, 5453–5459. [Google Scholar] [CrossRef]
- Nair, B.G.; Nagaoka, Y.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Aptamer conjugated magnetic nanoparticles as nanosurgeons. Nanotechnology 2010, 21, 455102. [Google Scholar] [CrossRef]
- Shangguan, D.; Li, Y.; Tang, Z.; Cao, Z.C.; Chen, H.W.; Mallikaratchy, P.; Sefah, K.; Yang, C.J.; Tan, W. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc. Natl. Acad. Sci. USA 2006, 103, 11838–11843. [Google Scholar] [CrossRef]
- Pan, Y.; Guo, M.; Nie, Z.; Huang, Y.; Pan, C.; Zeng, K.; Zhang, Y.; Yao, S. Selective collection and detection of leukemia cells on a magnet-quartz crystal microbalance system using aptamer-conjugated magnetic beads. Biosens. Bioelectron. 2010, 25, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Khoshfetrat, S.M.; Mehrgardi, M.A. Amplified detection of leukemia cancer cells using an aptamer-conjugated gold-coated magnetic nanoparticles on a nitrogen-doped graphene modified electrode. Bioelectrochemistry 2017, 114, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Shi, H.; He, X.; Wang, K.; He, D.; Yan, L.; Xu, F.; Lei, Y.; Tang, J.; Yu, Y. Iodide-Responsive Cu-Au Nanoparticle-Based Colorimetric Platform for Ultrasensitive Detection of Target Cancer Cells. Anal. Chem 2015, 87, 7141–7147. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, H.; Xu, D. Silver decahedral nanoparticles-enhanced fluorescence resonance energy transfer sensor for specific cell imaging. Anal. Chem. 2015, 87, 3826–3833. [Google Scholar] [CrossRef]
- Tan, J.; Yang, N.; Hu, Z.; Su, J.; Zhong, J.; Yang, Y.; Yu, Y.; Zhu, J.; Xue, D.; Huang, Y.; et al. Aptamer-Functionalized Fluorescent Silica Nanoparticles for Highly Sensitive Detection of Leukemia Cells. Nanoscale Res. Lett. 2016, 11, 298. [Google Scholar] [CrossRef]
- Fedorenko, S.V.; Mustafina, A.R.; Mukhametshina, A.R.; Jilkin, M.E.; Mukhametzyanov, T.A.; Solovieva, A.O.; Pozmogova, T.N.; Shestopalova, L.V.; Shestopalov, M.A.; Kholin, K.V.; et al. Cellular imaging by green luminescence of Tb(III)-doped aminomodified silica nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 551–558. [Google Scholar] [CrossRef]
- Runowski, M.; Ekner-Grzyb, A.; Mrowczynska, L.; Balabhadra, S.; Grzyb, T.; Paczesny, J.; Zep, A.; Lis, S. Synthesis and organic surface modification of luminescent, lanthanide-doped core/shell nanomaterials (LnF3@SiO2@NH2@organic acid) for potential bioapplications: Spectroscopic, structural, and in vitro cytotoxicity evaluation. Langmuir 2014, 30, 9533–9543. [Google Scholar] [CrossRef]
- Min, Y.; Li, J.; Liu, F.; Padmanabhan, P.; Yeow, E.K.; Xing, B. Recent Advance of Biological Molecular Imaging Based on Lanthanide-Doped Upconversion-Luminescent Nanomaterials. Nanomaterials 2014, 4, 129–154. [Google Scholar] [CrossRef]
- Kang, X.; Li, C.; Cheng, Z.; Ma, P.; Hou, Z.; Lin, J. Lanthanide-doped hollow nanomaterials as theranostic agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 80–101. [Google Scholar] [CrossRef]
- Bunzli, J.-C.G. Lanthanide light for biology and medical diagnosis. J. Lumin. 2016, 170, 866–878. [Google Scholar] [CrossRef]
- Parker, D.; Dickins, R.S.; Puschmann, H.; Crossland, C.; Howard, J.A. Being excited by lanthanide coordination complexes: Aqua species, chirality, excited-state chemistry, and exchange dynamics. Chem. Rev. 2002, 102, 1977–2010. [Google Scholar] [CrossRef] [PubMed]
- Rusakova, N.V.; Kost, S.S.; Mustafina, A.R.; Amirov, R.R.; Zairov, R.R.; Solovieva, S.E.; Antipin, I.S.; Konovalov, A.I.; Koroin, Y.V. Spectral-luminescence and magnetic relaxation properties of lanthanide-p-sulfonatothiacalix[4]arenes in aqueous solution of surfactants. Russ. Chem. Bull. 2008, 57, 567–572. [Google Scholar] [CrossRef]
- Iki, N.; Horiuchi, T.; Oka, H.; Koyama, K.; Morohashi, N.; Kabuto, C.; Miyano, S. Energy transfer luminescence of Tb3+ ion complexed with calix[4]arenetetrasulfonate and the thia and sulfonyl analogue. The effect of bridging groups. J. Chem. Soc. Perkin Trans. 2001, 2, 2219–2225. [Google Scholar] [CrossRef]
- Mukhametshina, A.; Petrov, A.; Fedorenko, S.; Petrov, K.; Nizameev, I.; Mustafina, A.; Sinyashin, O. Luminescent nanoparticles for rapid monitoring of endogenous acetylcholine release in mice atria. Lumin. J. Biol. Chem. Lumin. 2018, 33, 588–593. [Google Scholar] [CrossRef]
- An, Y.; Chen, M.; Xue, Q.; Liu, W. Preparation and self-assembly of carboxylic acid-functionalized silica. J. Colloid Interface Sci. 2007, 311, 507–513. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y. Fluorescent quantification of amino groups on silica nanoparticle surfaces. Anal. Bioanal. Chem. 2011, 399, 2503–2509. [Google Scholar] [CrossRef]
- BangsLab. TechNote 205, Covalent Coupling; Bangs Laboratory Inc.: Fishers, IN, USA, 2002. [Google Scholar]
- Rieger, A.M.; Nelson, K.L.; Konowalchuk, J.D.; Barreda, D.R. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J. Vis. Exp. JoVE 2011, 24, 2597. [Google Scholar] [CrossRef]
- Wartlick, H.; Spankuch-Schmitt, B.; Strebhardt, K.; Kreuter, J.; Langer, K. Tumour cell delivery of antisense oligonuclceotides by human serum albumin nanoparticles. J. Control. Release Off. J. Control. Release Soc. 2004, 96, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, D.; Cao, Z.; Meng, L.; Mallikaratchy, P.; Sefah, K.; Wang, H.; Li, Y.; Tan, W. Cell-specific aptamer probes for membrane protein elucidation in cancer cells. J. Proteome Res. 2008, 7, 2133–2139. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Joachim, E.; Choi, H.; Kim, K. Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Rim, K.T.; Koo, K.H.; Park, J.S. Toxicological evaluations of rare earths and their health impacts to workers: A literature review. Saf. Health Work 2013, 4, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Neizvestnova, E.M.; Grekhova, T.D.; Privalova, L.I.; Babakova, O.M.; Konovalova, N.E.; Petelina, E.V. Hygienic regulation of yttrium, terbium, ytterbium and lutetium fluorides in the air of the workplace. Med. Tr. Prom. Ekol. 1994, 7, 32–35. [Google Scholar]
- Shimada, H.; Nagano, M.; Funakoshi, T.; Kojima, S. Pulmonary toxicity of systemic terbium chloride in mice. J. Toxicol. Environ. Health 1996, 48, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Liu, X.T.; Lu, J.F.; Li, R.C.; Wang, K. The effects of Lanthanum, Cerium, Yttrium and Terbium ions on respiratory burst of peritoneal Macrophage (Mphi). J. Beijing Med. Univ. 2000, 32, 203–206. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grechkin, Y.A.; Grechkina, S.L.; Zaripov, E.A.; Fedorenko, S.V.; Mustafina, A.R.; Berezovski, M.V. Aptamer-Conjugated Tb(III)-Doped Silica Nanoparticles for Luminescent Detection of Leukemia Cells. Biomedicines 2020, 8, 14. https://doi.org/10.3390/biomedicines8010014
Grechkin YA, Grechkina SL, Zaripov EA, Fedorenko SV, Mustafina AR, Berezovski MV. Aptamer-Conjugated Tb(III)-Doped Silica Nanoparticles for Luminescent Detection of Leukemia Cells. Biomedicines. 2020; 8(1):14. https://doi.org/10.3390/biomedicines8010014
Chicago/Turabian StyleGrechkin, Yaroslav A., Svetlana L. Grechkina, Emil A. Zaripov, Svetlana V. Fedorenko, Asiya R. Mustafina, and Maxim V. Berezovski. 2020. "Aptamer-Conjugated Tb(III)-Doped Silica Nanoparticles for Luminescent Detection of Leukemia Cells" Biomedicines 8, no. 1: 14. https://doi.org/10.3390/biomedicines8010014
APA StyleGrechkin, Y. A., Grechkina, S. L., Zaripov, E. A., Fedorenko, S. V., Mustafina, A. R., & Berezovski, M. V. (2020). Aptamer-Conjugated Tb(III)-Doped Silica Nanoparticles for Luminescent Detection of Leukemia Cells. Biomedicines, 8(1), 14. https://doi.org/10.3390/biomedicines8010014





