GABA Levels in Left and Right Sensorimotor Cortex Correlate across Individuals
Abstract
1. Introduction
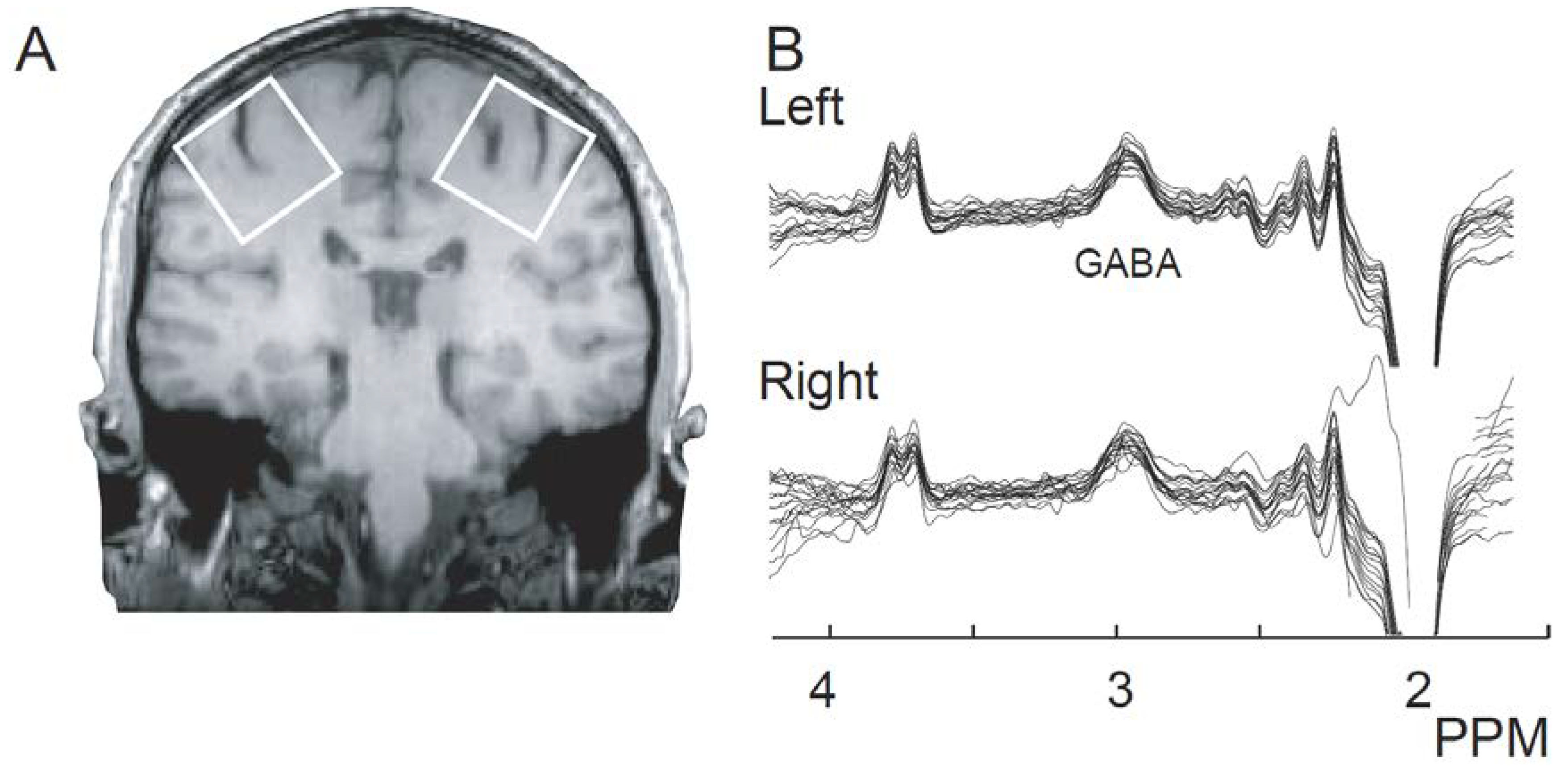
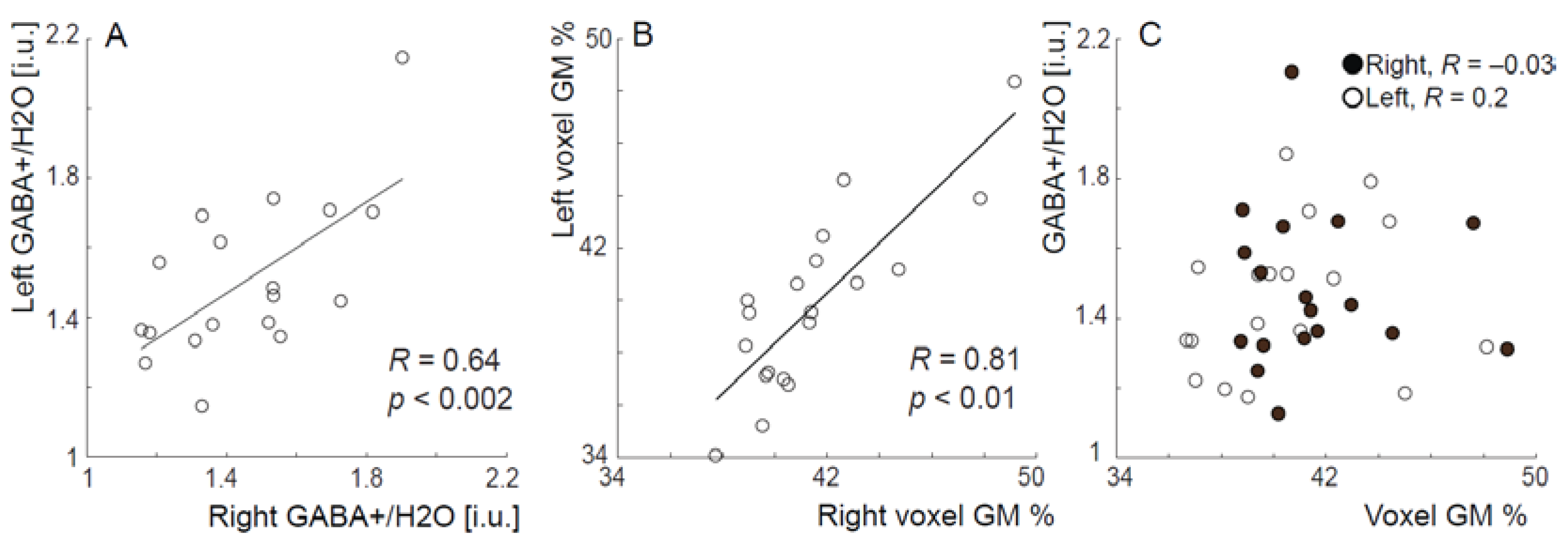
2. Results
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. MEGA-PRESS
4.3. Acquisition
4.4. Data Processing
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muthukumaraswamy, S.D.; Edden, R.A.E.; Jones, D.K.; Swettenham, J.B.; Singh, K.D. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8356–8361. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaraswamy, S.D.; Evans, C.J.; Edden, R.A.; Wise, R.G.; Singh, K.D. Individual variability in the shape and amplitude of the BOLD-HRF correlates with endogenous GABAergic inhibition. Hum. Brain Mapp. 2012, 33, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Northoff, G.; Walter, M.; Schulte, R.F.; Beck, J.; Dydak, U.; Henning, A.; Boeker, H.; Grimm, S.; Boesiger, P. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat. Neurosci. 2007, 10, 1515–1517. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Bachtiar, V.; Johansen-Berg, H. The role of GABA in human motor learning. Curr. Biol. 2011, 21, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Donahue, M.J.; Near, J.; Blicher, J.U.; Jezzard, P. Baseline GABA concentration and fMRI response. NeuroImage 2010, 53, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Gaetz, W.; Bloy, L.; Wang, D.J.; Port, R.G.; Blaskey, L.; Levy, S.E.; Roberts, T.P. GABA estimation in the brains of children on the autism spectrum: Measurement precision and regional cortical variation. NeuroImage 2013, 86, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Boy, F.; Evans, C.J.; Edden, R.A.; Singh, K.D.; Husain, M.; Sumner, P. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr. Biol. 2011, 20, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Edden, R.A.; Muthukumaraswamy, S.D.; Freeman, T.C.; Singh, K.D. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J. Neurosci. 2009, 29, 15721–15726. [Google Scholar] [CrossRef] [PubMed]
- Puts, N.A.J.; Edden, R.A.E.; John Evans, C.; McGlone, F.; McGonigle, D.J. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J. Neurosci. 2011, 31. [Google Scholar] [CrossRef] [PubMed]
- Sumner, P.; Edden, R.A.; Bompas, A.; Evans, C.J.; Singh, K.D. More GABA, less distraction: A neurochemical predictor of motor decision speed. Nat. Neurosci. 2010, 13, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Boy, F.; Evans, C.J.; Edden, R.A.; Lawrence, A.D.; Singh, K.D.; Husain, M.; Sumner, P. Dorsolateral Prefrontal gamma-Aminobutyric Acid in Men Predicts Individual Differences in Rash Impulsivity. Biol. Psychiatry 2011, 70, 866–872. [Google Scholar] [CrossRef]
- Goto, N.; Yoshimura, R.; Moriya, J.; Kakeda, S.; Hayashi, K.; Ueda, N.; Ikenouchi-Sugita, A.; Umene-Nakano, W.; Oonari, N.; Korogi, Y.; et al. Critical examination of a correlation between brain gamma-aminobutyric acid (GABA) concentrations and a personality trait of extroversion in healthy volunteers as measured by a 3 Tesla proton magnetic resonance spectroscopy study. Psychiatry Res. Neuroimaging 2010, 182, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Porges, E.C.; Woods, A.J.; Edden, R.A.E.; Puts, N.A.J.; Harris, A.D.; Chen, H.; Garcia, A.M.; Seider, T.R.; Lamb, D.G.; Williamson, J.B.; et al. Frontal Gamma-Aminobutyric Acid Concentrations Are Associated With Cognitive Performance in Older Adults. Biol. Psychiatry Cognit. Neurosci. Neuroimaging 2017, 2, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Floyer-Lea, A.; Wylezinska, M.; Kincses, T.; Matthews, P.M. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J. Neurophysiol. 2006, 95, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Michels, L.; Martin, E.; Klaver, P.; Edden, R.; Zelaya, F.; Lythgoe, D.J.; Lüchinger, R.; Brandeis, D.; O’Gorman, R.L. Frontal GABA Levels Change during Working Memory. PLoS ONE 2012, 7, e31933. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.D.; Puts, N.A.J.J.; Anderson, B.A.; Yantis, S.; Pekar, J.J.; Barker, P.B.; Edden, R.A.E.E. Multi-regional investigation of the relationship between functional MRI blood oxygenation level dependent (BOLD) activation and GABA concentration. PLoS ONE 2015, 10, e0117531. [Google Scholar] [CrossRef] [PubMed]
- Hlushchuk, Y.; Hari, R. Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. J. Neurosci. 2006, 26, 5819–5824. [Google Scholar] [CrossRef] [PubMed]
- Brodie, S.M.; Villamayor, A.; Borich, M.R.; Boyd, L.A. Exploring the specific time course of interhemispheric inhibition between the human primary sensory cortices. J. Neurophysiol. 2014, 112, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Conde, V.; Vollmann, H.; Taubert, M.; Sehm, B.; Cohen, L.G.; Villringer, A.; Ragert, P. Reversed timing-dependent associative plasticity in the human brain through interhemispheric interactions. J. Neurophysiol. 2013, 109, 2260–2271. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, Y.; Taoka, M.; Iriki, A.; Iriki, A. Bilateral activity and callosal connections in the somatosensory cortex. Neuroscientist 2001, 7, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Tommerdahl, M.; Favorov, O.V.; Whitsel, B.L. Dynamic representations of the somatosensory cortex. Neurosci. Biobehav. Rev. 2010, 34, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Poole, B.J.; Mather, M.; Livesey, E.J.; Harris, I.M.; Harris, J.A. Motor-evoked potentials reveal functional differences between dominant and non-dominant motor cortices during response preparation. Cortex 2018, 103, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, G.; Horniss, V.; Hoppe, J.; Heise, K.; Zimerman, M.; Gerloff, C.; Hummel, F.C. Distinct Temporospatial Interhemispheric Interactions in the Human Primary and Premotor Cortex during Movement Preparation. Cereb. Cortex 2010, 20, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Serrien, D.J.; Sovijärvi-Spapé, M.M. Cognitive control of response inhibition and switching: Hemispheric lateralization and hand preference. Brain Cognit. 2013, 82, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.D.; Puts, N.A.; Edden, R.A. Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. J. Magn. Reson. Imaging 2015, 42, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Edden, R.A.E.; Puts, N.A.J.; Harris, A.D.; Barker, P.B.; Evans, C.J. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J. Magn. Reson. Imaging 2014, 40, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.; Barker, P.B.; Bhattacharyya, P.K.; Brix, M.K.; Buur, P.F.; Cecil, K.M.; Chan, K.L.; Chen, D.Y.; Craven, A.R.; Cuypers, K.; et al. Big GABA: Edited MR spectroscopy at 24 research sites. NeuroImage 2017, 159, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Toga, A.W.; Thompson, P.M. Mapping brain asymmetry. Nat. Rev. Neurosci. 2003, 4, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Mechelli, A. Structural Covariance in the Human Cortex. J. Neurosci. 2005, 25, 8303–8310. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Imaging 1995, 34, 537–541. [Google Scholar] [CrossRef]
- Smith, S.M.; Miller, K.L.; Moeller, S.; Xu, J.; Auerbach, E.J.; Woolrich, M.W.; Beckmann, C.F.; Jenkinson, M.; Andersson, J.; Glasser, M.F.; et al. Temporally-independent functional modes of spontaneous brain activity. Proc. Natl. Acad. Sci. USA 2012, 109, 3131–3136. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, D.; Volkow, N.D. Association between Brain Activation and Functional Connectivity. Cereb. Cortex 2018. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, D.; Volkow, N.D. Functional connectivity hubs in the human brain. NeuroImage 2011, 57, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Zilles, K.; Amunts, K. Receptor mapping: Architecture of the human cerebral cortex. Curr. Opin. Neurol. 2009, 22, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.L.; Qi, H.-X.; Kaas, J.H. Spatiotemporal properties of neuron response suppression in owl monkey primary somatosensory cortex when stimuli are presented to both hands. J. Neurosci. 2011, 31, 3589–3601. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.S.; Serrien, D.J. Handedness and the excitability of cortical inhibitory circuits. Behav. Brain Res. 2012, 230, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Puts, N.A.J.; Edden, R.A.E. In vivo magnetic resonance spectroscopy of GABA: A methodological review. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 60, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Oeltzschner, G.; Puts, N.A.J.; Chan, K.L.; Boer, V.O.; Barker, P.B.; Edden, R.A.E. Dual-volume excitation and parallel reconstruction for J-difference-edited MR spectroscopy. Magn. Reson. Med. 2017, 77, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Gaddis, A.; Rosch, K.S.; Dirlikov, B.; Crocetti, D.; MacNeil, L.; Barber, A.D.; Muschelli, J.; Caffo, B.; Pekar, J.J.; Mostofsky, S.H. Motor overflow in children with attention-deficit/hyperactivity disorder is associated with decreased extent of neural activation in the motor cortex. Psychiatry Res. 2015, 233, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Mahone, E.M.; Puts, N.A.; Edden, R.A.E.; Ryan, M.; Singer, H.S. GABA and glutamate in children with Tourette syndrome: A 1H MR spectroscopy study at 7 T. Psychiatry Res. Neuroimaging 2018, 273, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Raczkowski, D.; Kalat, J.W.; Nebes, R. Reliability and validity of some handedness questionnaire items. Neuropsychologia 1974, 12, 43–47. [Google Scholar] [CrossRef]
- Mescher, M.; Merkle, H.; Kirsch, J.; Garwood, M.; Gruetter, R. Simultaneousin vivo spectral editing and water suppression. NMR Biomed. 1998, 11, 266–272. [Google Scholar] [CrossRef]
- Yousry, T.A.; Schmid, U.D.; Alkadhi, H.; Schmidt, D.; Peraud, A.; Buettner, A.; Winkler, P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 1997, 120 Pt 1, 141–157. [Google Scholar] [CrossRef]
- Near, J.; Edden, R.; Evans, C.J.; Paquin, R.; Harris, A.; Jezzard, P. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn. Reson. Med. 2015, 73, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Mullins, P.G.; McGonigle, D.J.; O’Gorman, R.L.; Puts, N.A.; Vidyasagar, R.; Evans, C.J.; Cardiff Symposium on MRS of GABA; Edden, R.A. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. NeuroImage 2014, 86, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Brady, M.; Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 2001, 20, 45–57. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puts, N.A.J.; Heba, S.; Harris, A.D.; Evans, C.J.; McGonigle, D.J.; Tegenthoff, M.; Schmidt-Wilcke, T.; Edden, R.A.E. GABA Levels in Left and Right Sensorimotor Cortex Correlate across Individuals. Biomedicines 2018, 6, 80. https://doi.org/10.3390/biomedicines6030080
Puts NAJ, Heba S, Harris AD, Evans CJ, McGonigle DJ, Tegenthoff M, Schmidt-Wilcke T, Edden RAE. GABA Levels in Left and Right Sensorimotor Cortex Correlate across Individuals. Biomedicines. 2018; 6(3):80. https://doi.org/10.3390/biomedicines6030080
Chicago/Turabian StylePuts, Nicolaas A. J., Stefanie Heba, Ashley D. Harris, Christopher John Evans, David J. McGonigle, Martin Tegenthoff, Tobias Schmidt-Wilcke, and Richard A. E. Edden. 2018. "GABA Levels in Left and Right Sensorimotor Cortex Correlate across Individuals" Biomedicines 6, no. 3: 80. https://doi.org/10.3390/biomedicines6030080
APA StylePuts, N. A. J., Heba, S., Harris, A. D., Evans, C. J., McGonigle, D. J., Tegenthoff, M., Schmidt-Wilcke, T., & Edden, R. A. E. (2018). GABA Levels in Left and Right Sensorimotor Cortex Correlate across Individuals. Biomedicines, 6(3), 80. https://doi.org/10.3390/biomedicines6030080





