Antihyperlipidemic and Antioxidant Effects of Averrhoa Carambola Extract in High-Fat Diet-Fed Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Standards
2.2. Collection of A. Carambola and Extract Preparation
2.3. Standardization and Quantification of Apigenin in A. Carambola Extract Using High Performance Liquid Chromatography (HPLC)
2.3.1. Development and Validation of the HPLC Method
2.3.2. Analysis and Standardization of Apigenin in MEACL
2.4. Animals and Treatments
2.4.1. Induction of Hyperlipidemia
2.4.2. Experimental Design
2.5. Determination of Serum Lipids and Atherogenic Index (AI)
2.6. Determination of the BMI
2.7. Histopathology
2.8. Determination of HMG-CoA Reductase and Pancreatic Lipase Activities
2.9. Determination of Lipid Peroxidation and Antioxidants
2.10. Determination of Hepatic and Fecal Lipids
2.11. Determination of Fecal Bile Acids
2.12. Statistical Analysis
3. Results
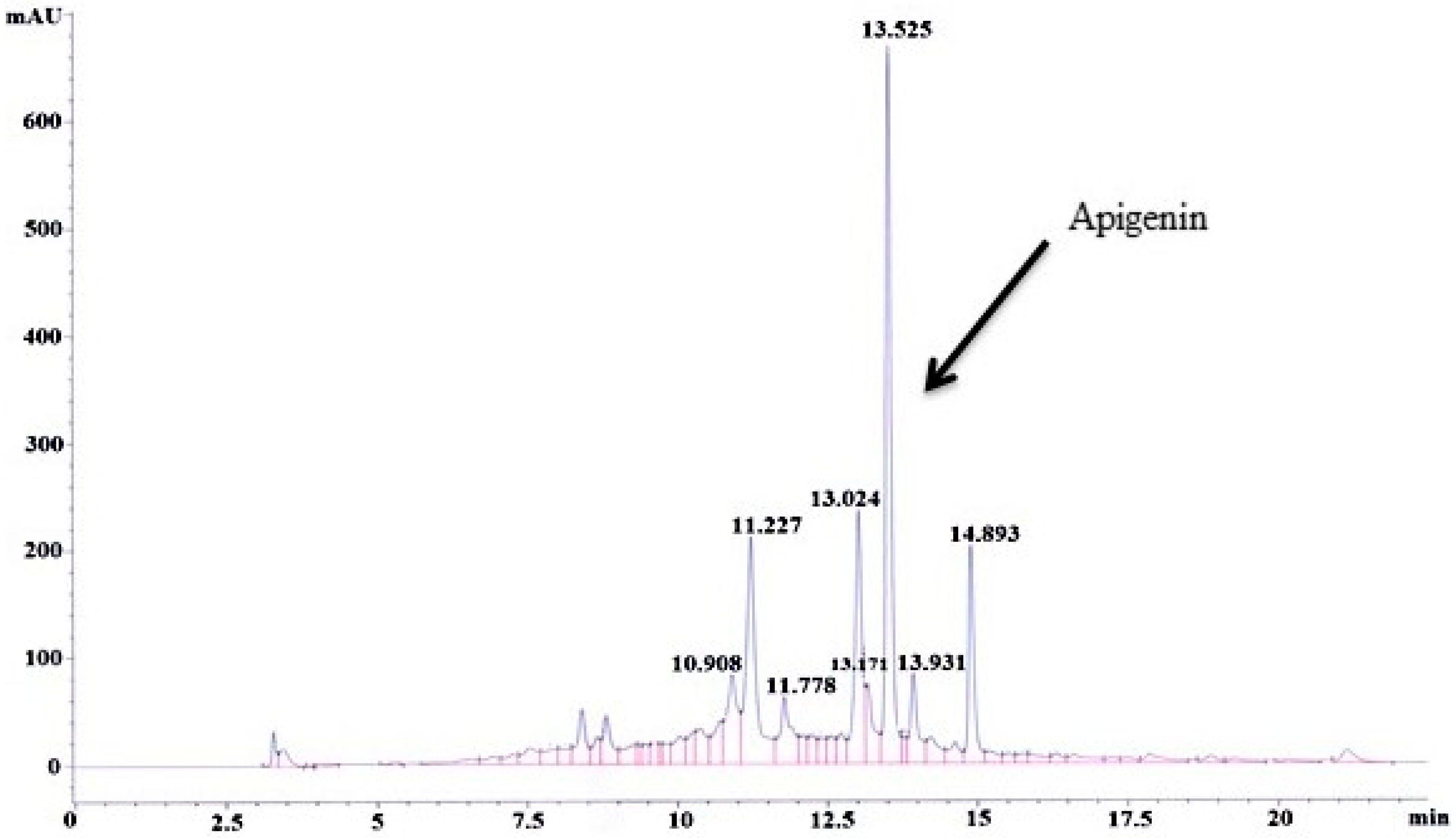
3.1. Standardization and Quantification of Apigenin in MEACL
3.1.1. HPLC Validation Method
3.1.2. HPLC–UV Analysis of MEACL
3.2. Effects of MEACL on Body Weight, Body Mass Index, Food Intake, and Relative Organ Weight
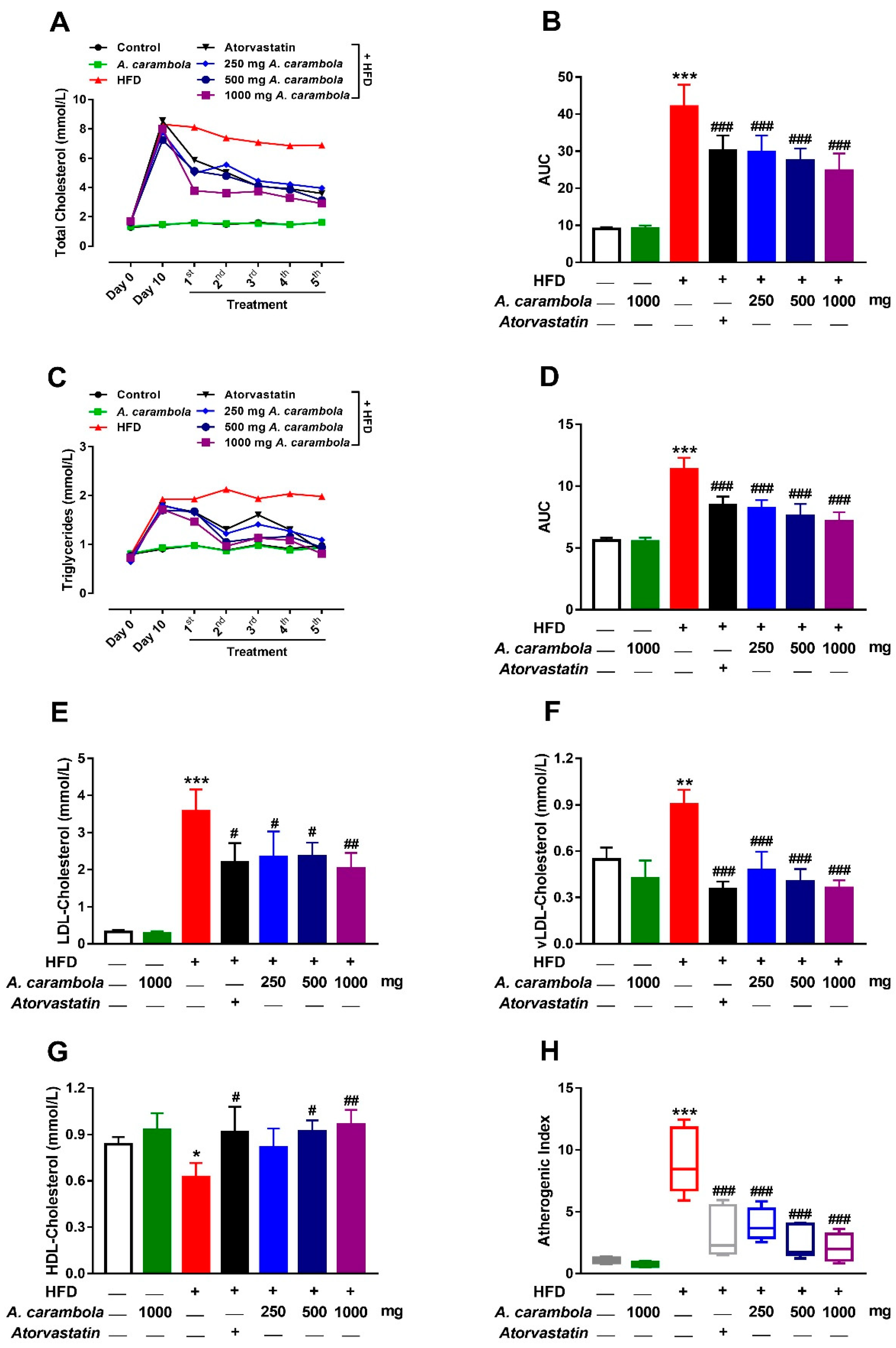
3.3. Antihyperlipidemic Effect of MEACL in HFD-Fed Rats
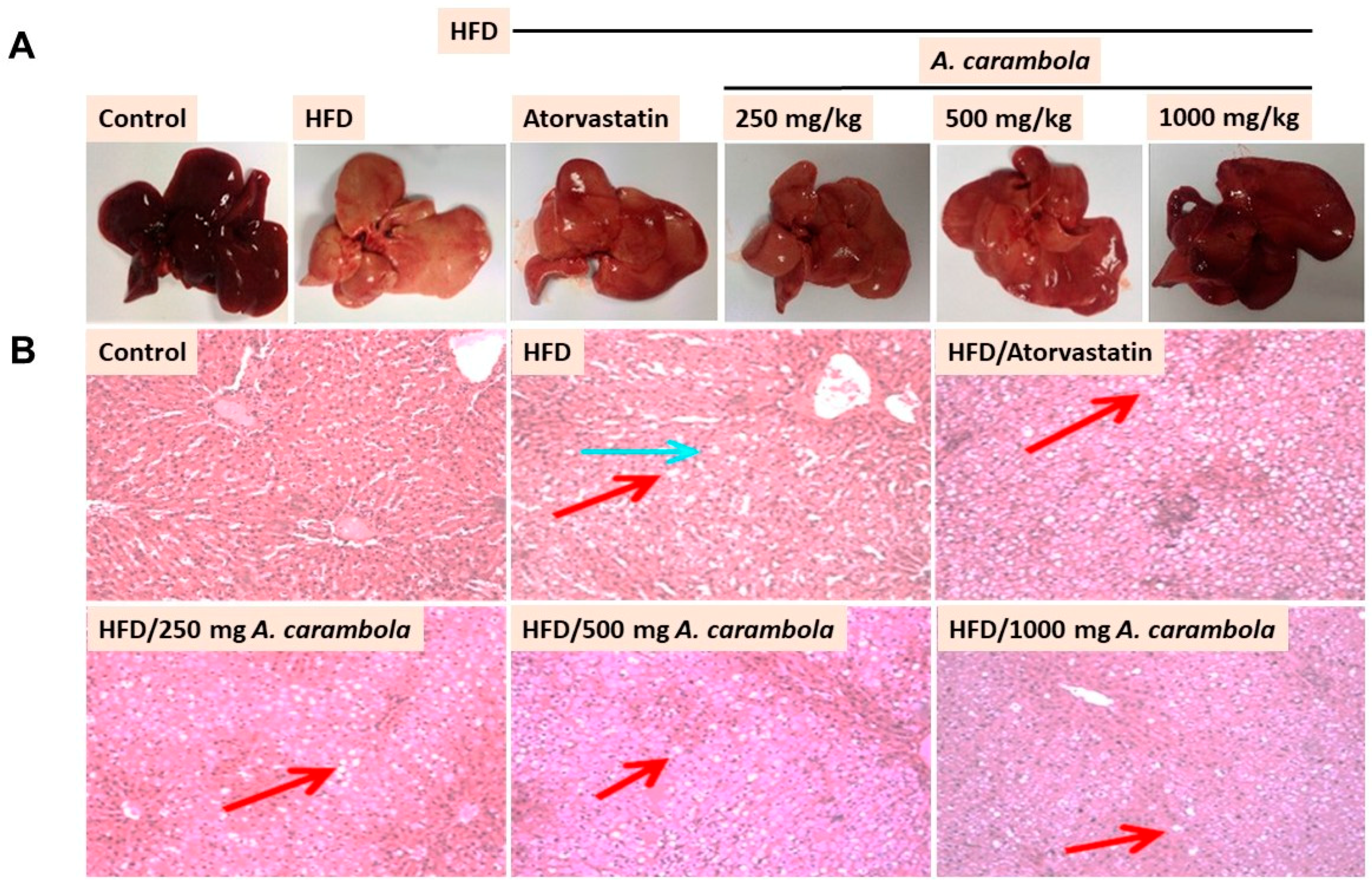
3.4. MEACL Prevents HFD-Induced Histopathological Alterations
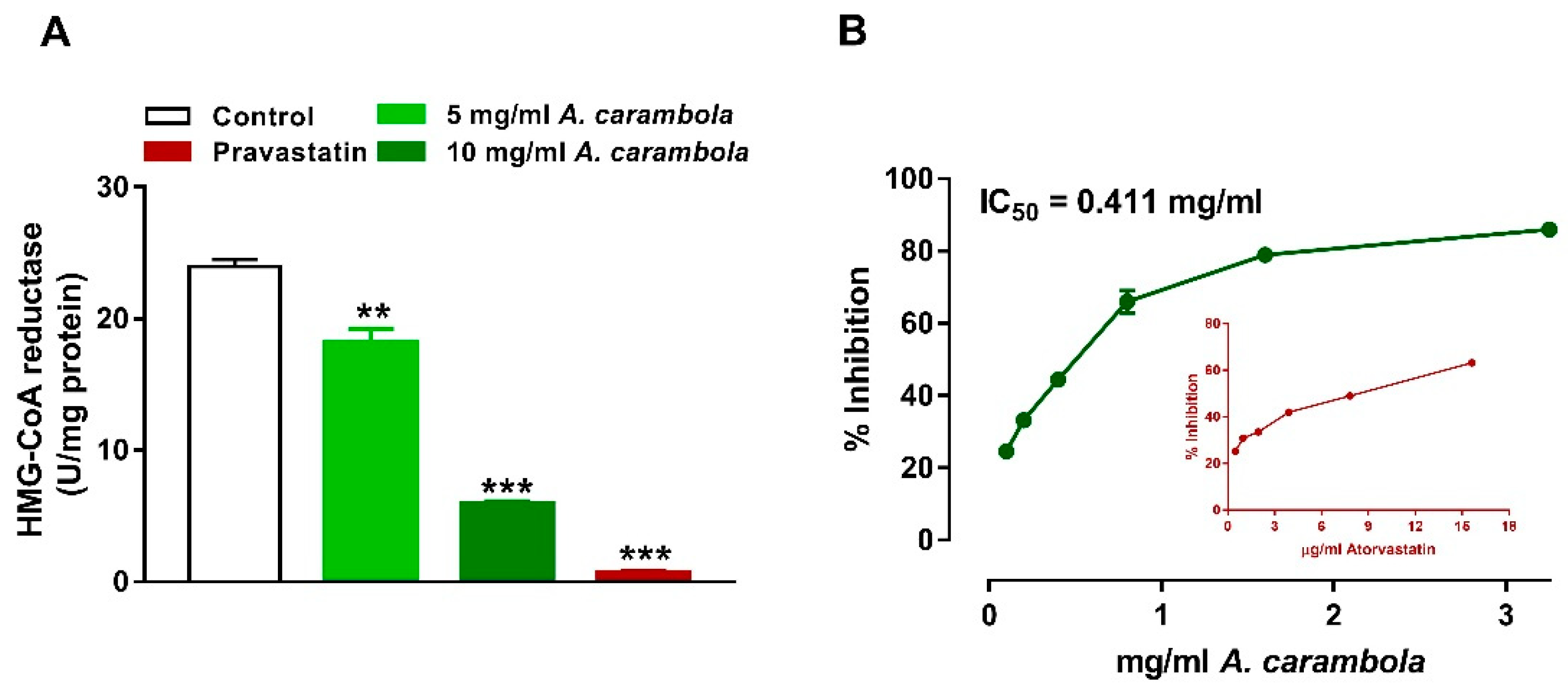
3.5. Inhibitory Activity of MEACL on HMG-CoA Reductase and Pancreatic Lipase
3.6. Effect of MEACL on Levels of Liver TC, TG, Fecal Cholesterol, and Fecal Bile Acids of HFD-induced Hyperlipidemic Rats
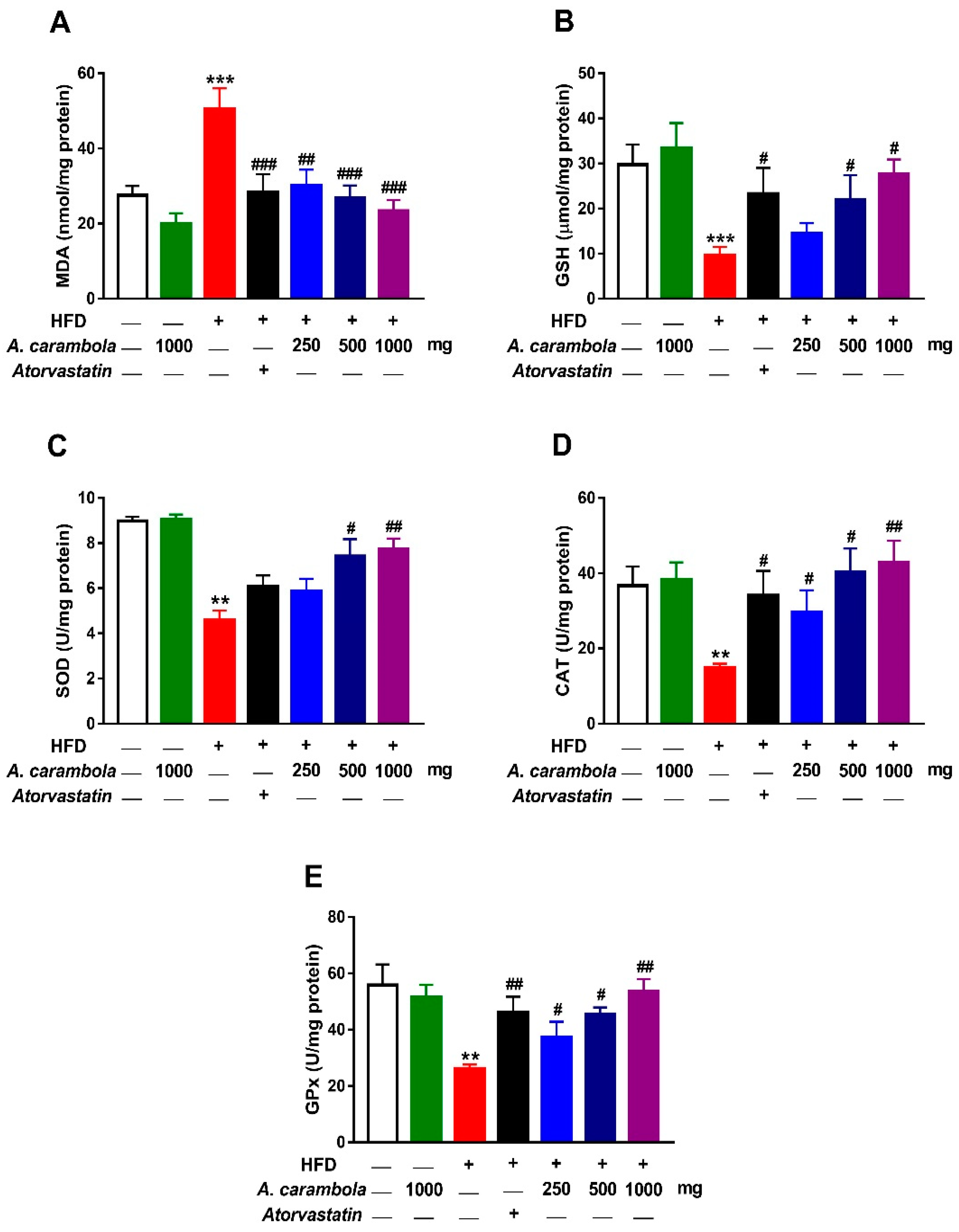
3.7. Antioxidant Activity of MEACL in HFD-Induced Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shukr, M.H.; Ismail, S.; Ahmed, S.M. Development and optimization of ezetimibe nanoparticles with improved antihyperlipidemic activity. J. Drug. Deliv. Sci. Technol. 2019, 49, 383–395. [Google Scholar] [CrossRef]
- Cosenza, G.P.; Viana, C.T.R.; Campos, P.P.; Kohlhoff, M.; Fagg, C.W.; Brandão, M.G.L. Chemical characterization, antihyperlipidaemic and antihyperglycemic effects of Brazilian bitter quina species in mice consuming a high-refined carbohydrate diet. J. Funct. Foods. 2019, 54, 220–230. [Google Scholar] [CrossRef]
- Surya, S.; Arun Kumar, R.; Carla, B.; Sunil, C. Antihyperlipidemic effect of Ficus dalhousiae miq. stem bark on Triton WR-1339 and high fat diet-induced hyperlipidemic rats. Bull. Fac. Pharm. Cairo. Univ. 2017, 55, 73–77. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.J.; Sun, Y.J.; Gong, J.H.; Du, K.; Zhang, Y.L.; Su, C.F.; Han, Q.Q.; Zheng, X.K.; Feng, W.S. Lignanamides with potent antihyperlipidemic activities from the root bark of Lycium chinense. Fitoterapia 2017, 122, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Valdés, S.; García-Torres, F.; Maldonado-Araque, C.; Goday, A.; Calle-Pascual, A.; Soriguer, F.; Castaño, L.; Catalá, M.; Gomis, R.; Rojo-Martínez, G. Prevalence of Obesity, Diabetes and Other Cardiovascular Risk Factors in Andalusia (Southern Spain). Comparison with National Prevalence Data. The Di@bet.es Study. Rev. Esp. Cardiol. 2014, 67, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Yang, H.; You, Y.; Xu, H.; Gong, L.; Yin, X.; Wang, W.; Gao, S.; Cheng, L.; et al. Prevention of atherosclerosis by Yindan Xinnaotong capsule combined with swimming in rats. BMC Complement. Altern. Med. 2015, 15, 109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Esakkimuthu, S.; Nagulkumar, S.; Darvin, S.S.; Buvanesvaragurunathan, K.; Sathya, T.N.; Navaneethakrishnan, K.R.; Kumaravel, T.S.; Murugan, S.S.; Shirota, O.; Balakrishna, K.; et al. Antihyperlipidemic effect of iridoid glycoside deacetylasperulosidic acid isolated from the seeds of Spermacoce hispida L.-A traditional antiobesity herb. J. Ethnopharmacol. 2019, 112170. [Google Scholar] [CrossRef]
- Tacherfiout, M.; Petrov, P.D.; Mattonai, M.; Ribechini, E.; Ribot, J.; Bonet, M.L.; Khettal, B. Antihyperlipidemic effect of a Rhamnus alaternus leaf extract in Triton-induced hyperlipidemic rats and human HepG2 cells. Biomed. Pharmacother. 2018, 101, 501–509. [Google Scholar] [CrossRef]
- Kamal, F.; Shahzad, M.; Ahmad, T.; Ahmed, Z.; Tareen, R.B.; Naz, R.; Ahmad, A. Antihyperlipidemic effect of Pistacia khinjuk. Biomed. Pharmacother. 2017, 96, 695–699. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Kong, K.W.; Ismail, A. Phytochemicals and medicinal properties of indigenous tropical fruits with potential for commercial development. Evid. Based Complement. Alternat. Med. 2016, 2016, 7591951. [Google Scholar] [CrossRef]
- Shui, G.; Leong, L.P. Analysis of polyphenolic antioxidants in star fruit using liquid chromatography and mass spectrometry. J Chromatogr. A. 2004, 1022, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Annegowda, H.V.; Bhat, R.; Min-Tze, L.; Karim, A.A.; Mansor, S.M. Influence of sonication treatments and extraction solvents on the phenolics and antioxidants in star fruits. J. Food Sci. Technol. 2012, 49, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Cabrini, D.A.; Moresco, H.H.; Imazu, P.; da Silva, C.D.; Pietrovski, E.F.; Mendes, D.A.; da Silveira Prudente, A.; Pizzolatti, M.G.; Brighente, I.M.; Otuki, M.F. Analysis of the potential topical anti-inflammatory activity of Averrhoa carambola l. in mice. Evid. Based Complement. Alternat. Med. 2011, 2011, 908059. [Google Scholar] [CrossRef] [PubMed]
- Pang, D.; You, L.; Zhou, L.; Li, T.; Zheng, B.; Liu, R.H. Averrhoa carambola free phenolic extract ameliorates nonalcoholic hepatic steatosis by modulating mircoRNA-34a, mircoRNA-33 and AMPK pathways in leptin receptor-deficient db/db mice. Food Func. 2017, 8, 4496–4507. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, H.; Wei, X.; Huang, X.; Chen, L.; Jiang, L.; Wu, X.; Zhou, X.; Qin, L.; Li, Y.; et al. 2-dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione isolated from Averrhoa carambola l. toot ameliorates diabetic nephropathy by inhibiting the TLR4/MyD88/NF-kappaB pathway. Diabetes Metab. Syndr. Obes. 2019, 12, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Saghir, S.A.M.; Sadikun, A.; Al-Suede, F.S.R.; Majid, A.M.S.A.; Murugaiyah, V. Antihyperlipidemic, antioxidant and cytotoxic activities of methanolic and aqueous extracts of different parts of Star fruit. Curr.Pharm. Biotechnol. 2016, 17, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Irudayaraj, S.S.; Sunil, C.; Duraipandiyan, V.; Ignacimuthu, S. In vitro antioxidant and antihyperlipidemic activities of Toddalia asiatica (L) Lam. leaves in Triton WR-1339 and high fat diet induced hyperlipidemic rats. Food. Chem. Toxicol. 2013, 60, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Patrolecco, L.; Ademollo, N.; Grenni, P.; Tolomei, A.; Barra Caracciolo, A.; Capri, S. Simultaneous determination of human pharmaceuticals in water samples by solid phase extraction and HPLC with UV-fluorescence detection. Microchem. J. 2013, 107, 165–171. [Google Scholar] [CrossRef]
- Pirol, O.; Sukuroglu, M.; Ozden, T. Simultaneous Determination of paracetamol, phenylephrine hydrochloride, oxolamine citrate and chlorpheniramine maleate by HPLC in pharmaceutical dosage forms. E. J. Chem. 2011, 8, 1275–1279. [Google Scholar] [CrossRef]
- Klimczak, I.; Gliszczyńska-Świgło, A. Comparison of UPLC and HPLC methods for determination of vitamin C. Food. Chem. 2015, 175, 100–105. [Google Scholar] [CrossRef]
- Kazakevich, Y.; LoBrutto, R. HPLC for Pharmaceutical Scientists; Wiley Online Library: Hoboken, NJ, USA, 2007; Volume 43. [Google Scholar]
- Khalid, H.; Zhari, I.; Amirin, S.; Pazilah, I. Accelerated stability and chemical kinetics of ethanol extracts of fruit of Piper sarmentosum using high performance liquid chromatography. Iran J Pharm Res 2011, 10, 403–413. [Google Scholar] [PubMed]
- Kheng, B.H. Phytochemical and Selected Pharmacological Studies of Standardized Fruit Extracts of Morinda Citrifolia Linn. Ph.D. Thesis, Universiti Sains Malaysia, Penang, Malaysia, 2012. [Google Scholar]
- Wu, Q.; Zhang, H.; Dong, X.; Chen, X.F.; Zhu, Z.Y.; Hong, Z.Y.; Chai, Y.F. UPLC-Q-TOF/MS based metabolomic profiling of serum and urine of hyperlipidemic rats induced by high fat diet. J. Pharm. Anal. 2014, 4, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.T.; Hetland, R.B.; Sabaredzovic, A.; Haug, L.S.; Steffensen, I.-L. In utero exposure to perfluorooctanoate (PFOA) or perfluorooctane sulfonate (PFOS) did not increase body weight or intestinal tumorigenesis in multiple intestinal neoplasia (Min/+) mice. Environ. Res. 2014, 132, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Ramakrishnan, S. Indirect assessment of hydroxymethylglutaryl-CoA reductase (NADPH) activity in liver tissue. Clin. Chem. 1975, 21, 1523–1525. [Google Scholar] [PubMed]
- Adisakwattana, S.; Chanathong, B. Alpha-glucosidase inhibitory activity and lipid-lowering mechanisms of Moringa oleifera leaf extract. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 803–808. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Jollow, D.; Mitchell, J.; Zampaglione, N.A.; Gillette, J. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Ōyanagui, Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal. Biochem. 1984, 142, 290–296. [Google Scholar] [CrossRef]
- Flohé, L.; Loschen, G.; Günzler, W.A.; Eichele, E. Glutathione peroxidase, V. The kinetic mechanism. Hoppe-Seyler’s Z. Physiol. Chem. 1972, 353, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Boil. Chem. 1957, 226, 497–509. [Google Scholar]
- Zhu, L.; Luo, X.; Jin, Z. Effect of resveratrol on serum and liver lipid profile and antioxidant activity in hyperlipidemia rats. Asian-Australas. J. Anim. Sci. 2008, 21, 890–895. [Google Scholar] [CrossRef]
- Sheltawy, M.J.; Losowsky, M. Determination of faecal bile acids by an enzymic method. Clin. Chim. Acta. 1975, 64, 127–132. [Google Scholar] [CrossRef]
- Miao, H.; Chen, H.; Pei, S.; Bai, X.; Vaziri, N.D.; Zhao, Y.Y. Plasma lipidomics reveal profound perturbation of glycerophospholipids, fatty acids, and sphingolipids in diet-induced hyperlipidemia. Chem. Biol. Interact. 2015, 228, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Afaq, F.; Mukhtar, H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem. Biophys. Res. Comm. 2001, 287, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Li, L.P.; Jiang, H.D. Determination and assay validation of luteolin and apigenin in human urine after oral administration of tablet of Chrysanthemum morifolium extract by HPLC. J. Pharm. Biomed. Anal. 2006, 41, 261–265. [Google Scholar] [CrossRef]
- Thiery-Vuillemin, A.; Nguyen, T.; Pivot, X.; Spano, J.; Dufresnne, A.; Soria, J. Molecularly targeted agents: Their promise as cancer chemopreventive interventions. Eur. J. Cancer. 2005, 41, 2003–2015. [Google Scholar] [CrossRef]
- Li, L.; Jiang, H.; Wu, H.; Zeng, S. Simultaneous determination of luteolin and apigenin in dog plasma by RP-HPLC. J. Pharm. Biomed. Anal. 2005, 37, 615–620. [Google Scholar] [CrossRef]
- Volpi, N.; Bergonzini, G. Analysis of flavonoids from propolis by on-line HPLC–electrospray mass spectrometry. J. Pharm. Biomed. Anal. 2006, 42, 354–361. [Google Scholar] [CrossRef]
- Nielsen, S.E.; Dragsted, L.O. Column-switching high-performance liquid chromatographic assay for determination of apigenin and acacetin in human urine with ultraviolet absorbance detection. J. Chromatogr. B Biomed. Appl. 1998, 713, 379–386. [Google Scholar] [CrossRef]
- Chen, Q.; Reis, S.E.; Kammerer, C.; Craig, W.; McNamara, D.M.; Holubkov, R.; Sharaf, B.L.; Sopko, G.; Pauly, D.F.; Merz, C.N.B. Association of anti-oxidized LDL and candidate genes with severity of coronary stenosis in the Women’s Ischemia Syndrome Evaluation study. J. Lipid Res. 2011, 52, 801–807. [Google Scholar] [CrossRef]
- Ntchapda, F.; Djedouboum, A.; Talla, E.; Dongmo, S.S.; Nana, P.; Adjia, H.; Nguimbou, R.M.; Bonabe, C.; Gaimatakon, S.; Yanou, N.N. Hypolipidemic and anti-atherogenic effect of aqueous extract leaves of Ficus glumosa (Moraceae) in rats. Exp. Gerontol. 2015, 62, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Deng, Z.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.; Liu, Q.; Tsao, R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on α-glucosidase and pancreatic lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, D.; Huang, B.; Chen, Y.; Lu, X.; Wang, Y. Inhibition of pancreatic lipase, α-glucosidase, α-amylase, and hypolipidemic effects of the total flavonoids from Nelumbo nucifera leaves. J. Ethnopharmacol. 2013, 149, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.N.C.; Palawat, S.; Paul, A.T. Design, synthesis, biological evaluation and molecular modelling studies of indole glyoxylamides as a new class of potential pancreatic lipase inhibitors. Bioorg. Chem. 2019, 85, 373–381. [Google Scholar] [CrossRef]
- Yang, R.L.; Shi, Y.H.; Hao, G.; Li, W.; Le, G.-W. Increasing oxidative stress with progressive hyperlipidemia in human: Relation between malondialdehyde and atherogenic index. J. Clin. Biochem. Nutr. 2008, 43, 154–158. [Google Scholar] [CrossRef]
- Ponce-Canchihuamán, J.C.; Pérez-Méndez, O.; Hernández-Muñoz, R.; Torres-Durán, P.V.; Juárez-Oropeza, M.A. Protective effects of Spirulina maxima on hyperlipidemia and oxidative-stress induced by lead acetate in the liver and kidney. Lipids Health. Dis. 2010, 9, 35. [Google Scholar] [CrossRef]
- Singh, U.; Kumar, S.; Dhakal, S. Study of oxidative stress in hypercholesterolemia. Int. J. Con-temp. Med. Res. 2017, 4, 1204–1207. [Google Scholar]
- Smathers, R.L.; Galligan, J.J.; Stewart, B.J.; Petersen, D.R. Overview of lipid peroxidation products and hepatic protein modification in alcoholic liver disease. Chem. Biol. Interact. 2011, 192, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Aladaileh, S.H.; Abukhalil, M.H.; Saghir, S.A.; Hanieh, H.; Alfwuaires, M.A.; Almaiman, A.A.; Bin-Jumah, M.; Mahmoud, A.M. Galangin activates Nrf2 signaling and attenuates oxidative damage, inflammation, and apoptosis in a rat model of cyclophosphamide-induced hepatotoxicity. Biomolecules 2019, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Terevinto, A.; Ramos, A.; Castroman, G.; Cabrera, M.; Saadoun, A. Oxidative status, in vitro iron-induced lipid oxidation and superoxide dismutase, catalase and glutathione peroxidase activities in rhea meat. Meat Sci. 2010, 84, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Vasant, R.A.; Narasimhacharya, A. Antidotal activity of Averrhoa carambola (Star fruit) on fluoride induced toxicity in rats. Interdiscip. Toxicol. 2014, 7, 103–110. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, J.; Goyal, P. Prophylactic role of Averrhoa carambola (star fruit) extract against chemically induced hepatocellular carcinoma in Swiss albino mice. Adv. Pharmacol. Sci. 2014, 2014, 158936. [Google Scholar] [PubMed]
- Lim, Y.S.; Lee, S.T. In vitro antioxidant capacities of star fruit (Averrhoa carambola), an underutilised tropical fruit. J. Biol. 2013, 1, 21–24. [Google Scholar]
- Gupta, V.K.; Kumria, R.; Garg, M.; Gupta, M. Recent updates on free radicals scavenging flavonoids: An overview. Asian J. Plant. Sci. 2010, 9, 108. [Google Scholar] [CrossRef]
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Huang, C.S.; Lii, C.K.; Lin, A.H.; Yeh, Y.W.; Yao, H.T.; Li, C.C.; Wang, T.S.; Chen, H.W. Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Arch. Toxicol. 2013, 87, 167–178. [Google Scholar] [CrossRef]
- Paredes-Gonzalez, X.; Fuentes, F.; Jeffery, S.; Saw, C.L.L.; Shu, L.; Su, Z.Y.; Kong, A.N.T. Induction of NRF2-mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharm. Drug Dispos. 2015, 36, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Rathee, J.S.; Hassarajani, S.A.; Chattopadhyay, S. Antioxidant activity of Nyctanthes arbortristis leaf extract. Food Chem. 2007, 103, 1350–1357. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, H.; Li, Q.; Qi, X.; Li, Y.; Zhang, Z.; Chen, J.; Ren, J. (5R)-5-hydroxytriptolide ameliorates liver lipid accumulation by suppressing lipid synthesis and promoting lipid oxidation in mice. Life Sci. 2019, 232, 116644. [Google Scholar] [CrossRef] [PubMed]
- Suckling, K.E.; Stange, E.F. Role of acyl-CoA: Cholesterol acyltransferase in cellular cholesterol metabolism. J. Lipid Res. 1985, 26, 647–671. [Google Scholar] [PubMed]
- Visavadiya, N.P.; Narasimhacharya, A. Sesame as a hypocholesteremic and antioxidant dietary component. Food Chem. Toxicol. 2008, 46, 1889–1895. [Google Scholar] [CrossRef] [PubMed]


| Time (min) | A (%) | B (%) |
|---|---|---|
| 0.0 | 90.0 | 10.0 |
| 5.0 | 90.0 | 10.0 |
| 12.0 | 55.0 | 45.0 |
| 17.0 | 55.0 | 45.0 |
| 20.0 | 90.0 | 10.0 |
| Amount Added (µg/mL) | Amount Measured (µg/mL) | Mean Recovery % | RSD % | CV |
|---|---|---|---|---|
| 50 | 49.98 | 99.96 | 3.20 | 3.21 |
| 5 | 5.2 | 104.00 | 3.10 | 2.94 |
| 0.625 | 0.628 | 100.48 | 3.99 | 4.15 |
| Concentration (µg/mL) | Within-day | Between-day | ||
|---|---|---|---|---|
| Accuracy (%) | Precision CV % | Accuracy (%) | Precision (CV%) | |
| 50 | 95.08 | 3.24 | 104.92 | 1.32 |
| 20 | 104.07 | 3.88 | 95.24 | 1.98 |
| 5 | 102.12 | 4.03 | 99.18 | 4.54 |
| 2.5 | 100.24 | 2.64 | 97.65 | 2.99 |
| 1.25 | 99.38 | 3.28 | 95.75 | 5.04 |
| 0.625 | 99.65 | 1.01 | 101.84 | 2.17 |
| Organ | Control | HFD | HFD + Atorvastatin | HFD + 250 mg/kg MEACL | HFD + 500 mg/kg MEACL | HFD + 1000 mg/kg MEACL |
|---|---|---|---|---|---|---|
| Liver | 3.06 ± 0.10 | 4.78 ± 0.18 | 3.88 ± 0.24 | 3.91± 0.22 | 4.0 ± 0.13# | 3.69 ± 0.15## |
| Brain | 0.46 ± 0.02 | 0.38 ± 0.04 | 0.46 ± 0.01 | 0.59 ± 0.04 | 0.55 ± 0.05 | 0.48 ± 0.06 |
| Heart | 0.32 ± 0.02 | 0.35 ± 0.01 | 0.38 ± 0.01 | 0.40 ± 0.02 | 0.39 ± 0.01 | 0.35 ± 0.02 |
| Lung | 0.52 ± 0.02 | 0.48 ± 0.02 | 0.56 ± 0.02 | 0.57 ± 0.04 | 0.62 ± 0.04 | 0.60 ± 0.06 |
| Thymus | 0.12 ± 0.01 | 0.10 ± 0.01 | 0.14 ± 0.02 | 0.14 ± 0.03 | 0.11 ± 0.01 | 0.11 ± 0.02 |
| R. kidney | 0.28 ± 0.01 | 0.26 ± 0.01 | 0.36 ± 0.01 | 0.34 ± 0.02 | 0.34 ±0.01 | 0.33 ± 0. 02 |
| L. kidney | 0.29 ± 0.01 | 0.26 ± 0.01 | 0.34 ± 0.01 | 0.33 ± 0.01 | 0.34 ± 0.02 | 0.32 ± 0.02 |
| R. adrenal | 0.004 ± 0.0002 | 0.004 ± 0.0002 | 0.005 ± 0.0001 | 0.005 ± 0.0001 | 0.005 ± 0.0002 | 0.006 ± 0.0003 |
| L. adrenal | 0.005 ± 0.0002 | 0.005 ± 0.0003 | 0.005 ± 0.0002 | 0.006 ± 0.0004 | 0.005 ± 0.0001 | 0.005 ± 0.0002 |
| Spleen | 0.21 ± 0.01 | 0.19 ± 0.02 | 0.24 ± 0.01 | 0.27 ± 0.03 | 0.26 ± 0.02 | 0.21 ± 0.01 |
| Stomach | 1.28 ± 0.09 | 1.40 ± 0.10 | 0.63 ± 0.06 | 1.06 ± 0.16 | 1.19 ± 0.16 | 1.18 ± 0.14 |
| Adipose tissue | 1.54 ± 0.20 | 1.50 ± 0.14 | 1.59 ± 0.23 | 1.54 ± 0.201 | 1.55 ± 0.27 | 0.89 ± 0.08# |
| Gut | 5.42 ± 0.23 | 5.00 ± 0.13 | 4.58 ± 0.16 | 5.08 ± 0.35 | 5.73 ± 0.36 | 5.56 ± 0.44 |
| R. tests | 0.38 ± 0.03 | 0.35 ± 0.02 | 0.50 ± 0.01 | 0.50 ±0.03 | 0.54 ± 0.02 | 0.53 ± 0.03 |
| L. tests | 0.43 ± 0.04 | 0.35 ± 0.02 | 0.51 ± 0.01 | 0.51 ± 0.02 | 0.55 ± 0.02 | 0.54 ± 0.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aladaileh, S.H.; Saghir, S.A.M.; Murugesu, K.; Sadikun, A.; Ahmad, A.; Kaur, G.; Mahmoud, A.M.; Murugaiyah, V. Antihyperlipidemic and Antioxidant Effects of Averrhoa Carambola Extract in High-Fat Diet-Fed Rats. Biomedicines 2019, 7, 72. https://doi.org/10.3390/biomedicines7030072
Aladaileh SH, Saghir SAM, Murugesu K, Sadikun A, Ahmad A, Kaur G, Mahmoud AM, Murugaiyah V. Antihyperlipidemic and Antioxidant Effects of Averrhoa Carambola Extract in High-Fat Diet-Fed Rats. Biomedicines. 2019; 7(3):72. https://doi.org/10.3390/biomedicines7030072
Chicago/Turabian StyleAladaileh, Saleem H., Sultan A. M. Saghir, Kisantini Murugesu, Amirin Sadikun, Ashfaq Ahmad, Gurjeet Kaur, Ayman M. Mahmoud, and Vikneswaran Murugaiyah. 2019. "Antihyperlipidemic and Antioxidant Effects of Averrhoa Carambola Extract in High-Fat Diet-Fed Rats" Biomedicines 7, no. 3: 72. https://doi.org/10.3390/biomedicines7030072
APA StyleAladaileh, S. H., Saghir, S. A. M., Murugesu, K., Sadikun, A., Ahmad, A., Kaur, G., Mahmoud, A. M., & Murugaiyah, V. (2019). Antihyperlipidemic and Antioxidant Effects of Averrhoa Carambola Extract in High-Fat Diet-Fed Rats. Biomedicines, 7(3), 72. https://doi.org/10.3390/biomedicines7030072





