Circulating Exosomal miRNA Profiles Predict the Occurrence and Recurrence of Hepatocellular Carcinoma in Patients with Direct-Acting Antiviral-Induced Sustained Viral Response
Abstract
1. Introduction
2. Experimental Section
2.1. Sample Information
2.2. RNA Preparation and Microarray Assay
2.3. Cell Proliferation Assay
2.4. Caspase Assay
2.5. HCC Prediction by Linear SVM
2.6. Statistics
3. Results
3.1. Predicting HCC Recurrence in Patients with DAA-Induced SVR Liver Cirrhosis
3.2. Predicting HCC Recurrence in DAA-Induced SVR Liver Cirrhosis and Non-Cirrhosis Patients
3.3. Predicting HCC in HCC-Naïve Patients with a DAA-Induced SVR
3.4. Hepatic miRNA Expression Pattern in SVR-non-HCC and SVR-HCC Patients
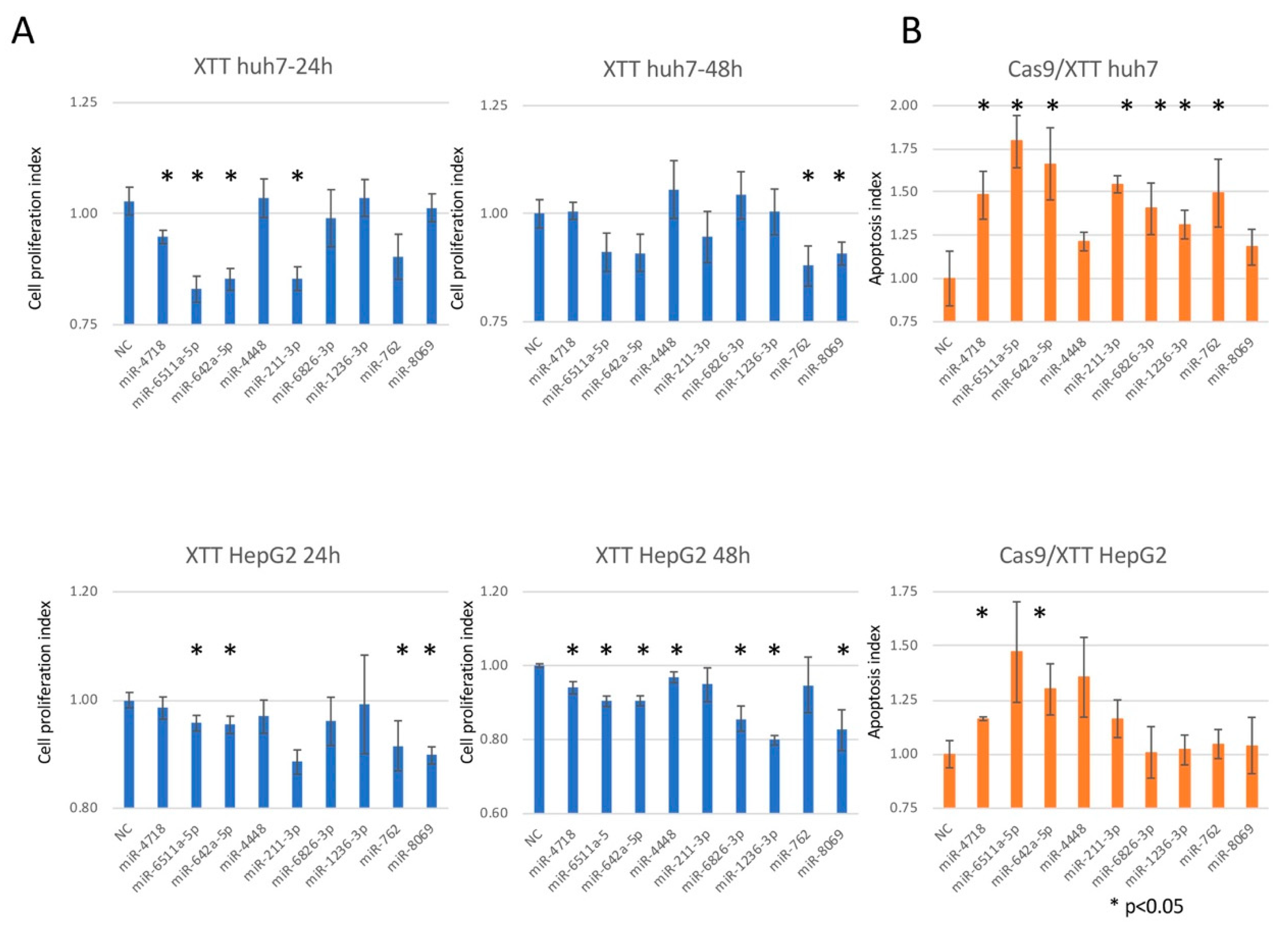
3.5. Hepatocarcinogenic Potential of the miRNAs Used to Predict SVR-HCC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hiramatsu, N.; Oze, T.; Takehara, T. Suppression of hepatocellular carcinoma development in hepatitis C patients given interferon-based antiviral therapy. Hepatol. Res. 2015, 45, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Stroffolini, T.; Colombo, M.; Bollani, S.; Benvegnu, L.; Mazzella, G.; Ascione, A.; Santantonio, T.; Piccinino, F.; Andreone, P.; et al. Italian Association of the Study of the Liver, D., Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: A retrospective study. Hepatology 2007, 45, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Kumada, T.; Tokuda, A.; Horiguchi, Y.; Nakano, H.; Honda, T.; Nakano, S.; Hayashi, K.; Katano, Y.; Nakano, I.; et al. Long-term follow-up of sustained responders to interferon therapy, in patients with chronic hepatitis C. J. Viral Hepat. 2000, 7, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Green, P.K.; Berry, K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J. Hepatol. 2018, 68, 25–32. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005. [Google Scholar] [CrossRef]
- Reig, M.; Marino, Z.; Perello, C.; Inarrairaegui, M.; Ribeiro, A.; Lens, S.; Diaz, A.; Vilana, R.; Darnell, A.; Varela, M.; et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 2016, 65, 719–726. [Google Scholar] [CrossRef]
- Cabibbo, G.; Petta, S.; Calvaruso, V.; Cacciola, I.; Cannavo, M.R.; Madonia, S.; Distefano, M.; Larocca, L.; Prestileo, T.; Tine, F.; et al. Is early recurrence of hepatocellular carcinoma in HCV cirrhotic patients affected by treatment with direct-acting antivirals? A prospective multicentre study. Aliment. Pharmacol. Ther. 2017, 46, 688–695. [Google Scholar] [CrossRef]
- Hwang, I. Cell-cell communication via extracellular membrane vesicles and its role in the immune response. Mol. Cells 2013, 36, 105–111. [Google Scholar] [CrossRef]
- Shirvani-Dastgerdi, E.; Schwartz, R.E.; Ploss, A. Hepatocarcinogenesis associated with hepatitis B, delta and C viruses. Curr. Opin. Virol. 2016, 20, 1–10. [Google Scholar] [CrossRef]
- Vescovo, T.; Refolo, G.; Vitagliano, G.; Fimia, G.M.; Piacentini, M. Molecular mechanisms of hepatitis C virus-induced hepatocellular carcinoma. Clin. Microbiol. Infect. 2016, 22, 853–861. [Google Scholar] [CrossRef]
- Brimacombe, C.L.; Grove, J.; Meredith, L.W.; Hu, K.; Syder, A.J.; Flores, M.V.; Timpe, J.M.; Krieger, S.E.; Baumert, T.F.; Tellinghuisen, T.L.; et al. Neutralizing antibody-resistant hepatitis C virus cell-to-cell transmission. J. Virol. 2011, 85, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Toyoda, H.; Tanahashi, T.; Tanaka, J.; Kumada, T.; Yoshioka, Y.; Kosaka, N.; Ochiya, T.; Taguchi, Y.H. Comprehensive miRNA expression analysis in peripheral blood can diagnose liver disease. PLoS ONE 2012, 7, e48366. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, Y.H.; Murakami, Y. Principal component analysis based feature extraction approach to identify circulating microRNA biomarkers. PLoS ONE 2013, 8, e66714. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, Y.H.; Murakami, Y. Universal disease biomarker: Can a fixed set of blood microRNAs diagnose multiple diseases? BMC Res. Notes 2014, 7, 581. [Google Scholar] [CrossRef] [PubMed]
- Chaiteerakij, R.; Addissie, B.D.; Roberts, L.R. Update on biomarkers of hepatocellular carcinoma. Clin. Gastroenterol Hepatol. 2015, 13, 237–245. [Google Scholar] [CrossRef]
- Wang, H.; Hou, L.; Li, A.; Duan, Y.; Gao, H.; Song, X. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed. Res. Int. 2014, 2014, 864894. [Google Scholar] [CrossRef]
- Fornari, F.; Ferracin, M.; Trere, D.; Milazzo, M.; Marinelli, S.; Galassi, M.; Venerandi, L.; Pollutri, D.; Patrizi, C.; Borghi, A.; et al. Circulating microRNAs, miR-939, miR-595, miR-519d and miR-494, Identify Cirrhotic Patients with HCC. PLoS ONE 2015, 10, e0141448. [Google Scholar] [CrossRef]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Huang, S.; Cai, N.; Pacheco, P.P.; Narrandes, S.; Wang, Y.; Xu, W. Applications of Support Vector Machine (SVM) Learning in Cancer Genomics. Cancer Genomics Proteom. 2018, 15, 41–51. [Google Scholar]
- Sasaki, R.; Yamasaki, K.; Abiru, S.; Komori, A.; Nagaoka, S.; Saeki, A.; Hashimoto, S.; Bekki, S.; Kugiyama, Y.; Kuno, A.; et al. Serum Wisteria Floribunda Agglutinin-Positive Mac-2 Binding Protein Values Predict the Development of Hepatocellular Carcinoma among Patients with Chronic Hepatitis C after Sustained Virological Response. PLoS ONE 2015, 10, e0129053. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, Y.; Oikawa, T.; Yoshino, H.; Hashimoto, S.; Handa, H.; Yamamoto, H.; Hashimoto, A.; Sabe, H. Frequent overexpression of AMAP1, an Arf6 effector in cell invasion, is characteristic of the MMTV-PyMT rather than the MMTV-Neu human breast cancer model. Cell Commun. Signal. 2018, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhuang, L.; Szatmary, P.; Wen, L.; Sun, H.; Lu, Y.; Xu, Q.; Chen, X. Upregulation of heat shock proteins (HSPA12A, HSP90B1, HSPA4, HSPA5 and HSPA6) in tumour tissues is associated with poor outcomes from HBV-related early-stage hepatocellular carcinoma. Int. J. Med. Sci. 2015, 12, 256–263. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, J.; Li, J.; Shao, J.; Fang, L. MiR-130a-3p inhibits migration and invasion by regulating RAB5B in human breast cancer stem cell-like cells. Biochem. Biophys. Res. Commun. 2018, 501, 486–493. [Google Scholar] [CrossRef]
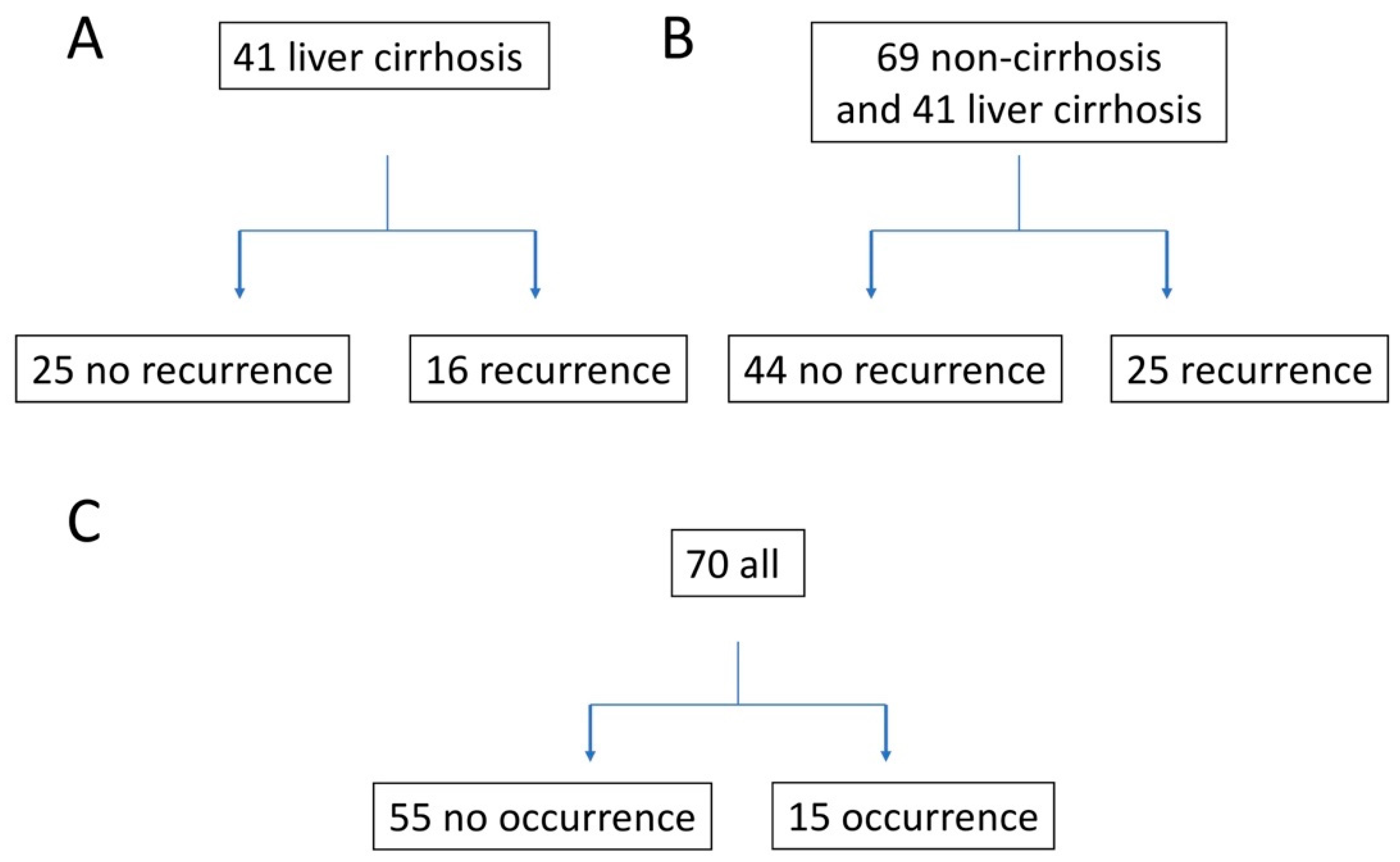


| Summary of Analysis Groups | |||||
|---|---|---|---|---|---|
| Presence or Absence of HCC Recurrence | No. | Age | Sex | Liver Disease | DAA Treatment |
| No | 25 | 73.6 ± 7.2 | F:13/M:12 | liver cirrhosis | AD:8/SL:15/SR:2 |
| Yes | 16 | 72.1 ± 9.0 | F:8/M:8 | liver cirrhosis | AD:8/SL:4/SR:4 |
| Clinical Data by Analysis Group | |||||
| Treatment | Items | No Recurrence | Recurrence | p-Value | |
| pre- | ALT | 46.8 ± 23.3 | 55.8 ± 31.1 | 3.02 × 10−1 | |
| T.Bil | 0.8 ± 0.4 | 0.8 ± 0.3 | 6.67 × 10−1 | ||
| Alb | 3.7 ± 0.3 | 3.3 ± 0.9 | 5.27 × 10−3 | ||
| PT | 82.6 ± 8.1 | 75.9 ± 19.8 | 1.33 × 10−1 | ||
| PLT | 9.9 ± 2.9 | 9.1 ± 4.1 | 4.69 × 10−1 | ||
| AFP | 27.3 ± 58.7 | 42.5 ± 68.3 | 4.63 × 10−1 | ||
| M2BPGi | 6.0 ± 3.9 | 7.6 ± 3.9 | 2.24 × 10−1 | ||
| FIB-4 | 6.6 ± 2.6 | 8.3 ± 4.3 | 1.27 × 10−1 | ||
| post- | ALT | 44.6 ± 106.3 | 26.3 ± 15.8 | 5.01 × 10−1 | |
| T.Bil | 0.9 ± 0.4 | 0.9 ± 0.3 | 4.17 × 10−1 | ||
| Alb | 3.8 ± 0.3 | 3.8 ± 0.4 | 7.78 × 10−1 | ||
| PT | 80.4 ± 19.2 | 78.1 ± 15.7 | 5.85 × 10−1 | ||
| PLT | 11.0 ± 3.5 | 9.8 ± 33.6 | 3.60 × 10−1 | ||
| AFP | 8.6 ± 7.2 | 14.5 ± 14.9 | 1.37 × 10−1 | ||
| M2BPGi | 3.8 ± 2.6 | 4.7 ± 3.1 | 3.00 × 10−1 | ||
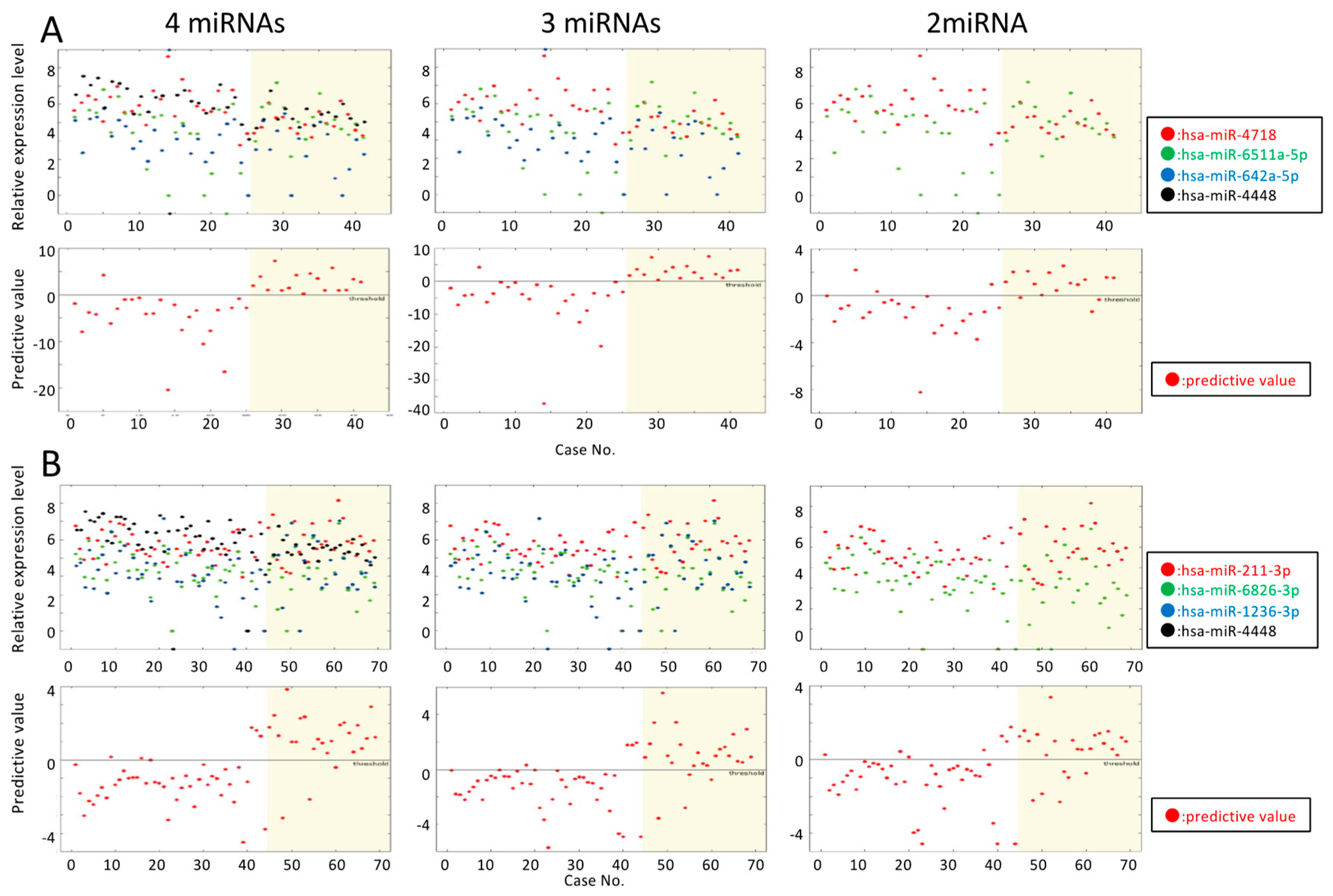
| #miRNAs | Equation | ACC (%) | REC (%) | PRE (%) | SPE (%) | LOOCV (%) | ACCALL (%) |
|---|---|---|---|---|---|---|---|
| 4 | y = −2.75 × [miR-4718] + 3.02 × [miR-6511a-5p] −1.38 × [miR-642a-5p] −1.21 × [miR-4448]+12.60 | 87.2 | 87.8 | 83.1 | 92.7 | 97.6 | |
| 3 | y = −4.12 × [miR-4718] + 3.46 × [miR-6511a-5p] − 2.08 × [miR-642a-5p] + 13.51 | 88.5 | 89.5 | 83.0 | 87.8 | 92.7 | 97.6 |
| 2 | y = −1.72 × [miR-4718] + 0.74 × [miR-6511a-5p] + 5.83 | 79.6 | 77.0 | 73.6 | 80.5 | 82.9 |
| Summary of Analysis Groups | |||||
|---|---|---|---|---|---|
| Presence or Absence of HCC Recurrence | No. | Age | Sex | Liver Disease | DAA Treatment |
| No | 44 | 73.5 ± 7.76 | F:20/M:24 | non-cirrhosis:19 /liver cirrhosis:25 | AD:15/SL:26/SR:3 |
| Yes | 25 | 72.7 ± 8.20 | F:11/M:14 | non-cirrhosis:9 /liver cirrhosis:16 | AD:10/SL:9/SR:6 |
| Clinical Data by Analysis Group | |||||
| Treatment | Items | No Recurrence | Recurrence | p-Value | |
| pre- | ALT | 49.1 ± 33.5 | 53.4 ± 35.4 | 6.13 × 10−1 | |
| T.Bil | 0.7 ± 0.3 | 0.8 ± 0.3 | 8.76 × 10−1 | ||
| Alb | 3.7 ± 0.3 | 3.5 ± 0.8 | 3.61 × 10−2 | ||
| PT | 86.7 ± 13.4 | 81.2 ± 19.3 | 1.73 × 10−1 | ||
| PLT | 13.2 ± 6.1 | 10.8 ± 4.6 | 9.26 × 10−2 | ||
| AFP | 19.6 ± 45.1 | 39.6 ± 71.4 | 1.75 × 10−1 | ||
| M2BPGi | 4.5 ± 3.7 | 5.9 ± 4.1 | 1.44 × 10−1 | ||
| FIB-4 | 5.3 ± 2.8 | 7.0 ± 4.0 | 3.50 × 10−2 | ||
| post- | ALT | 37.1 ± 81.6 | 25.5 ± 15.5 | 4.84 × 10−1 | |
| T.Bil | 0.9 ± 0.4 | 0.9 ± 0.3 | 7.92 × 10−1 | ||
| Alb | 3.8 ± 0.4 | 3.8 ± 0.4 | 9.71 × 10−1 | ||
| PT | 85.9 ± 19.2 | 82.2 ± 14.8 | 3.20 × 10−1 | ||
| PLT | 14.0 ± 6.6 | 11.9 ± 5.4 | 1.93 × 10−1 | ||
| AFP | 7.5 ± 6.1 | 14.5 ± 18.0 | 3.52 × 10−2 | ||
| M2BPGi | 2.7 ± 2.2 | 3.5 ± 2.8 | 1.65 × 10−1 | ||
| #miRNAs | Equation | ACC (%) | REC (%) | PRE (%) | SPE (%) | LOOCV (%) | ACCALL (%) |
|---|---|---|---|---|---|---|---|
| 4 | y = 1.62 × [miR-211-3p] − 1.43 × [miR-6826-3p] + 0.65 × [miR-1236-3p] − 0.84 × [miR-4448] − 1.19 | 85.3 | 86.1 | 77.8 | 85.3 | 87.0 | 87.0 |
| 3 | y = 1.93 × [miR-211-3p] − 2.16 × [miR-6826-3p] + 0.80 × [miR-1236-3p] − 5.48 | 82.9 | 77.8 | 76.2 | 85.5 | 88.4 | |
| 2 | y = 1.84 × [miR-211-3p] − 1.35 × [miR-6826-3p] − 5.07 | 79.0 | 72.8 | 71.2 | 81.2 | 81.2 |
| Summary of Analysis Groups | |||||
|---|---|---|---|---|---|
| Presence or Absence HCC Occurrence | No. | Age | Sex | Liver Disease | DAA Treatment |
| No | 55 | 66.2 ± 10.6 | F:29/M:26 | non-cirrhosis: 8 /liver cirrhosis: 47 | AD:32/SL:20/SR:3 |
| Yes | 15 | 71.8 ± 8.0 | F:8/M:7 | non-cirrhosis: 10 /liver cirrhosis: 5 | AD:5/SL:8/SR:2 |
| Clinical Data by Analysis Group | |||||
| Treatment | Items | No Occurrence | Occurrence | p-Value | |
| pre- | ALT | 57.1 ± 31.9 | 54.5 ± 25.2 | 7.73 × 10−1 | |
| T.Bil | 0.9 ± 0.4 | 0.7 ± 0.3 | 8.08 × 10−2 | ||
| Alb | 3.6 ± 0.6 | 3.8 ± 0.5 | 2.88 × 10−1 | ||
| PT | 82.1 ± 26.6 | 81.2 ± 26.8 | 8.63 × 10−1 | ||
| PLT | 10.9 ± 5.5 | 12.9 ± 6.3 | 2.36 × 10−1 | ||
| AFP | 34.2 ± 63.3 | 18.6 ± 19.2 | 3.54 × 10−1 | ||
| M2BPGi | 6.0 ± 3.3 | 3.9 ± 2.0 | 1.84 × 10−2 | ||
| FIB-4 | 6.5 ± 3.9 | 5.4 ± 3.4 | 3.13 × 10−1 | ||
| post- | ALT | 29.7 ± 26.1 | 23.3 ± 11.9 | 3.61 × 10−1 | |
| T.Bil | 0.9 ± 0.5 | 0.8 ± 0.3 | 4.62 × 10−1 | ||
| Alb | 4.5 ± 5.0 | 4.0 ± 0.5 | 6.87 × 10−1 | ||
| PT | 81.2 ± 25.8 | 88.5 ± 33.8 | 1.18 × 10−1 | ||
| PLT | 11.7 ± 5.7 | 14.2 ± 6.0 | 1.51 × 10−1 | ||
| AFP | 7.8 ± 6.3 | 25.6 ± 49.1 | 1.64 × 10−2 | ||
| M2BPGi | 3.4 ± 2.3 | 2.37 ± 1.7 | 1.50 × 10−1 | ||
| #miRNAs | Chosen mRNAs | ACC (%) | REC (%) | PRE (%) | SPE (%) | LOOCV (%) | ACCALL (%) |
|---|---|---|---|---|---|---|---|
| 4 | miR-762, miR-8069, miR-7847-3p, miR-7846-3p | 85.5 | 74.5 | 66.2 | 87.1 | 91.4 | |
| 3 | miR-762, miR-8069, miR-6090 | 83.4 | 68.9 | 64.0 | 87.1 | 91.4 | |
| 2 | miR-762, miR-8069 | 83.3 | 71.4 | 60.6 | 87.1 | 85.7 | 85.7 |
| Summary of Analysis Groups | |||||
|---|---|---|---|---|---|
| Presence or Absence of HCC Occurrence | No. | Age | Sex | Liver Disease | DAA Treatment |
| No | 36 | 62.3 ± 5.9 | F:23/M:13 | F0/F1/F2/F3 1/22/7/6 | AD:23/SL:5/SR:3/VIK:5 |
| Yes | 7 | 62.7 ± 5.3 | F:2/M:5 | F0/F1/F2/F3 0/3/3/1 | AD:5/SL:1/SR:1 |
| Clinical Data by Analysis Group | |||||
| Items | SVR-non-HCC | SVR-HCC | p-Value | ||
| pre-treatment | fibrosis stage | 1.5 ± 0.8 | 1.7 ± 0.8 | 5.22 × 10−1 | |
| ALT | 48.8 ± 33.5 | 93.1 ± 50.4 | 5.30 × 10−3 | ||
| T.BIL | 0.7 ± 0.3 | 0.9 ± 0.3 | 1.57 × 10−1 | ||
| PLT | 18.5 ± 5.0 | 12.8 ± 4.2 | 6.97 × 10−3 | ||
| ALB | 4.3 ± 0.3 | 4.0 ± 0.2 | 1.02 × 10−2 | ||
| AFP | 5.8 ± 7.7 | 17.6 ± 23.6 | 1.84 × 10−2 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itami-Matsumoto, S.; Hayakawa, M.; Uchida-Kobayashi, S.; Enomoto, M.; Tamori, A.; Mizuno, K.; Toyoda, H.; Tamura, T.; Akutsu, T.; Ochiya, T.; et al. Circulating Exosomal miRNA Profiles Predict the Occurrence and Recurrence of Hepatocellular Carcinoma in Patients with Direct-Acting Antiviral-Induced Sustained Viral Response. Biomedicines 2019, 7, 87. https://doi.org/10.3390/biomedicines7040087
Itami-Matsumoto S, Hayakawa M, Uchida-Kobayashi S, Enomoto M, Tamori A, Mizuno K, Toyoda H, Tamura T, Akutsu T, Ochiya T, et al. Circulating Exosomal miRNA Profiles Predict the Occurrence and Recurrence of Hepatocellular Carcinoma in Patients with Direct-Acting Antiviral-Induced Sustained Viral Response. Biomedicines. 2019; 7(4):87. https://doi.org/10.3390/biomedicines7040087
Chicago/Turabian StyleItami-Matsumoto, Saori, Michiyo Hayakawa, Sawako Uchida-Kobayashi, Masaru Enomoto, Akihiro Tamori, Kazuyuki Mizuno, Hidenori Toyoda, Takeyuki Tamura, Tatsuya Akutsu, Takahiro Ochiya, and et al. 2019. "Circulating Exosomal miRNA Profiles Predict the Occurrence and Recurrence of Hepatocellular Carcinoma in Patients with Direct-Acting Antiviral-Induced Sustained Viral Response" Biomedicines 7, no. 4: 87. https://doi.org/10.3390/biomedicines7040087
APA StyleItami-Matsumoto, S., Hayakawa, M., Uchida-Kobayashi, S., Enomoto, M., Tamori, A., Mizuno, K., Toyoda, H., Tamura, T., Akutsu, T., Ochiya, T., Kawada, N., & Murakami, Y. (2019). Circulating Exosomal miRNA Profiles Predict the Occurrence and Recurrence of Hepatocellular Carcinoma in Patients with Direct-Acting Antiviral-Induced Sustained Viral Response. Biomedicines, 7(4), 87. https://doi.org/10.3390/biomedicines7040087







