Management of Bleeding from Unresectable Gastric Cancer
Abstract
1. Introduction
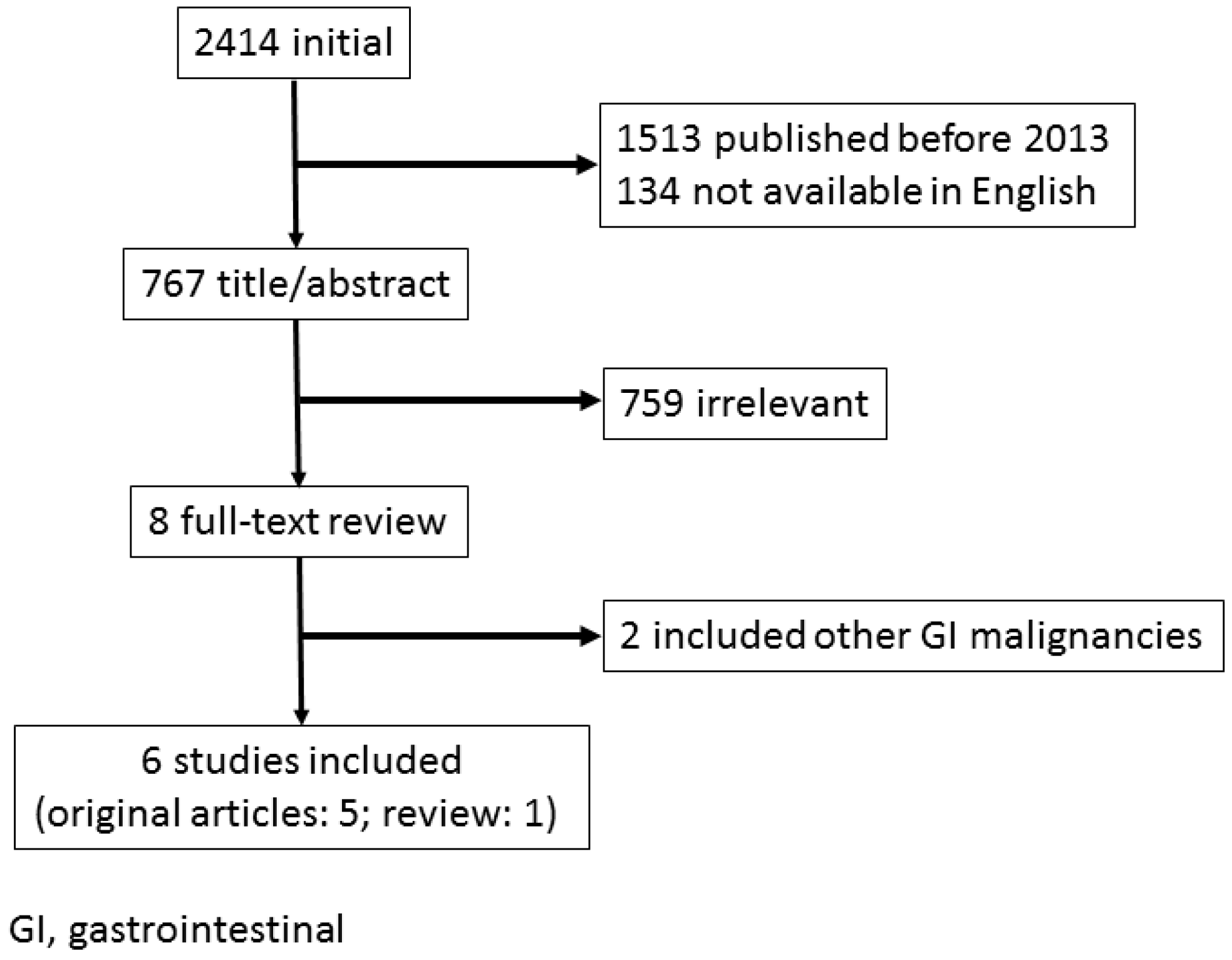
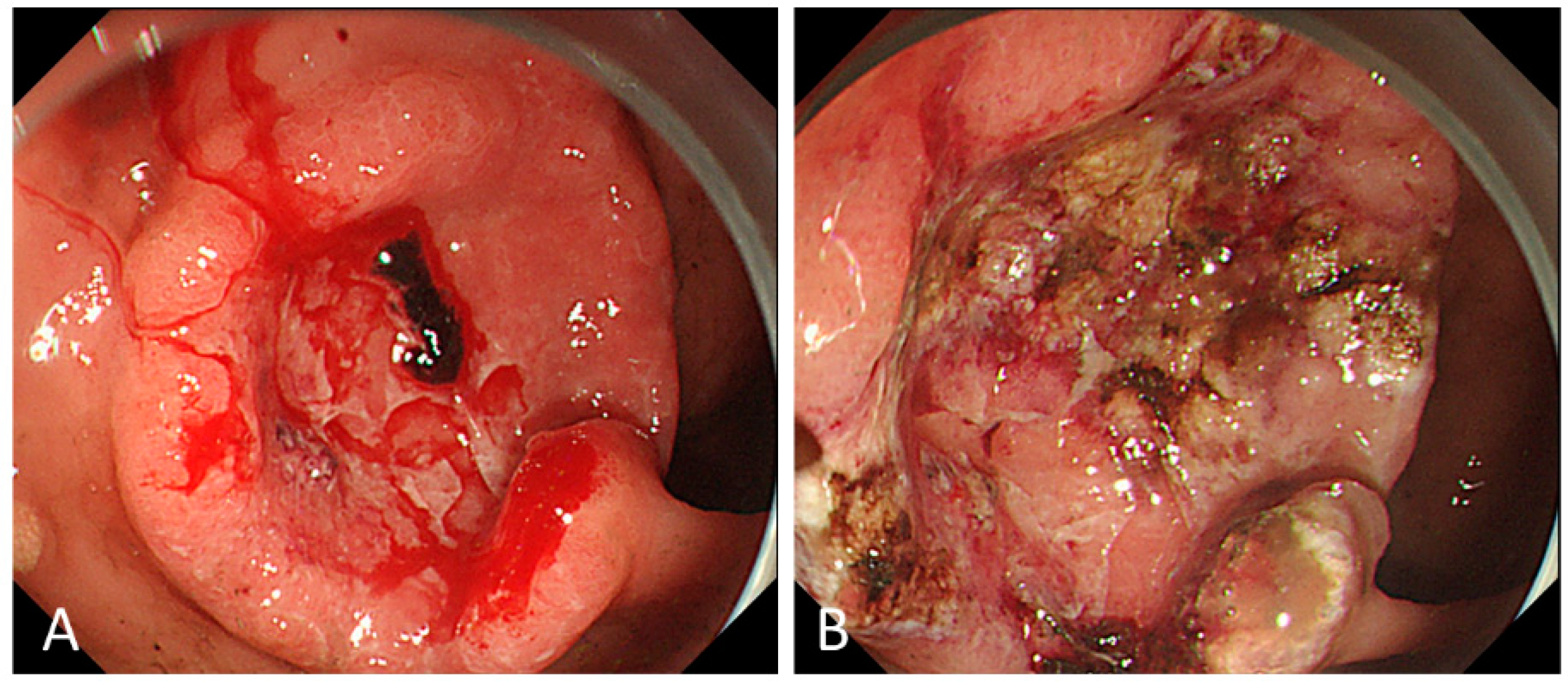
2. Endoscopic Management for Bleeding from Unresectable GC
2.1. Outcomes of ET
2.2. Indications of ET
2.3. Modalities of ET
2.4. Complications of ET
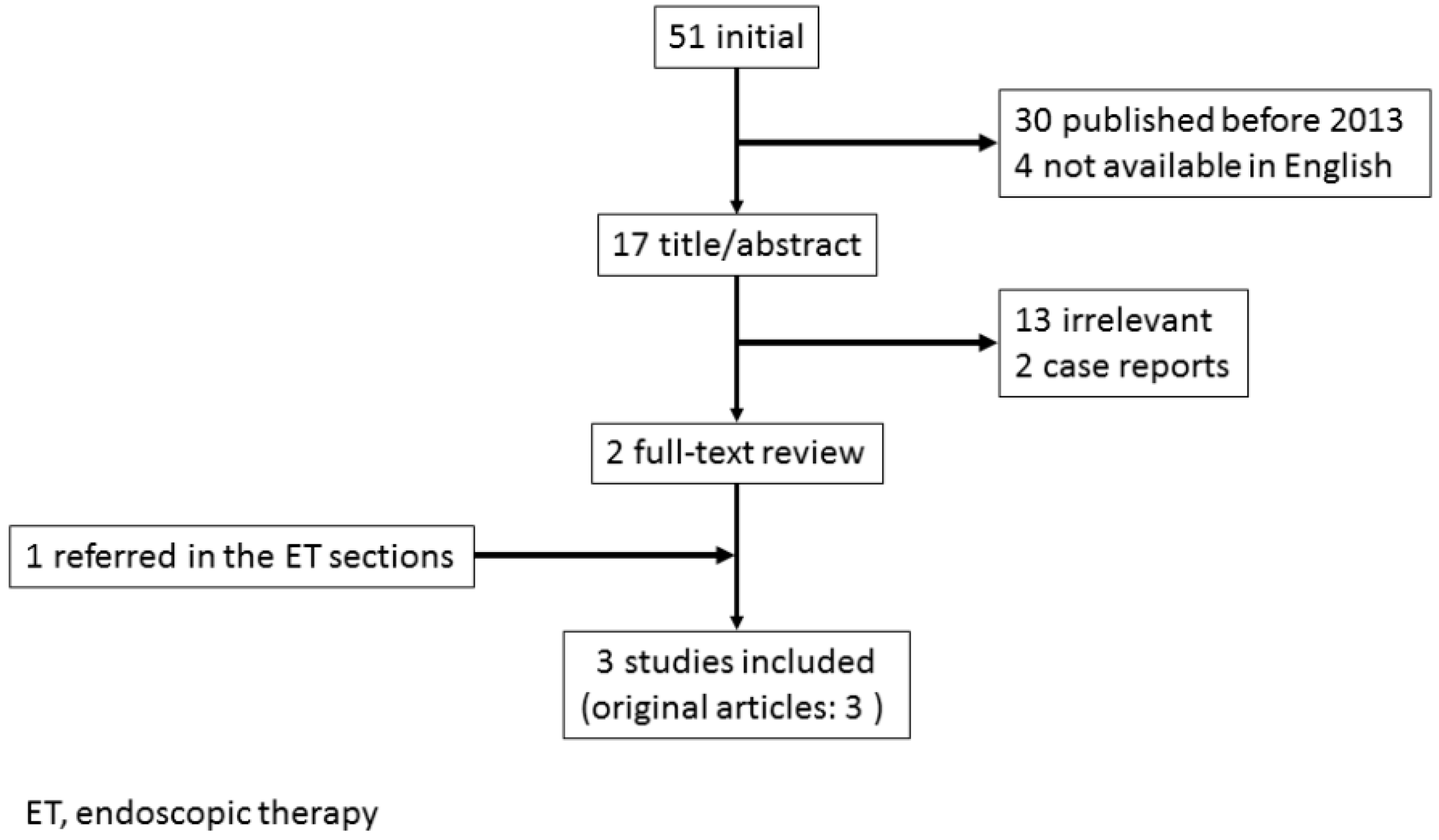
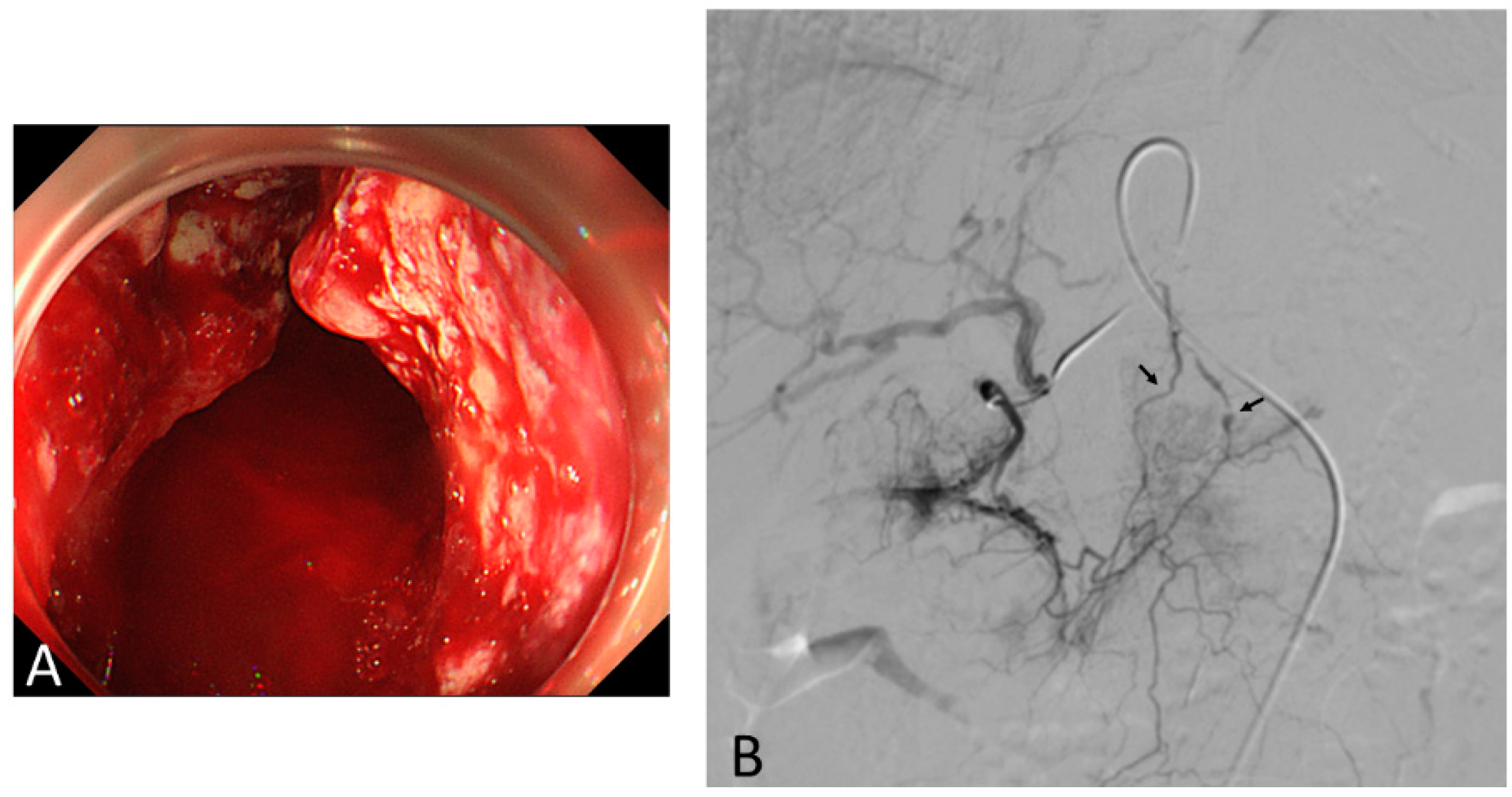
3. Transcatheter Embolotherapy for Bleeding from Unresectable GC
3.1. Outcomes of TAE
3.2. Indications of TAE
3.3. TAE Procedure
3.4. Complications of TAE
4. Palliative RT for Bleeding from Unresectable GC
4.1. Outcomes of Palliative RT
4.2. Condition of RT
4.3. Toxicity
4.4. The Assessment of the QOL
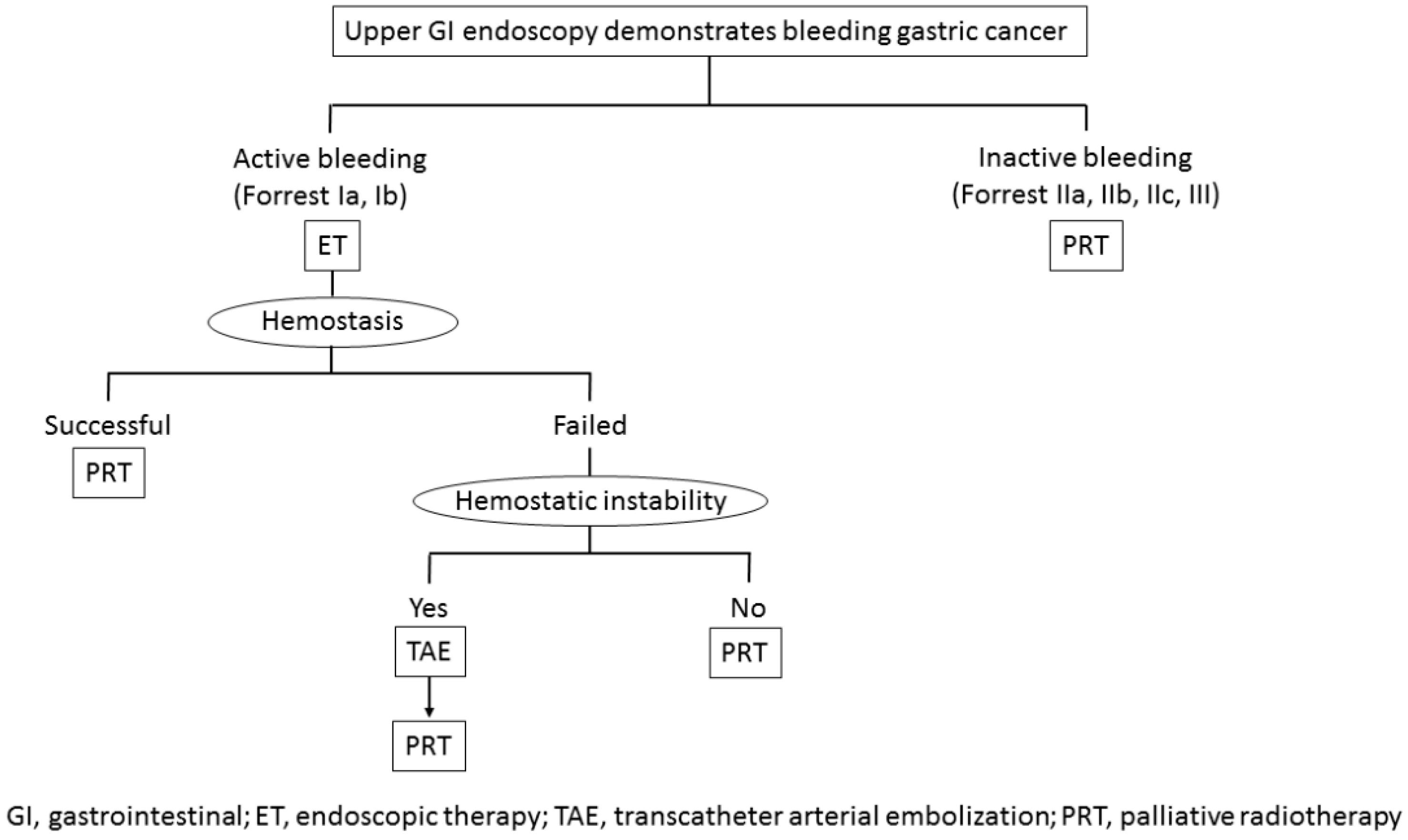
5. Treatment Strategy for Bleeding from Unresectable Gastric Cancer
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- New Global Cancer Data: GLOBOCAN. 2018. Available online: https://www.uicc.org/new-global-cancer-data-globocan-2018 (accessed on 27 April 2019).
- Sverdén, E.; Markar, S.R.; Agreus, L.; Lagergren, J. Acute upper gastrointestinal bleeding. BMJ 2018, 363, k4023. [Google Scholar] [CrossRef] [PubMed]
- Sheibani, S.; Kim, J.J.; Chen, B.; Park, S.; Saberi, B.; Keyashian, K.; Buxbaum, J.; Laine, L. Natural history of acute upper GI bleeding due to tumours: Short-term success and long-term recurrence with or without endoscopic therapy. Aliment Pharm. Ther. 2013, 38, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Ahn, J.Y.; Jung, H.Y.; Chun, J.H.; Nam, K.; Lee, J.H.; Jung, K.W.; Kim, D.H.; Choi, K.D.; Song, H.J.; et al. Can Endoscopic Bleeding Control Improve the Prognosis of Advanced Gastric Cancer Patients? A Retrospective Case-Control Study. J. Clin. Gastroenterol. 2017, 51, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.A.; Hao, J.Q.; Zhang, L.N.; Li, M.L.; Wu, X.S.; Weng, H.; Lv, W.J.; Zhang, W.J.; Chen, L.; et al. Long-term outcomes after radical gastrectomy in gastric cancer patients with overt bleeding. World J. Gastroenterol. 2015, 21, 13316–13324. [Google Scholar] [CrossRef] [PubMed]
- Gralnek, I.M.; Dumonceau, J.M.; Kuipers, E.J.; Lanas, A.; Sanders, D.S.; Kurien, M.; Rotondano, G.; Hucl, T.; Dinis-Ribeiro, M.; Marmo, R.; et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015, 47, a1–a46. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, M.; Iguchi, M.; Kakushima, N.; Kato, M.; Sakata, Y.; Hoteya, S.; Kataoka, M.; Shimaoka, S.; Yahagi, N.; Fujimoto, K. Guidelines for endoscopic management of non-variceal upper gastrointestinal bleeding. Dig. Endosc. 2016, 28, 363–378. [Google Scholar] [CrossRef]
- Hwang, J.H.; Fisher, D.A.; Ben-Menachem, T.; Chandrasekhara, V.; Chathadi, K.; Decker, G.A.; Early, D.S.; Evans, J.A.; Fanelli, R.D.; Foley, K.; et al. Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy. The role of endoscopy in the management of acute non-variceal upper GI bleeding. Gastrointest. Endosc. 2012, 75, 1132–1138. [Google Scholar] [CrossRef]
- Koh, K.H.; Kim, K.; Kwon, D.H.; Chung, B.S.; Sohn, J.Y.; Ahn, D.S.; Jeon, B.J.; Kim, S.H.; Kim, I.H.; Kim, S.W.; et al. The successful endoscopic hemostasis factors in bleeding from advanced gastric cancer. Gastric Cancer 2013, 16, 397–403. [Google Scholar] [CrossRef]
- Kim, Y.I.; Choi, I.J.; Cho, S.J.; Lee, J.Y.; Kim, C.G.; Kim, M.J.; Ryu, K.W.; Kim, Y.W.; Park, Y.I. Outcome of endoscopic therapy for cancer bleeding in patients with unresectable gastric cancer. J. Gastroenterol. Hepatol. 2013, 28, 1489–1495. [Google Scholar] [CrossRef]
- Song, I.J.; Kim, H.J.; Lee, J.A.; Park, J.C.; Shin, S.K.; Lee, S.K.; Lee, Y.C.; Chung, H. Clinical Outcomes of Endoscopic Hemostasis for Bleeding in Patients with Unresectable Advanced Gastric Cancer. J. Gastric Cancer 2017, 17, 374–383. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, J.C.; Kim, E.H.; Shin, S.K.; Lee, S.K.; Lee, Y.C. Hemostatic powder application for control of acute upper gastrointestinal bleeding in patients with gastric malignancy. Endosc. Int. Open 2018, 6, E700–E705. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Choi, I.J. Endoscopic management of tumor bleeding from inoperable gastric cancer. Clin. Endosc. 2015, 48, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.A.; Finlayson, N.D.; Shearman, D.J. Endoscopy in gastrointestinal bleeding. Lancet 1974, 2, 394–397. [Google Scholar] [CrossRef]
- Barkun, A.N.; Moosavi, S.; Martel, M. Topical hemostatic agents: A systematic review with particular emphasis on endoscopic application in GI bleeding. Gastrointest. Endosc. 2013, 77, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Al-Bakir, I.; Bhuva, M.; Patel, J.; Fullard, M.; Leahy, A. Early clinical experience of the safety and efficacy of EndoClot in the management of non-variceal upper gastrointestinal bleeding. Endosc. Int. Open 2015, 3, E605–E609. [Google Scholar] [CrossRef] [PubMed]
- Kirschniak, A.; Kratt, T.; Stüker, D.; Braun, A.; Schurr, M.O.; Königsrainer, A. A new endoscopic over-the-scope clip system for treatment of lesions and bleeding in the GI tract: First clinical experiences. Gastrointest. Endosc. 2007, 66, 162–167. [Google Scholar] [CrossRef]
- Goenka, M.K.; Rai, V.K.; Goenka, U.; Tiwary, I.K. Endoscopic Management of Gastrointestinal Leaks and Bleeding with the Over-the-Scope Clip: A Prospective Study. Clin. Endosc. 2017, 50, 58–63. [Google Scholar] [CrossRef]
- Chan, S.M.; Chiu, P.W.; Teoh, A.Y.; Lau, J.Y. Use of the Over-The-Scope Clip for treatment of refractory upper gastrointestinal bleeding: A case series. Endoscopy 2014, 46, 428–431. [Google Scholar] [CrossRef]
- Marmo, R.; Rotondano, G.; Piscopo, R.; Bianco, M.A.; D’Angella, R.; Cipolletta, L. Dual therapy versus monotherapy in the endoscopic treatment of high-risk bleeding ulcers: A meta-analysis of controlled trials. Am. J. Gastroenterol. 2007, 102, 279–289. [Google Scholar] [CrossRef]
- Mirsadraee, S.; Tirukonda, P.; Nicholson, A.; Everett, S.M.; McPherson, S.J. Embolization for non-variceal upper gastrointestinal tract haemorrhage: A systematic review. Clin. Radiol. 2011, 66, 500–509. [Google Scholar] [CrossRef]
- Lu, Y.; Loffroy, R.; Lau, J.Y.; Barkun, A. Multidisciplinary management strategies for acute non-variceal upper gastrointestinal bleeding. Br. J. Surg. 2014, 101, e34–e50. [Google Scholar] [CrossRef]
- Yap, F.Y.; Omene, B.O.; Patel, M.N.; Yohannan, T.; Minocha, J.; Knuttinen, M.G.; Owens, C.A.; Bui, J.T.; Gaba, R.C. Transcatheter embolotherapy for gastrointestinal bleeding: A single center review of safety, efficacy, and clinical outcomes. Dig. Dis. Sci. 2013, 58, 1976–1984. [Google Scholar] [CrossRef]
- Meehan, T.; Stecker, M.S.; Kalva, S.P.; Oklu, R.; Walker, T.G.; Ganguli, S. Outcomes of transcatheter arterial embolization for acute hemorrhage originating from gastric adenocarcinoma. J. Vasc. Interv. Radiol. 2014, 25, 847–851. [Google Scholar] [CrossRef]
- Park, S.; Shin, J.H.; Gwon, D.I.; Kim, H.J.; Sung, K.B.; Yoon, H.K.; Ko, G.Y.; Ko, H.K. Transcatheter Arterial Embolization for Gastrointestinal Bleeding Associated with Gastric Carcinoma: Prognostic Factors Predicting Successful Hemostasis and Survival. J. Vasc. Interv. Radiol. 2017, 28, 1012–1021. [Google Scholar] [CrossRef]
- Orron, D.E.; Bloom, A.I.; Neeman, Z. The Role of Transcatheter Arterial Embolization in the Management of Nonvariceal Upper Gastrointestinal Bleeding. Gastrointest. Endosc. Clin. 2018, 28, 331–349. [Google Scholar] [CrossRef]
- Chaw, C.L.; Niblock, P.G.; Chaw, C.S.; Adamson, D.J. The role of palliative radiotherapy for haemostasis in unresectable gastric cancer: A single-institution experience. Ecancermedicalscience 2014, 8, 384. [Google Scholar] [CrossRef]
- Tey, J.; Choo, B.A.; Leong, C.N.; Loy, E.Y.; Wong, L.C.; Lim, K.; Lu, J.J.; Koh, W.Y. Clinical outcome of palliative radiotherapy for locally advanced symptomatic GC in the modern era. Medicine 2014, 93, e118. [Google Scholar] [CrossRef]
- Kondoh, C.; Shitara, K.; Nomura, M.; Takahari, D.; Ura, T.; Tachibana, H.; Tomita, N.; Kodaira, T.; Muro, K. Efficacy of palliative radiotherapy for gastric bleeding in patients with unresectable advanced gastric cancer: A retrospective cohort study. BMC Palliat. Care 2015, 14, 37. [Google Scholar] [CrossRef]
- Kawabata, H.; Uno, K.; Yasuda, K.; Yamashita, M. Experience of Low-Dose, Short-Course Palliative Radiotherapy for Bleeding from Unresectable Gastric Cancer. J. Palliat. Med. 2017, 20, 177–180. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, J.W.; Jang, H.S. Palliative external beam radiotherapy for the treatment of tumor bleeding in inoperable advanced gastric cancer. BMC Cancer 2017, 17, 541. [Google Scholar] [CrossRef]
- Hiramoto, S.; Kikuchi, A.; Tetsuso, H.; Yoshioka, A.; Kohigashi, Y.; Maeda, I. Efficacy of palliative radiotherapy and chemo-radiotherapy for unresectable gastric cancer demonstrating bleeding and obstruction. Int. J. Clin. Oncol. 2018, 23, 1090–1094. [Google Scholar] [CrossRef]
- Tey, J.; Zheng, H.; Soon, Y.Y.; Leong, C.N.; Koh, W.Y.; Lim, K.; So, J.B.Y.; Shabbir, A.; Tham, I.W.K.; Lu, J. Palliative radiotherapy in symptomatic locally advanced gastric cancer: A phase II trial. Cancer Med. 2019, 8, 1447–1458. [Google Scholar] [CrossRef]
- Tey, J.; Soon, Y.Y.; Koh, W.Y.; Leong, C.N.; Choo, B.A.; Ho, F.; Vellayappan, B.; Lim, K.; Tham, I.W. Palliative radiotherapy for gastric cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 25797–25805. [Google Scholar] [CrossRef]
- Sapienza, L.G.; Ning, M.S.; Jhingran, A.; Lin, L.L.; Leão, C.R.; da Silva, B.B.; Pellizzon, A.C.A.; Gomes, M.J.L.; Baiocchi, G. Short-course palliative radiation therapy leads to excellent bleeding control: A single centre retrospective study. Clin. Transl. Radiat. Oncol. 2018, 14, 40–46. [Google Scholar] [CrossRef]
- Fairchild, A.; Harris, K.; Barnes, E.; Wong, R.; Lutz, S.; Bezjak, A.; Cheung, P.; Chow, E. Palliative thoracic radiotherapy for lung cancer: A systematic review. J. Clin. Oncol. 2008, 26, 4001–4011. [Google Scholar] [CrossRef]
- Duchesne, G.M.; Bolger, J.J.; Griffiths, G.O.; Trevor Roberts, J.; Graham, J.D.; Hoskin, P.J.; Fossâ, S.D.; Uscinska, B.M.; Parmar, M.K. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: Results of medical research council trial BA09. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 379–388. [Google Scholar] [CrossRef]
- Kawabata, H.; Hitomi, M.; Yamamoto, Y.; Fujii, T.; Inoue, N.; Kawakatsu, Y.; Okazaki, Y.; Miyata, M.; Motoi, S.; Shimizu, Y. Experience of palliative radiotherapy for tumor-ssociated bleeding from biliary tract cancer. J. Med. Cases 2018, 9, 328–331. [Google Scholar] [CrossRef]
- Kosugi, T.; Shikama, N.; Saito, T.; Nakamura, N.; Nakura, A.; Harada, H.; Wada, H.; Nozaki, M.; Uchida, N.; Nakamura, K. A Nationwide Survey in Japan of Palliative Radiotherapy for Bleeding in Gastrointestinal and Genitourinary Tumor Patients. World J. Oncol. 2016, 29–33. [Google Scholar] [CrossRef]
- Asakura, H.; Hashimoto, T.; Harada, H.; Mizumoto, M.; Furutani, K.; Hasuike, N.; Matsuoka, M.; Ono, H.; Boku, N.; Nishimura, T. Palliative radiotherapy for bleeding from advanced gastric cancer: Is a schedule of 30 Gy in 10 fractions adequate? J. Cancer Res. Clin. Oncol. 2011, 137, 125–130. [Google Scholar] [CrossRef]
- Kim, M.M.; Rana, V.; Janjan, N.A.; Das, P.; Phan, A.T.; Delclos, M.E.; Mansfield, P.F.; Ajani, J.A.; Crane, C.H.; Krishnan, S. Clinical benefit of palliative radiation therapy in advanced gastric cancer. Acta Oncol. 2008, 47, 421–427. [Google Scholar] [CrossRef]
- Kawabata, H.; Kakihara, N.; Nishitani, Y.; Asano, K.; Nose, M.; Takanashi, A.; Kanda, E.; Nishimura, M.; Tokunaga, E.; Matsurugi, A.; et al. A Soup Service for Advanced Digestive Cancer Patients with Severe Anorexia in Palliative Care. J. Palliat. Med. 2018, 21, 380–382. [Google Scholar] [CrossRef]
- Kawabata, H.; Kitamura, K.; Yamamoto, Y.; Okamoto, Y.; Shimaji, A.; Aoki, K.; Kato, Y.; Tokuyama, Y.; Shimizu, Y. Experience of End-of-Life Home Care in an Acute General Community Hospital in Japan. J. Med. Cases 2019, 10, 75–77. [Google Scholar] [CrossRef]
| Author/Year | Patients | Modalities | Successful Hemostasis, n (%) | Rebleeding, n (%) | Prognosis | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number | Stage, n (%) | Previous Treatment | Forrest Class I a/b, n (%) | Median OS (Months) | 30-Day Mortality Rate | ||||
| Koh KH 2013 [9] | 45 | NR | NR | 20/25 (100%) | Thermal therapy, clipping, injection, spray | 14 (31%) | NR | NR | 18.8% |
| Kim YI 2013 [10] | 113 | 110 (97.3%) | CT; 22 | 17/59 (67%) | Thermal therapy, clipping, injection, spray | 105 (92.9%) | 43 (41%) | 3.2 | 15.9% |
| Song IJ 2017 [11] | 106 | 105 (99.1%) | CT; 53, RT; 1, CRT; 10 | 6/59 (61%) | Thermal therapy, clipping, injection | 88 (83.0%) | 30 (28.3%) | 3.9 in non-rebleeding patients 2.7 in rebleeding patients | 22.6% |
| Park H 2017 [4] | 64 | 41 (64.1%) | CT; 52, RT; 3 | NR | Thermal therapy, clipping, injection, spray | 47 (73.4%) | 17 (36.2%) | 6.5 | NR |
| Kim YJ 2018 [12] | 12* | NR | CT; 6 | 0/12 (100%) | Hemostatic powder application 5 required combined therapy (thermal therapy, clipping, injection) | 12 (100%) | 2 (16%) | NR | 0% |
| Author/Year | Patients | Successful Hemostasis, n (%) | Rebleeding, n (%) | Prognosis | Complications | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Stage, n (%) | Endoscopic Evaluation | Forrest Class I a/b, n (%) | ET n (%) | Technical | Clinical | Median OS (Months) | 30-Day Mortality Rate | |||
| Koh KH 2013 [9] | 31 | NR | 31 | 10/21 (100%) | 31 (100%) | 31 (100%) | 31/31 (100%) | 5 (16%) | NR | 25.8% | NR |
| Meehan T 2014 [24] | 10 | NR | 10 | NR | 10 (100%) | 10/10 (100%) | 4/10 (40%) | 43 (41%) | 0.6 | 60.0% | 0 |
| Park S 2017 [25] | 43 | 28 (68.3%) | 23 | 4/16 (86%) | 9 (20.9%) | 34/40 (85%) | 26/40 (65%) | 7 (26%) | 2.8 | 25.0% | Splenic infarction; 2 |
| Author/Year | Index Symptom | Patients | Radiotherapy | Chemotherapy | Successful Hemostasis, % (n) | Rebleeding % (n) | Survival (Months) | Duration (Months) | QOL Assessment | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Stage | PS ≥ 3, n (%) | Dose/Fraction | BED (Gy10) | Previous | Concurrent | Additional | |||||||
| Chaw CL 2014 [27] | Bleeding | 52 | Mixed | NR | 8 Gy/1 fr or 20 Gy/5 fr | 8.8 or 28 | 14 | 0 | 3 | 50% (22/44) | NR | 5.3 | NR | NR |
| Tey J 2014 [28] | Bleeding, pain, obstruction | 115 | Mixed | 11 (9.5%) | 8–40 Gy/1–16 fr | 14.4–50 | 9 | 0 | 10 | 80.6% (83/103) | 30% (25/83) BED ≤ 39 36% (17/47) BED > 39 22% (8/36) | 2.8 | 3.3 | NR |
| Kondoh C 2015 [29] | Bleeding | 15 | Metastatic | 10 (66%) | Median 30 Gy (30–40 Gy/10–20 fr) | Median 39 (23–48) | 9 | 5 | 5 | 73% (11/15- 7 in RT, 4 in CRT) | 36% (4/11- 2 in RT, 2 in CRT) | 2.1 | 0.9 | NR |
| Kawabata H 2017 [30] | Bleeding | 18 | Mixed | 4 (22%) | 6 Gy/3 fr | 7.2 | 13 | 2 | 8 | 55% (10/18) | 60% (6/10) | NR | NR | PS, Dietary intake |
| Lee YH 2017 [31] | Bleeding | 42 | Mixed | 8 (19%) | Median 39.6 Gy/20 fr (14–50.4 Gy/7–28 fr) | Median 47 (16.8–59.4) | 31 | 7 | NR | 69% (29/42) | 37% (11/29) | 3.1 | 3.7 | NR |
| Hiramoto S 2018 [32] | Bleeding, obstruction | 23 | Mixed | 1 (4.3%) | Median 42 Gy/20 fr (30–60 Gy/10–30 fr) | Median 50.8 (39–72) | 10 | 15 | 8 | 88.8% (16/18) | 25% (4/16) | 3.9 | 3.4 | NR |
| Tey J 2019 [33] | Bleeding, pain, obstruction | 50 | Mixed | 5 (10%) | 36 Gy/12 fr | 48.6 | 5 | 0 | 7 | 80% (40/50) | 5% (2/40) | 2.7 | 3.4 | EORTCQLQ-C30 |
| Author/Year | Toxicity, n (%) | Acute Toxicity (CTC) | Late Toxicity | ||
|---|---|---|---|---|---|
| Gastrointestinal (Grade ≥ 3) | Skin/Connective Tissue (Grade ≥ 3) | Others (Grade ≥ 3) | |||
| Chaw CL 2014 [27] | NR | NR | NR | NR | NR |
| Tey J 2014 [28] | 3 (2%) | Vomitting; 1 Gastritis; 1 | 0 | Anorexia; 1 | 0 |
| Kondoh C 2015 [29] | 3 (20%) | 0 | 0 | Neutropenia; 3 in CRT | 0 |
| Kawabata H 2017 [30] | 2 (11%) | GI obstruction; 1 | 0 | Leukocytopenia; 1 in CRT | 0 |
| Lee YH 2017 [31] | 0 | 0 | 0 | 0 | 0 |
| Hiramoto S 2018 [33] | 0 | 0 | 0 | 0 | 0 |
| Tey J 2019 [33] | 2 (4%) | Gastritis; 1 | 0 | Anorexia; 1 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawabata, H.; Hitomi, M.; Motoi, S. Management of Bleeding from Unresectable Gastric Cancer. Biomedicines 2019, 7, 54. https://doi.org/10.3390/biomedicines7030054
Kawabata H, Hitomi M, Motoi S. Management of Bleeding from Unresectable Gastric Cancer. Biomedicines. 2019; 7(3):54. https://doi.org/10.3390/biomedicines7030054
Chicago/Turabian StyleKawabata, Hideaki, Misuzu Hitomi, and Shigehiro Motoi. 2019. "Management of Bleeding from Unresectable Gastric Cancer" Biomedicines 7, no. 3: 54. https://doi.org/10.3390/biomedicines7030054
APA StyleKawabata, H., Hitomi, M., & Motoi, S. (2019). Management of Bleeding from Unresectable Gastric Cancer. Biomedicines, 7(3), 54. https://doi.org/10.3390/biomedicines7030054





