Therapeutic Targeting Strategies of Cancer Stem Cells in Gastrointestinal Malignancies
Abstract
1. Introduction
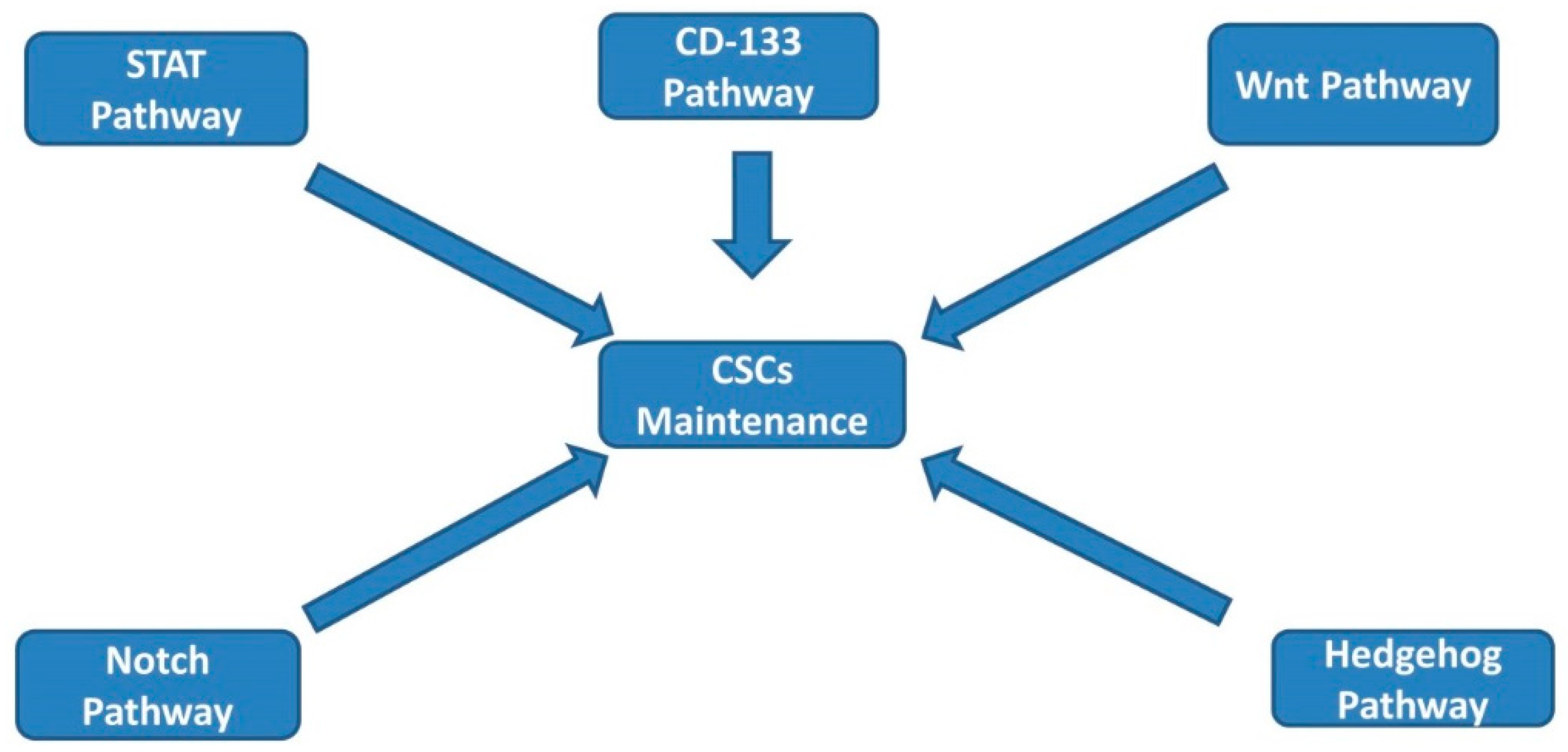
2. CSC Signaling Pathways
3. Napabucasin
3.1. Pancreatic Cancer
3.2. Gastric and GEJ Cancers
3.3. Colorectal Cancers
3.4. Hepatocellular Carcinoma
4. Amcasertib
5. CART-133
6. Other Agents in Development
7. Conclusions
Funding
Conflicts of Interest
References
- Putzer, B.M.; Solanki, M.; Herchenroder, O. Advances in cancer stem cell targeting: How to strike the evil at its root. Adv. Drug Deliv. Rev. 2017, 120, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Bekaii-Saab, T.; El-Rayes, B. Identifying and targeting cancer stem cells in the treatment of gastric cancer. Cancer 2017, 123, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wulfkuhle, J.; Zhang, H.; Gu, P.; Yang, Y.; Deng, J.; Margolick, J.B.; Liotta, L.A.; Petricoin, E., 3rd; Zhang, Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc. Natl. Acad. Sci. USA 2007, 104, 16158–16163. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.X.; Yang, S.W.; Li, P.A.; Luo, X.; Li, Z.Y.; Hao, Y.X.; Yu, P.W. The promotion of the transformation of quiescent gastric cancer stem cells by IL-17 and the underlying mechanisms. Oncogene 2017, 36, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wu, J.; Shi, J.; Huo, Y.M.; Dai, W.; Geng, J.; Lu, P.; Yang, M.W.; Fang, Y.; Wang, W.; et al. IL22RA1/STAT3 Signaling Promotes Stemness and Tumorigenicity in Pancreatic Cancer. Cancer Res. 2018, 78, 3293–3305. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.L.; Kissil, J.L. Notch signaling in pancreatic cancer: Oncogene or tumor suppressor? Trends Mol. Med. 2013, 19, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Borah, A.; Raveendran, S.; Rochani, A.; Maekawa, T.; Kumar, D.S. Targeting self-renewal pathways in cancer stem cells: Clinical implications for cancer therapy. Oncogenesis 2015, 4, e177. [Google Scholar] [CrossRef] [PubMed]
- Apelqvist, A.; Li, H.; Sommer, L.; Beatus, P.; Anderson, D.J.; Honjo, T.; Hrabe de Angelis, M.; Lendahl, U.; Edlund, H. Notch signalling controls pancreatic cell differentiation. Nature 1999, 400, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Li, F.; Luo, B.; Wang, X.H.; Sun, H.C.; Liu, S.; Cui, Y.Q.; Xu, X.X. A side population of cells from a human pancreatic carcinoma cell line harbors cancer stem cell characteristics. Neoplasma 2009, 56, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Kong, D.; Banerjee, S.; Ahmad, A.; Azmi, A.S.; Ali, S.; Abbruzzese, J.L.; Gallick, G.E.; Sarkar, F.H. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009, 69, 2400–2407. [Google Scholar] [CrossRef] [PubMed]
- Kusoglu, A.; Biray Avci, C. Cancer stem cells: A brief review of the current status. Gene 2019, 681, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, H.Y.; Park, K.K.; Choi, Y.K.; Nam, J.S.; Hong, I.S. CWP232228 targets liver cancer stem cells through Wnt/beta-catenin signaling: A novel therapeutic approach for liver cancer treatment. Oncotarget 2016, 7, 20395–20409. [Google Scholar] [CrossRef] [PubMed]
- Klaus, A.; Birchmeier, W. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer 2008, 8, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Gujral, T.S.; Chan, M.; Peshkin, L.; Sorger, P.K.; Kirschner, M.W.; MacBeath, G. A noncanonical Frizzled2 pathway regulates epithelial-mesenchymal transition and metastasis. Cell 2014, 159, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.V.; Vanner, R.; Dirks, P.; Eaves, C.J. Cancer stem cells: An evolving concept. Nat. Rev. Cancer 2012, 12, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Katoh, Y.; Katoh, M. Hedgehog signaling pathway and gastrointestinal stem cell signaling network (review). Int. J. Mol. Med. 2006, 18, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Sekulic, A.; Migden, M.R.; Oro, A.E.; Dirix, L.; Lewis, K.D.; Hainsworth, J.D.; Solomon, J.A.; Yoo, S.; Arron, S.T.; Friedlander, P.A.; et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012, 366, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rogoff, H.A.; Keates, S.; Gao, Y.; Murikipudi, S.; Mikule, K.; Leggett, D.; Li, W.; Pardee, A.B.; Li, C.J. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc. Natl. Acad. Sci. USA 2015, 112, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Sonbol, M.B.; Bekaii-Saab, T. A clinical trial protocol paper discussing the BRIGHTER study. Future Oncol. 2018, 14, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Bekaii-Saab, T.S.; Starodub, A.; El-Rayes, B.F.; Shahda, S.; O’Neil, B.H.; Noonan, A.M.; Shaib, W.L.; Hanna, W.T.; Mikhail, S.; Neki, A.S.; et al. Phase 1b/2 trial of cancer stemness inhibitor napabucasin (NAPA) + nab-paclitaxel (nPTX) and gemcitabine (Gem) in metastatic pancreatic adenocarcinoma (mPDAC). J. Clin. Oncol. 2018, 36, 4110. [Google Scholar] [CrossRef]
- Becerra, C.; Stephenson, J.; Jonker, D.J.; Cohn, A.L.; Asmis, T.R.; Bekaii-Saab, T.S.; Conkling, P.R.; Garbo, L.E.; Lenz, H.-J.; Richards, D.A.; et al. Phase Ib/II study of cancer stem cell (CSC) inhibitor BBI608 combined with paclitaxel in advanced gastric and gastroesophageal junction (GEJ) adenocarcinoma. J. Clin. Oncol. 2015, 33, 4069. [Google Scholar] [CrossRef]
- Shah, M.A.; Shitara, K.; Lordick, F.; Bang, Y.-J.; Tebbutt, N.C.; Metges, J.-P.; Muro, K.; Shen, L.; Tjulandin, S.; Hays, J.L.; et al. The BRIGHTER trial: A phase 3 randomized double-blind study of napabucasin (NAPA) plus paclitaxel (PTX) versus placebo (PBO) plus PTX in patients (pts) with pretreated advanced gastric and gastroesophageal junction (GEJ) adenocarcinoma. J. Clin. Oncol. 2018, 36, 4010. [Google Scholar] [CrossRef]
- Bendell, J.C.; Hubbard, J.M.; O’Neil, B.H.; Jonker, D.J.; Starodub, A.; Peyton, J.D.; Pitot, H.C.; Halfdanarson, T.R.; Nadeau, B.R.; Zubkus, J.D.; et al. Phase 1b/II study of cancer stemness inhibitor napabucasin (BBI-608) in combination with FOLFIRI +/− bevacizumab (bev) in metastatic colorectal cancer (mCRC) patients (pts). J. Clin. Oncol. 2017, 35, 3529. [Google Scholar] [CrossRef]
- Grothey, A.; Shah, M.A.; Yoshino, T.; Cutsem, E.V.; Taieb, J.; Xu, R.; Tebbutt, N.C.; Falcone, A.; Cervantes, A.; Borodyansky, L.; et al. CanStem303C trial: A phase III study of napabucasin (BBI-608) in combination with 5-fluorouracil (5-FU), leucovorin, irinotecan (FOLFIRI) in adult patients with previously treated metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2017, 35, TPS3619. [Google Scholar] [CrossRef]
- Jonker, D.J.; Nott, L.; Yoshino, T.; Gill, S.; Shapiro, J.; Ohtsu, A.; Zalcberg, J.; Vickers, M.M.; Wei, A.C.; Gao, Y.; et al. Napabucasin versus placebo in refractory advanced colorectal cancer: A randomised phase 3 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 263–270. [Google Scholar] [CrossRef]
- El-Rayes, B.F.; Richards, D.A.; Cohn, A.L.; Richey, S.L.; Feinstein, T.; Kundranda, M.N.; El-Khoueiry, A.B.; Melear, J.M.; Braiteh, F.S.; Hitron, M.; et al. BBI608-503-103HCC: A phase Ib/II clinical study of napabucasin (BBI608) in combination with sorafenib or amcasertib (BBI503) in combination with sorafenib (Sor) in adult patients with hepatocellular carcinoma (HCC). J. Clin. Oncol. 2017, 35, 4077. [Google Scholar] [CrossRef]
- Laurie, S.A.; Jonker, D.J.; Edenfield, W.J.; Stephenson, J.; Keller, D.; Hitron, M.; Li, W.; Li, Y.; Gada, K.; Gao, Y.; et al. A phase 1 dose-escalation study of BBI503, a first-in-class cancer stemness kinase inhibitor in adult patients with advanced solid tumors. J. Clin. Oncol. 2014, 32, 2527. [Google Scholar] [CrossRef]
- Cote, G.M.; Chau, N.G.; Spira, A.I.; Edenfield, W.J.; Laurie, S.A.; Richards, D.A.; Richey, S.L.; Gao, Y.; Li, Y.; Li, W.; et al. A phase 1b/2 study of amcasertib, a first-in-class cancer stemness kinase inhibitor in advanced head and neck cancer. J. Clin. Oncol. 2017, 35, 6032. [Google Scholar] [CrossRef]
- Ferrandina, G.; Petrillo, M.; Bonanno, G.; Scambia, G. Targeting CD133 antigen in cancer. Expert. Opin. Ther. Targets 2009, 13, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Schmohl, J.U.; Vallera, D.A. CD133, Selectively Targeting the Root of Cancer. Toxins 2016, 8, 165. [Google Scholar] [CrossRef] [PubMed]
- Kohga, K.; Tatsumi, T.; Takehara, T.; Tsunematsu, H.; Shimizu, S.; Yamamoto, M.; Sasakawa, A.; Miyagi, T.; Hayashi, N. Expression of CD133 confers malignant potential by regulating metalloproteinases in human hepatocellular carcinoma. J. Hepatol. 2010, 52, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.R.; Xu, Y.; Yu, B.; Zhou, J.; Qiu, S.J.; Shi, G.M.; Zhang, B.H.; Wu, W.Z.; Shi, Y.H.; Wu, B.; et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut 2010, 59, 953–962. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. YESCARTA (Axicabtagene Ciloleucel). Available online: https://www.fda.gov/biologicsbloodvaccines/cellulargenetherapyproducts/approvedproducts/ucm581222.htm (accessed on 14 January 2019).
- Wang, Y.; Chen, M.; Wu, Z.; Tong, C.; Dai, H.; Guo, Y.; Liu, Y.; Huang, J.; Lv, H.; Luo, C.; et al. CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunology 2018, 7, e1440169. [Google Scholar] [CrossRef] [PubMed]
- Cubillo Gracian, A.; Dean, A.; Muñoz, A.; Hidalgo, M.; Pazo-Cid, R.; Martin, M.; Macarulla Mercade, T.; Lipton, L.; Harris, M.; Manzano-Mozo, J.L.; et al. 620PDYOSEMITE: A 3 arm double-blind randomized phase 2 study of gemcitabine, paclitaxel protein-bound particles for injectable suspension, and placebo (GAP) versus gemcitabine, paclitaxel protein-bound particles for injectable suspension and either 1 or 2 truncated courses of demcizumab (GAD). Ann. Oncol. 2017, 28, mdx369.004. [Google Scholar] [CrossRef]
- Kim, E.J.; Sahai, V.; Abel, E.V.; Griffith, K.A.; Greenson, J.K.; Takebe, N.; Khan, G.N.; Blau, J.L.; Craig, R.; Balis, U.G.; et al. Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin. Cancer Res. 2014, 20, 5937–5945. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, D.V.; Junttila, M.R.; Karrison, T.; Bahary, N.; Horiba, M.N.; Nattam, S.R.; Marsh, R.; Wallace, J.; Kozloff, M.; Rajdev, L.; et al. Randomized Phase Ib/II Study of Gemcitabine Plus Placebo or Vismodegib, a Hedgehog Pathway Inhibitor, in Patients With Metastatic Pancreatic Cancer. J. Clin. Oncol. 2015, 33, 4284–4292. [Google Scholar] [CrossRef] [PubMed]
| Study ID | Phase | Treatment Administered | Number of patients | Outcome |
|---|---|---|---|---|
| GEJ/Gastric | ||||
| Shitara 2015 [1] | Phase 1 | Napabucasin + paclitaxel | 6 | ORR 33.3% (2 PR) |
| Hitron 2014 [2] | Phase 1b dose escalation | Napabucasin + paclitaxel | 24 patients with advanced cancers (5 with GEJ or gastric cancers) | ORR 40% (2/5 PR) |
| Becerra 2015 [3] | Phase Ib/II extension study | Napabucasin + paclitaxel | 46 patients | ORR 15%; 11% (2/19) in patients with prior taxane; 31% (5/16) with no prior taxane exposure |
| Shah 2018 [4] (the BRIGHTER trial) | Phase III | Napabucasin + paclitaxel versus placebo | 714 patients | Median OS 6.93 months vs. 7.36 in napabucasin vs. placebo (statistically not significant) |
| Pancreatic | ||||
| Bekaii-Saab 2018 [5] | Phase IB/II study | Napabucasin + nab-paclitaxel and gemcitabine | 59 (50 evaluable patients) | 2 CR (4%) and 26 PR (52%) |
| CanStem111P study (NCT02993731) | Phase III | Napabucasin + nab-paclitaxel and gemcitabine | Pending | primary endpoint of OS (results pending) |
| CRC | ||||
| Bendell 2017 [6] | Phase I/II | Napabucasin + FOLFIRI +/− bevacizumab | 82 (66 evaluable patients) | 1 CR (1.5%), 13 PR (20%), and 27 SD (41%) |
| CanStem303C (NCT02753127) [7] | Phase III | Napabucasin in combination with FOLFIRI | Pending | primary endpoint of OS (results pending) |
| HCC | ||||
| El-Rayes 2017 [8] | Phase I/II | Napabucasin + sorafenib | 6 | DCR 67%; PFS 24.9 weeks; OS 32.6 weeks |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonbol, M.B.; Ahn, D.H.; Bekaii-Saab, T. Therapeutic Targeting Strategies of Cancer Stem Cells in Gastrointestinal Malignancies. Biomedicines 2019, 7, 17. https://doi.org/10.3390/biomedicines7010017
Sonbol MB, Ahn DH, Bekaii-Saab T. Therapeutic Targeting Strategies of Cancer Stem Cells in Gastrointestinal Malignancies. Biomedicines. 2019; 7(1):17. https://doi.org/10.3390/biomedicines7010017
Chicago/Turabian StyleSonbol, Mohamad B., Daniel H. Ahn, and Tanios Bekaii-Saab. 2019. "Therapeutic Targeting Strategies of Cancer Stem Cells in Gastrointestinal Malignancies" Biomedicines 7, no. 1: 17. https://doi.org/10.3390/biomedicines7010017
APA StyleSonbol, M. B., Ahn, D. H., & Bekaii-Saab, T. (2019). Therapeutic Targeting Strategies of Cancer Stem Cells in Gastrointestinal Malignancies. Biomedicines, 7(1), 17. https://doi.org/10.3390/biomedicines7010017





