Histology of Non-Melanoma Skin Cancers: An Update
Abstract
:1. Introduction
2. Discussion
2.1. Basal Cell Carcinoma and Its Histological Variants
2.1.1. General Considerations
2.1.2. Main Histologic Variants
2.2. The Histologic Features of Actinic Keratosis, Bowen’s Disease, and Squamous Cell Carcinoma
2.2.1. General Considerations
2.2.2. Actinic Keratosis
2.2.3. Main Histologic Variant and Differential Diagnoses of NMSC with Squamous Differentiation
Acknowledgments
Conflicts of Interest
References
- Apalla, Z.; Nashan, D.; Weller, R.B.; Castellsagué, X. Skin Cancer: Epidemiology, Disease Burden, Pathophysiology, Diagnosis, and Therapeutic Approaches. Dermatol. Ther. (Heidelberg) 2017, 7 (Suppl. 1), 5–19. [Google Scholar] [CrossRef] [PubMed]
- Barnhill, R.; Crowson, N.A.; Magro, M.C.; Piepkor, M.W. Dermatopathology, 3rd ed.; McGraw-Hill Education/Medical: New York, NY, USA, 2010. [Google Scholar]
- Kempf, W.; Hantschke, M.; Kutzner, H.; Burgdorf, W. Dermatopathology; Steinkopff Darmstadt: Darmstadt, Germany, 2008. [Google Scholar]
- Yada, K.; Kashima, K.; Daa, T.; Kitano, S.; Fujiwara, S.; Yokoyama, S. Expression of CD10 in basal cell carcinomas. Am. J. Dermatopathol. 2004, 26, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Mavrikakis, I.; Malhotra, R.; Selva, D.; Huilgol, S.C.; Barlow, R. Linear basal cell carcinoma: A distinct clinical entity. J. Plast. Reconst. Aesthet. Surg. 2006, 59, 419–423. [Google Scholar] [CrossRef]
- Niazi, Z.B.; Lamberty, B.G. Perineural infiltration in basal cell carcinomas. Br. J. Plast. Surg. 1993, 46, 156–157. [Google Scholar] [CrossRef]
- Miller, S.J. Biology of basal cell carcinoma (Part I). J. Am. Acad. Dermatol. 1991, 24, 1–13. [Google Scholar] [CrossRef]
- Bleehen, S.S. Pigmented basal cell epithelioma. Br. J. Dermatol. 1975, 93, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.J.; Graham, J.H. Granular cell basal cell carcinoma. Arch. Dermatol. 1979, 115, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Elston, D.M.; Bergfeld, W.F.; Petroff, N. Basal cell carcinoma with monster cells. J. Cutan. Pathol. 1993, 20, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.A.; Cohen, P.R.; Herzberg, A.J.; Wallis, M.E.; Rapini, R.P. Pleomorphic basal cell carcinoma. J. Am. Acad. Dermatol. 2006, 32, 740–746. [Google Scholar] [CrossRef]
- Mercu, T.R.; Dast, S.; Ciurea, M.E.; Mărgăritescu, C.; Popescu, F.D.; Manolea, H.O.; Scrieciu, M.; Ghelase, Ş.M. Histopathological aspects of some rare forms of facial basal cell carcinoma. Rom. J. Morphol. Embryol. 2017, 58, 425–432. [Google Scholar]
- Siegle, R.J.; MacMillan, J.; Pollack, S.V. Infiltrative basal cell carcinoma: A nonsclerosing subtype. J. Dermatol. Surg. Oncol. 1986, 12, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Sexton, M.; Jones, D.B.; Maloney, M.E. Histologic pattern analysis of basal cell carcinoma. J. Am. Acad. Dermatol. 1990, 23, 1118–1126. [Google Scholar] [CrossRef]
- Teo, W.L.; Wong, C.H.; Song, C. Morpheaform facial basal cell carcinoma—A 16-year experience in an Asian center. Int. J. Dermathol. 2012, 51, 1396–1398. [Google Scholar] [CrossRef] [PubMed]
- De Faria, J.L. Basal cell carcinoma of the skin with areas of squamous cell carcinoma: A basosquamous cell carcinoma? J. Clin. Pathol. 1985, 38, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, P.; Singh, A.; Ramesh, V. Basal cell carcinoma in the North Indian population: Clinicopathologic review and immunohistochemical analysis. Indian J. Dermatol. Venereol. Leprol. 2011, 77, 328–330. [Google Scholar] [PubMed]
- Goldberg, L.H. Basal cell carcinoma. Lancet 1996, 9002, 663–667. [Google Scholar] [CrossRef]
- Artis, A.H.; Van Marion, A.M.; Lohman, B.G.; Thissen, M.R.; Steijlen, P.M.; Nelemans, P.J.; Kelleners-Smeets, N.W. Differentiation between basal cell carcinoma and tricoepithelioma by immunohistochemical staining of the androgen receptor: An overview. Eur. J. Dermatol. 2011, 21, 870–873. [Google Scholar]
- Ansai, S.; Takayama, R.; Kimura, T.; Kawana, S. Ber-EP4 is a useful marker for follicular germinative cell differentiation of cutaneous epithelial neoplasms. J. Dermatol. 2012, 39, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Heidarpour, M.; Rajabi, P.; Sajadi, F. CD10 expression help to differentiate basal cell carcinoma from tricopeithelioma. J. Res. Med. Sci. 2011, 16, 938–944. [Google Scholar] [PubMed]
- Parekh, V.; Seykora, J.T. Cutaneous Squamous Cell Carcinoma. Clin. Lab. Med. 2017, 37, 503–525. [Google Scholar] [CrossRef] [PubMed]
- De Hertog, S.A.; Wensveen, C.A.; Bastiaens, M.T.; Kielich, C.J.; Berkhout, M.J.; Westendorp, R.G.; Vermeer, B.J.; Bouwes Bavinck, J.N.; Leiden Skin Cancer Study. Relation between smoking and skin cancer. J. Clin. Oncol. 2001, 19, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Hakim, I.A.; Harris, R.B.; Ritenbaugh, C. Fat intake and risk of squamous cell carcinoma of the skin. Nutr. Cancer 2000, 36, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Roest, M.A.; Keane, F.M.; Agnew, K.; Hawk, J.L.; Griffiths, W.A. Multiple squamous skin carcinomas following excess sunbed use. J. R. Soc. Med. 2001, 94, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Ibiebele, T.I.; van der Pols, J.C.; Hughes, M.C.; Marks, G.C.; Green, A.C. Dietary pattern in association with squamous cell carcinoma of the skin: A prospective study in Australian adults. Int. J. Cancer 2007, 125, 1401–1408. [Google Scholar]
- Hama, N.; Ohtsuka, T.; Yamazaki, S. Detection of mucosal human papilloma virus DNA in bowenoid papulosis, Bowen’s disease and squamous cell carcinoma of the skin. J. Dermatol. 2006, 33, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Kwa, R.E.; Campana, K.; Moy, R.L. Biology of cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 1992, 26, 1–16. [Google Scholar] [CrossRef]
- Smith, K.J.; Skelton, H.G., III; Morgan, A.M.; Barrett, T.L.; Lupton, G.P. Spindle cell neoplasms coexpressing cytokeratin and vimentin (metaplastic squamous cell carcinoma). J. Cutan. Pathol. 1992, 19, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.C.; Helwig, E.B. Adenoid squamous cell carcinoma (adenoacanthoma): A clinicopathologic study of 155 patients. Cancer 1966, 19, 1639–1650. [Google Scholar] [CrossRef]
- Caya, J.G.; Hidayat, A.A.; Weiner, M.J. A clinicopathologic study of 21 cases of adenoid squamous cell carcinoma of the eyelid and periorbital region. Am. J. Ophthalmol. 1985, 99, 291–297. [Google Scholar] [CrossRef]
- Pyne, J.H.; Myint, E.; Barr, E.M. Acantholytic invasive squamous cell carcinoma: Tumor diameter, invasion depth, grade of differentiation, surgical margins, perineural invasion, recurrence and death rate. J. Cutan. Pathol. 2017, 44, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.W., III; Farmer, E.R.; Rady, P.L.; Tyring, S.K. Cutaneous papillary squamous cell carcinoma in an immunosuppressed host. J. Cutan. Pathol. 1994, 21, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.M.; McCalmont, T.; Yu, S.S. Adenosquamous carcinoma of the skin: A case series. Arch. Dermatol. 2009, 145, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.M.; Molina, I.; Monteagudo, C.; Marti, N.; Lopez, V.; Jorda, E. Buschke-Lowenstein tumor. J. Dermatol. Case Rep. 2008, 2, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.; Bhagat, A.; Vasudevan, B.; Sridhar, J.; Madan, R.; Ray, M. A Rare Case of Plantar Epithelioma Cuniculatum Arising from a Wart. Indian J. Dermatol. 2015, 60, 485–487. [Google Scholar] [PubMed]
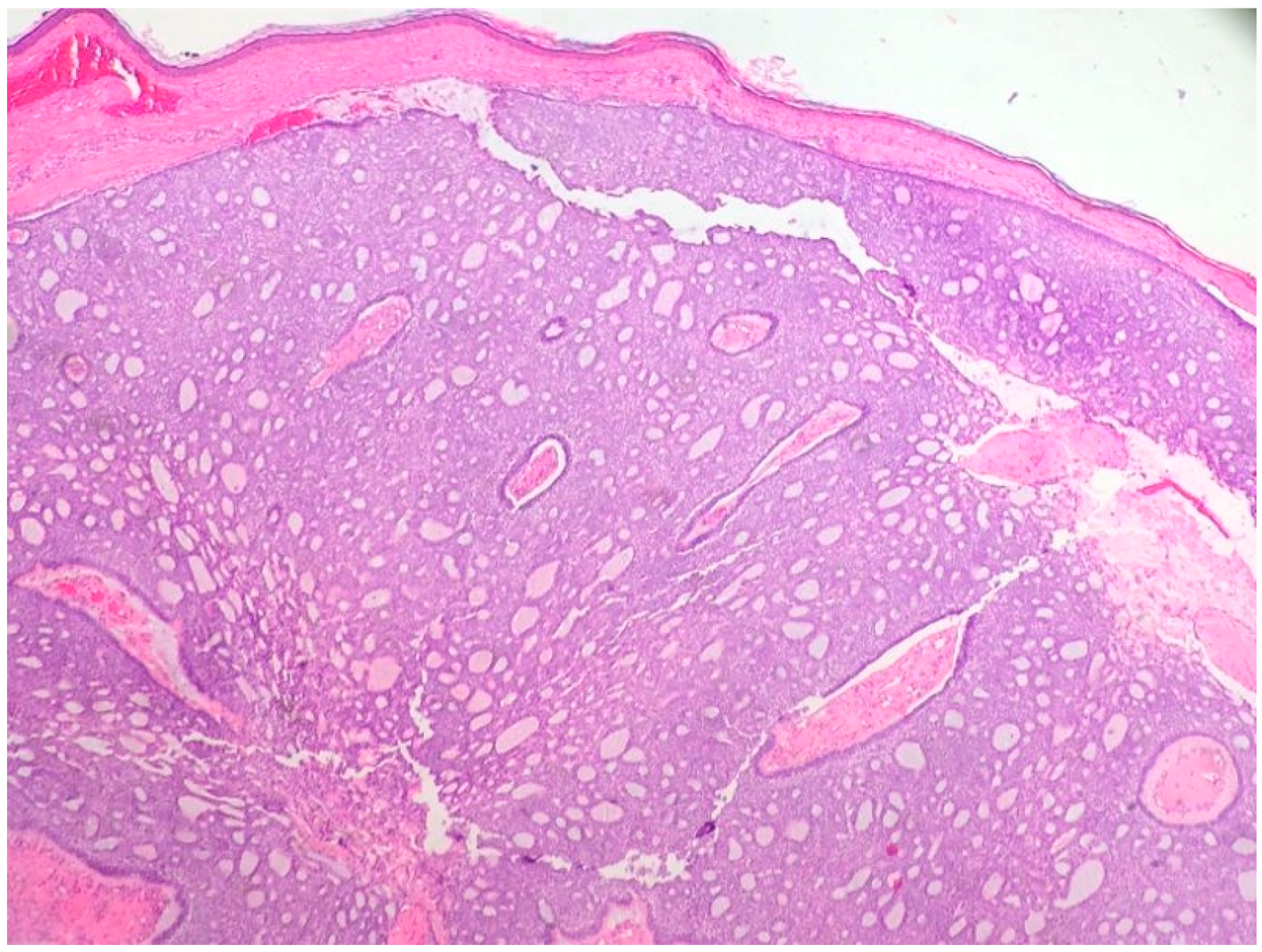
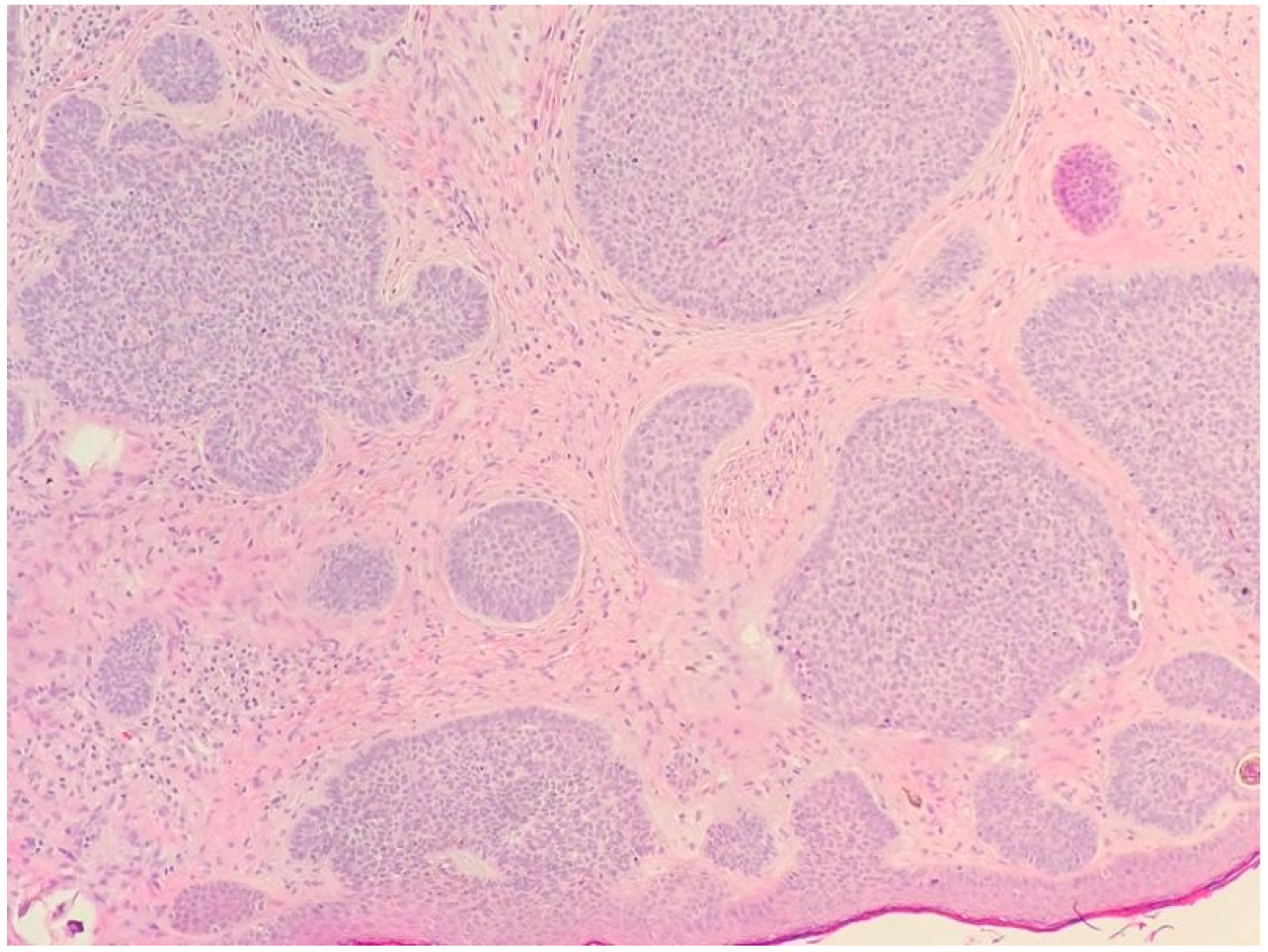
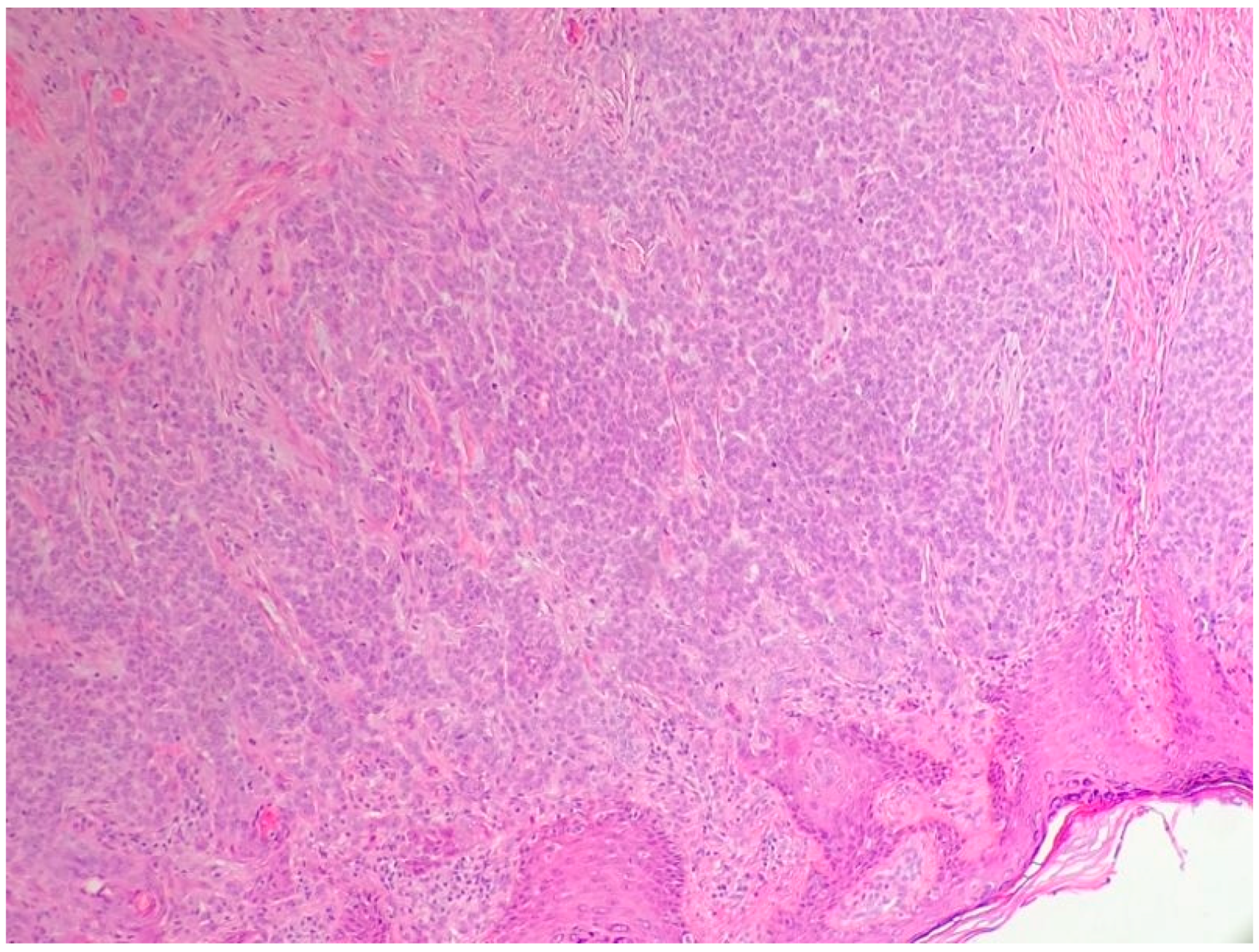
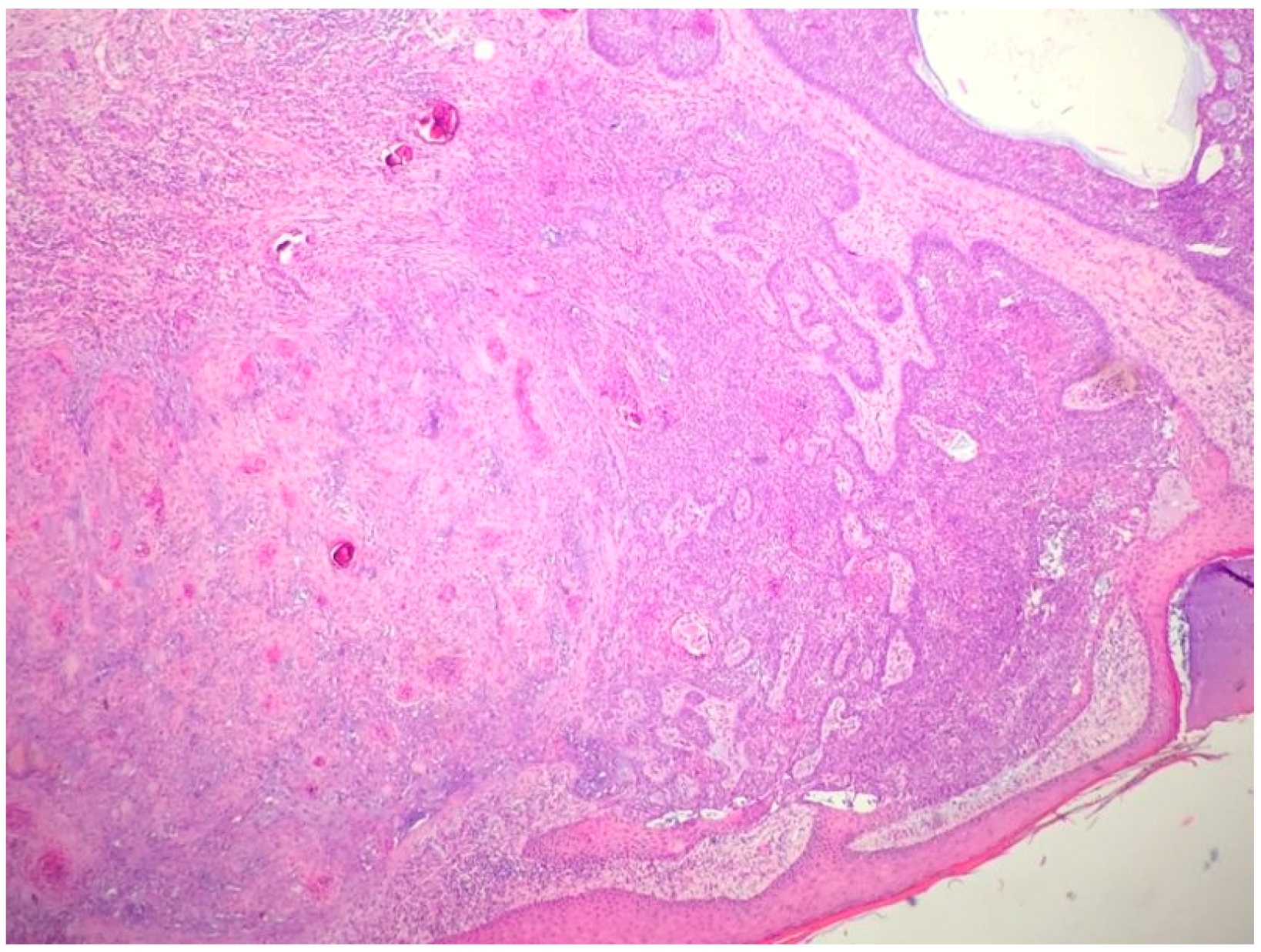
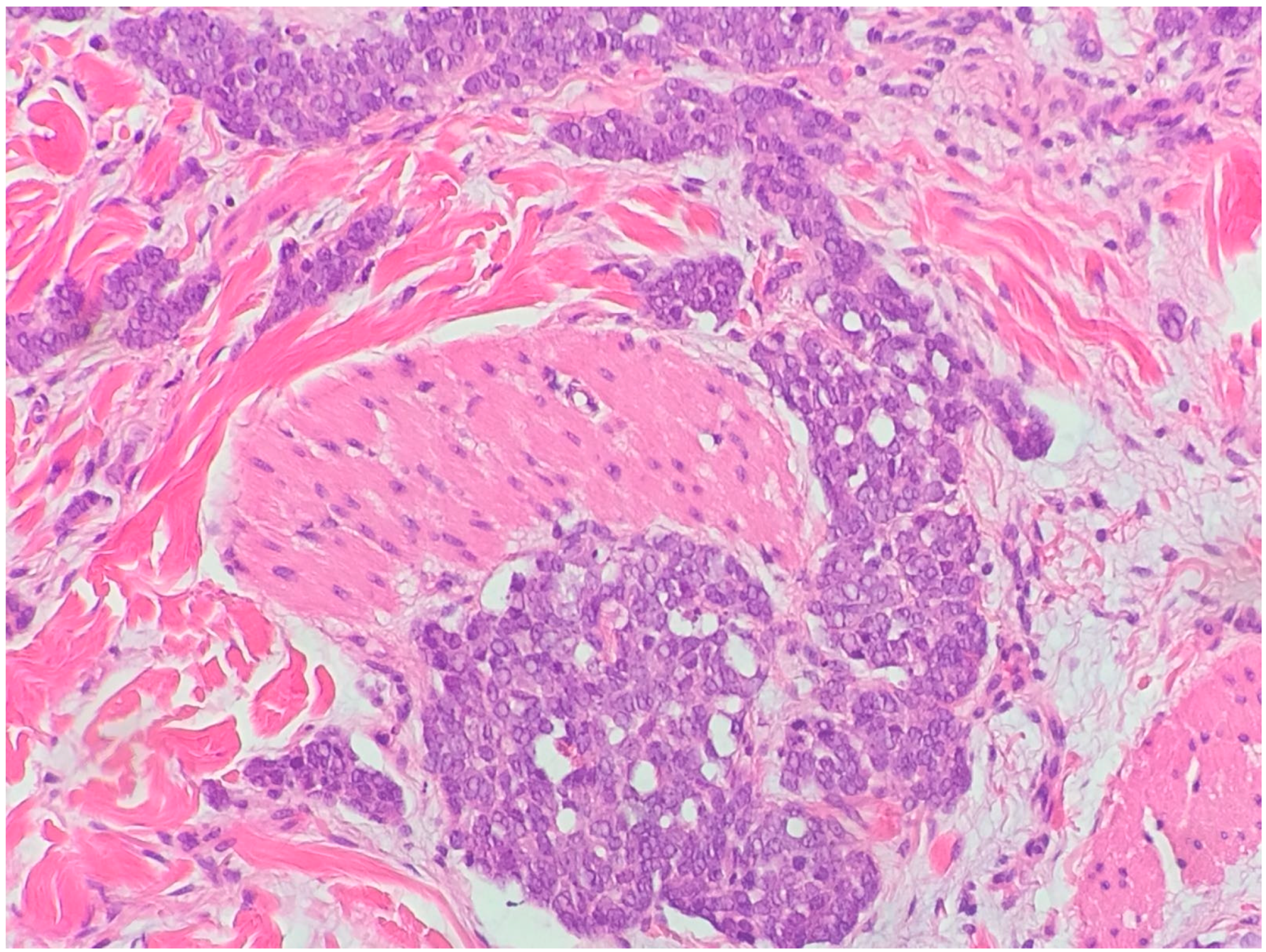
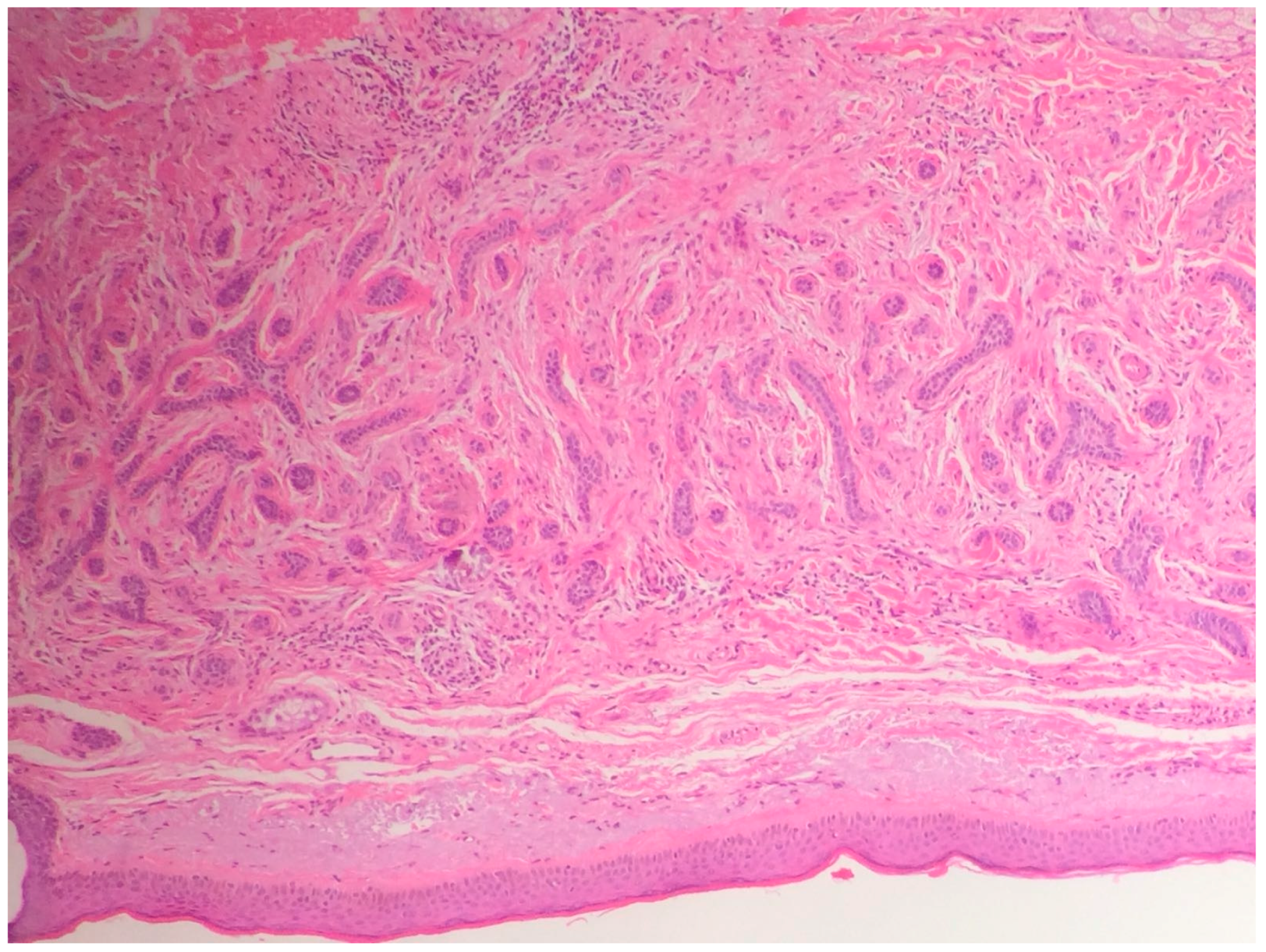

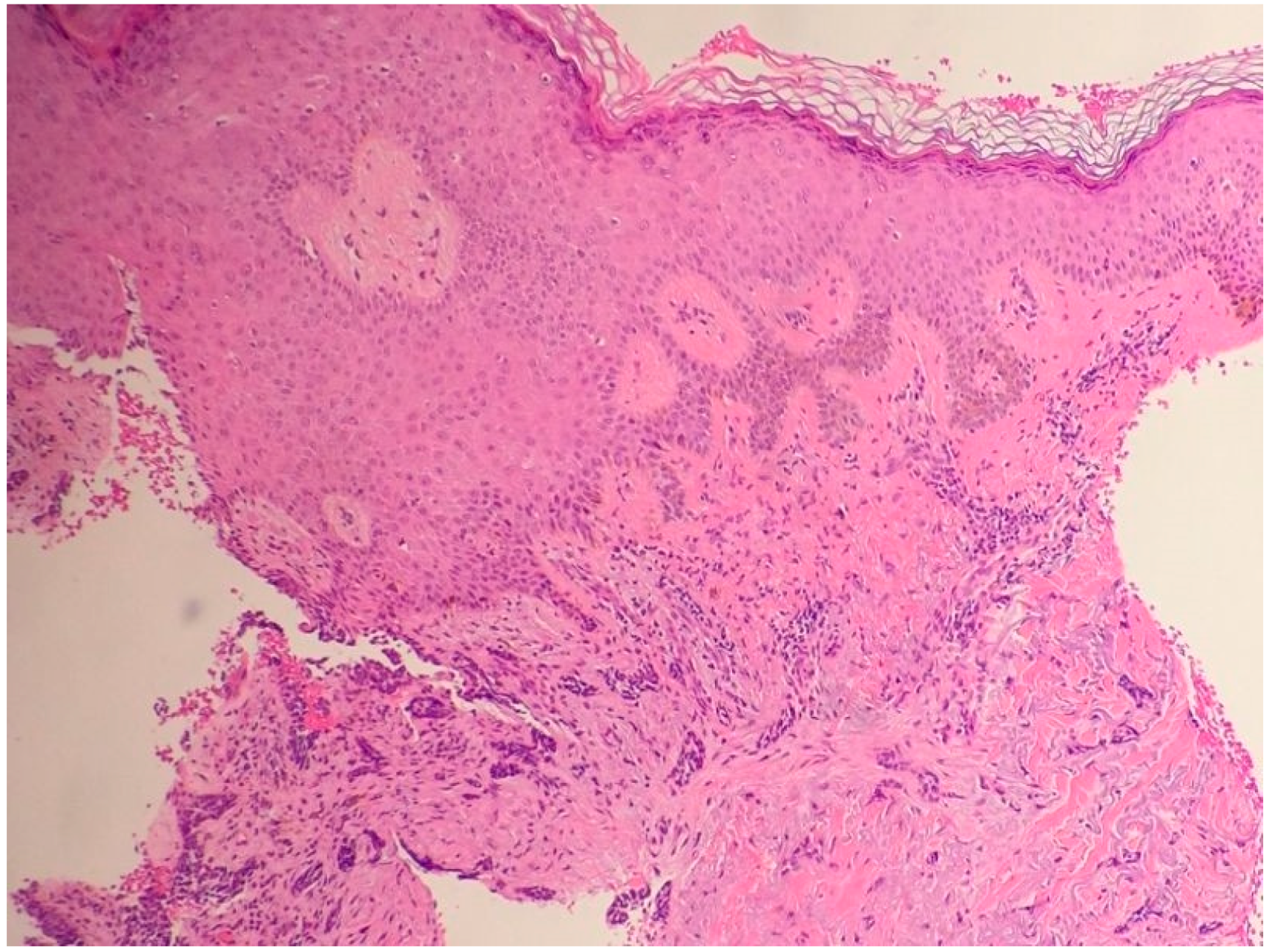
| Differential Diagnosis | Pathological Features |
|---|---|
| Tricoblastoma | Absence of cleft, rudimentary hair germs, papillary mesenchymal bodies. |
| ADC | Lack of basaloid cells disposed in peripheral palisades; adenoid-cystic lesion without connection to the epidermis; absence of artefactual clefts |
| MAC | Bland keratinocytes, keratin cysts, ductal differentiation CEA+, EMA+ and BerEp4- |
| Tricoepithelioma * | Rims of collagen bundles, calcification, follicular/sebaceous/infundibular differentiation and cut artefacts. Cytokeratin (CK)20+, p75+, Pleckstrin homology-like domain family A member 1 + (PHLDA1+), common acute lymphoblastic leukemiaantigen + (CD10+) in tumor stroma, CK 6-, Ki-67– and Androgen Rceptor - (AR-) |
| MCC | Cells arranged in a diffuse, trabecular and/or nested pattern, involving also the subcutis. Mouse Anti-Cytokeratin (CAM) 5.2+, CK20+, S100-, human leukocyte common antigen – ( LCA-), thyroid transcription factor 1- (TTF1-) |
| Differential Diagnosis | Clinico-Pathological Features |
|---|---|
| AK | Atypical keratinocytes confined on basal layer. |
| Bowen | Atypical keratinocytes at every layer of epidermis. |
| KA | Symmetrical and circumscribed proliferation of keratinocytes, with central horn plug, with epidermis that extends over the tumor. Highly differentiated SCC. |
| Invasive SCC | Atypical and pleomorphic keratinocytes, involving the dermis and the sub-cutis with a potential metastatic spread. |
| QE | As Bowen, but in the mucosa. |
| ACs | Squamous differentiation, but does not show connection with the epidermis and highlights adnexal features. |
| AD SCC | Mixed glandular and squamous differentiation. |
| VSCC * | Exophytic squamous proliferation with marked papillomatosis and low atypia and the presence of koilocyte-like changes |
| EC | SCC of the foot. Histologically is characterized hyperkeratosis, acanthosis with an undulating, densely keratinized, well differentiated squamous epithelium, deeply penetrating the soft tissues. |
| IFK | Sharply circumscribed endophytic verrucous proliferation with prominent squamous features. |
| SK | Acanthosis, absence of atypia, pseudo-horn cysts, in inflamed lesions, mitoses may be present. |
| BP | Atypical keratinocytes and mitoses. Histology similar to Bowen’s disease. |
| Metastasis | Personal medical history of the patient, nodular proliferation without connection to epidermis, immunohistochemical evaluation. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolino, G.; Donati, M.; Didona, D.; Mercuri, S.R.; Cantisani, C. Histology of Non-Melanoma Skin Cancers: An Update. Biomedicines 2017, 5, 71. https://doi.org/10.3390/biomedicines5040071
Paolino G, Donati M, Didona D, Mercuri SR, Cantisani C. Histology of Non-Melanoma Skin Cancers: An Update. Biomedicines. 2017; 5(4):71. https://doi.org/10.3390/biomedicines5040071
Chicago/Turabian StylePaolino, Giovanni, Michele Donati, Dario Didona, Santo Raffaele Mercuri, and Carmen Cantisani. 2017. "Histology of Non-Melanoma Skin Cancers: An Update" Biomedicines 5, no. 4: 71. https://doi.org/10.3390/biomedicines5040071
APA StylePaolino, G., Donati, M., Didona, D., Mercuri, S. R., & Cantisani, C. (2017). Histology of Non-Melanoma Skin Cancers: An Update. Biomedicines, 5(4), 71. https://doi.org/10.3390/biomedicines5040071







