The Role of Antifibrotic Therapy in Pulmonary Fibrosis and Lung Cancer: A Multicenter Retrospective Analysis
Abstract
1. Introduction
1.1. Lung Cancer and Interstitial Lung Disease (ILD): Pathogenetic Mechanisms
1.2. Incidence and Survival of Lung Cancer in ILDs
1.3. Antifibrotic Therapy in Lung Cancer and the Role of PD-L1
1.4. Pirfenidone
1.5. Nintedanib
2. Materials and Methods
2.1. Study Design and Setting
2.2. Eligibility Criteria
- Diagnosis of fibrotic ILD, confirmed by high-resolution computed tomography (HRCT) and/or histopathology, according to international consensus guidelines.
- Diagnosis of primary lung cancer, confirmed by histology or cytology.
- Availability of clinical and treatment data regarding both ILD and lung cancer.
2.3. Exposure Definition
- Antifibrotic group (AF): patients who had received antifibrotic therapy.
- No-antifibrotic group (NO): patients who had never received antifibrotic therapy.
2.4. Outcomes
- Primary outcome: occurrence of acute exacerbation of ILD (AE-ILD) after the initiation of first-line oncologic therapy. AE-ILD was defined according to international consensus criteria as: (i) acute worsening of dyspnea within 30 days, (ii) new bilateral ground-glass opacities or consolidations superimposed on a background of fibrotic ILD on HRCT, and (iii) exclusion of alternative causes such as infection, pulmonary embolism, or heart failure.
- Secondary outcomes:
- PD-L1 expression in tumor tissue, recorded when available. Expression was categorized using the standard thresholds of ≥1% and ≥50% tumor proportion score.
- Autoimmunity was assessed using standard serological panels, including antinuclear antibodies (ANA), extractable nuclear antigen antibodies (ENA), antineutrophil cytoplasmic antibodies (ANCA), and rheumatoid factor. Results were categorized as positive or negative according to established laboratory cut-off values.
- Exploratory outcome: survival, expressed in months from cancer diagnosis to death. Importantly, survival time was available only for deceased patients, whereas for survivors the database recorded “alive” without a date of last contact. As a result, Kaplan–Meier survival curves and log-rank tests were not feasible, and survival is presented descriptively.
2.5. Oncologic Treatment Categories
- Surgery (S)
- Chemotherapy (CHT)
- Radiotherapy (RT)
- Immunotherapy (IC)
2.6. Data Collection and Management of Missing Data
2.7. Statistical Analysis
- Group comparisons:
- ○
- Binary outcomes (e.g., AE-ILD yes/no) between AF and NO groups were compared using Fisher’s exact test, given the limited sample size.
- ○
- Continuous baseline variables were compared in terms of variance using Levene’s test (median-centered) to assess the equality of variances between AF and NO groups.
- ○
- No formal between-group mean comparison was attempted for continuous variables with high missingness or small denominators.
- Subgroup analyses: exploratory and descriptive, stratified by first-line oncologic treatment, PD-L1 status, and autoimmunity. Given small subgroup sizes, no inferential statistics were applied, and results were interpreted cautiously.
- Survival: survival times were recorded numerically only for deceased patients. As censoring dates for survivors were systematically missing, Kaplan–Meier curves, median overall survival estimates, and log-rank comparisons could not be performed. Descriptive statistics (median survival among deceased patients) were reported, but no inferential time-to-event methods were applied.
- Software: All analyses were performed using JMP® (v18.2.2, SAS Institute, Cary, NC, USA) and Jamovi (version 2.5). A two-sided p < 0.05 was considered statistically significant.
- Baseline variance analysis: Levene’s tests showed no evidence of unequal variances between antifibrotic (AF) and no-antifibrotic (NO) groups for BMI (p = 0.988), pack-years (p = 0.619), or DLCO% (p = 0.367). Age at ILD diagnosis showed a borderline, non-significant difference (p = 0.077). These findings suggest overall comparability of variance between groups, supporting the validity of subsequent group comparisons.
- Advanced statistical analyses were performed using the partitioning algorithm in the JMP software (JMP-Statistical Discoveries, SAS, www.jmp.com). Decision trees were employed as a classification method, using a series of hierarchical variable selections structured in a tree-like model. Variables (branches) were defined as splitting criteria.
2.8. Limitations
3. Results
3.1. Patient Characteristics
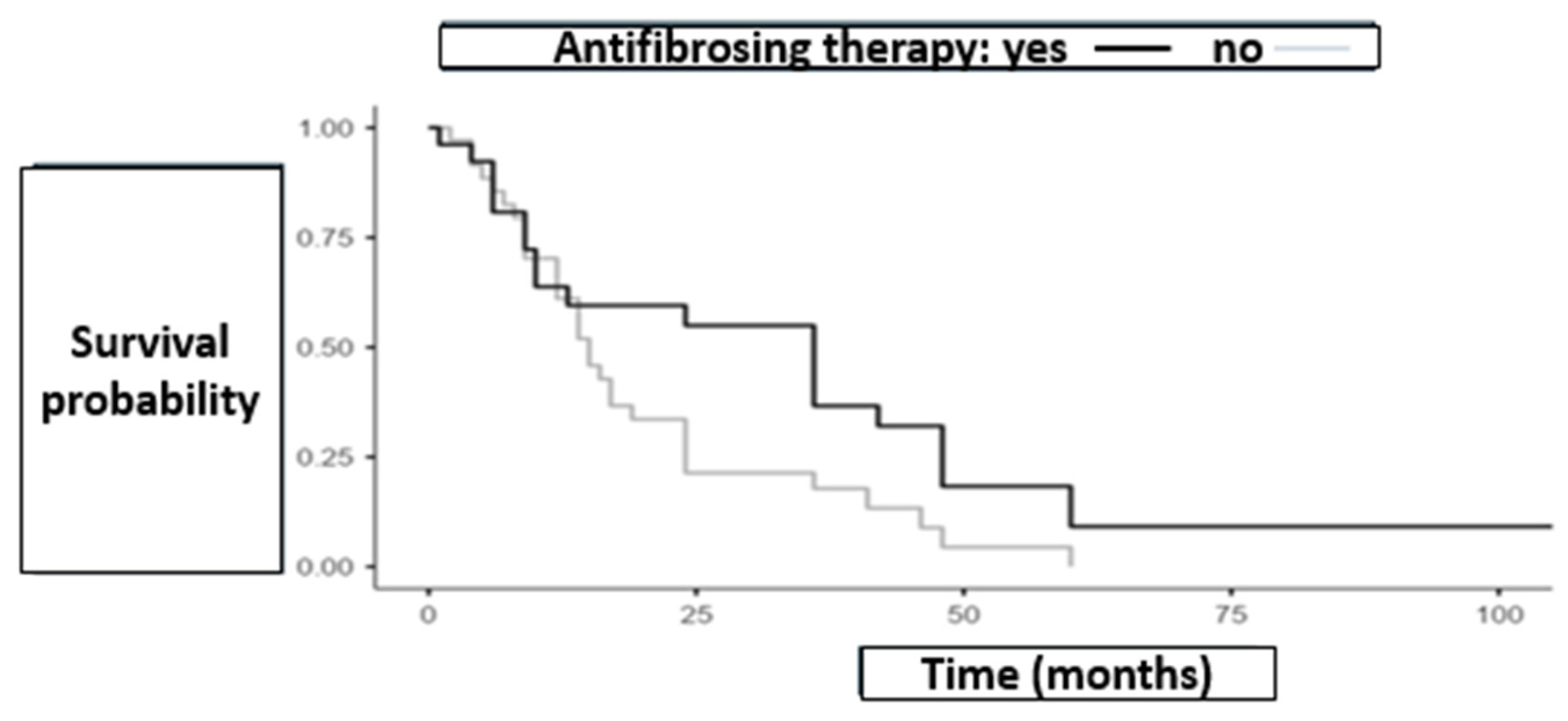
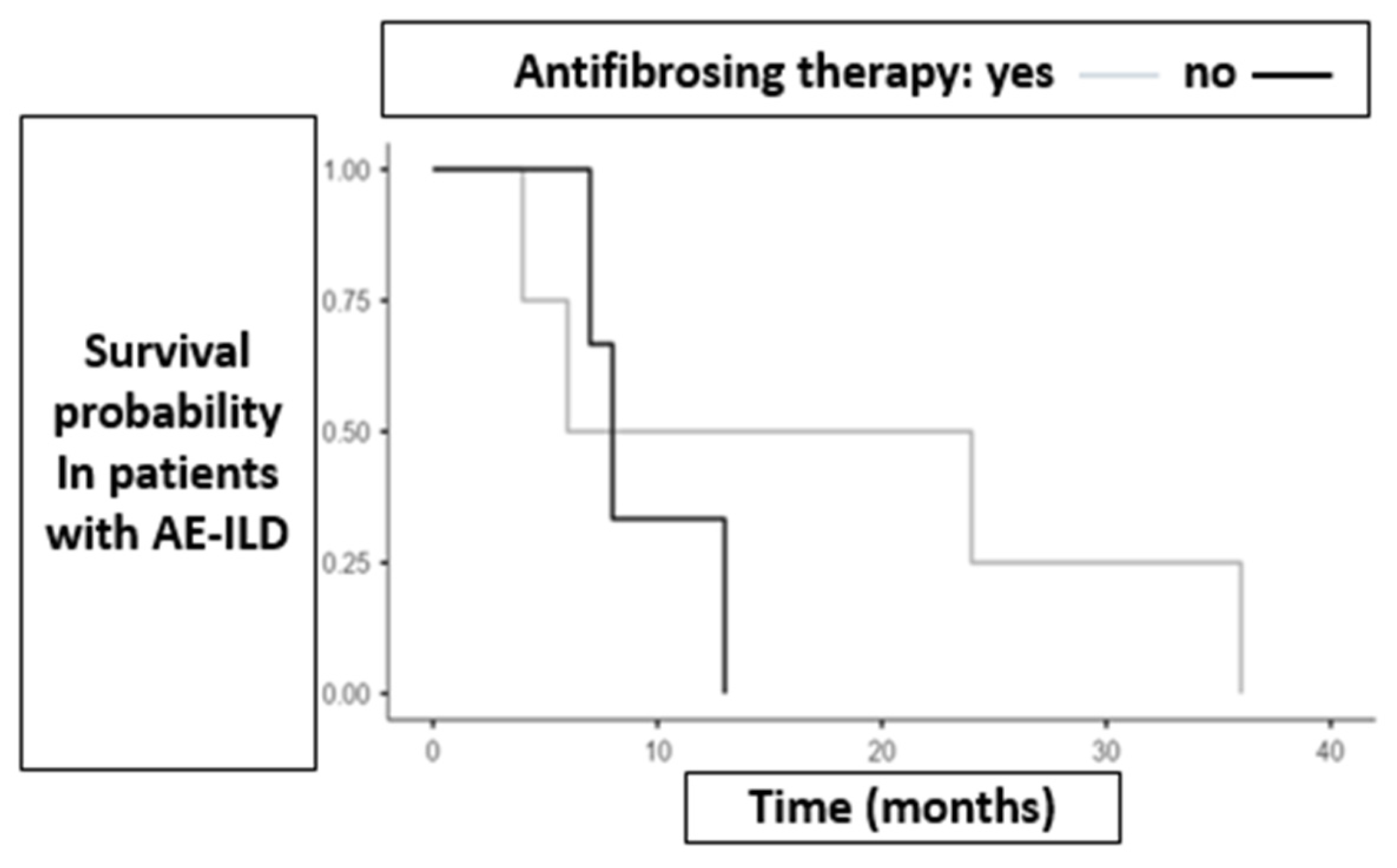
3.2. Survival and AE-ILD
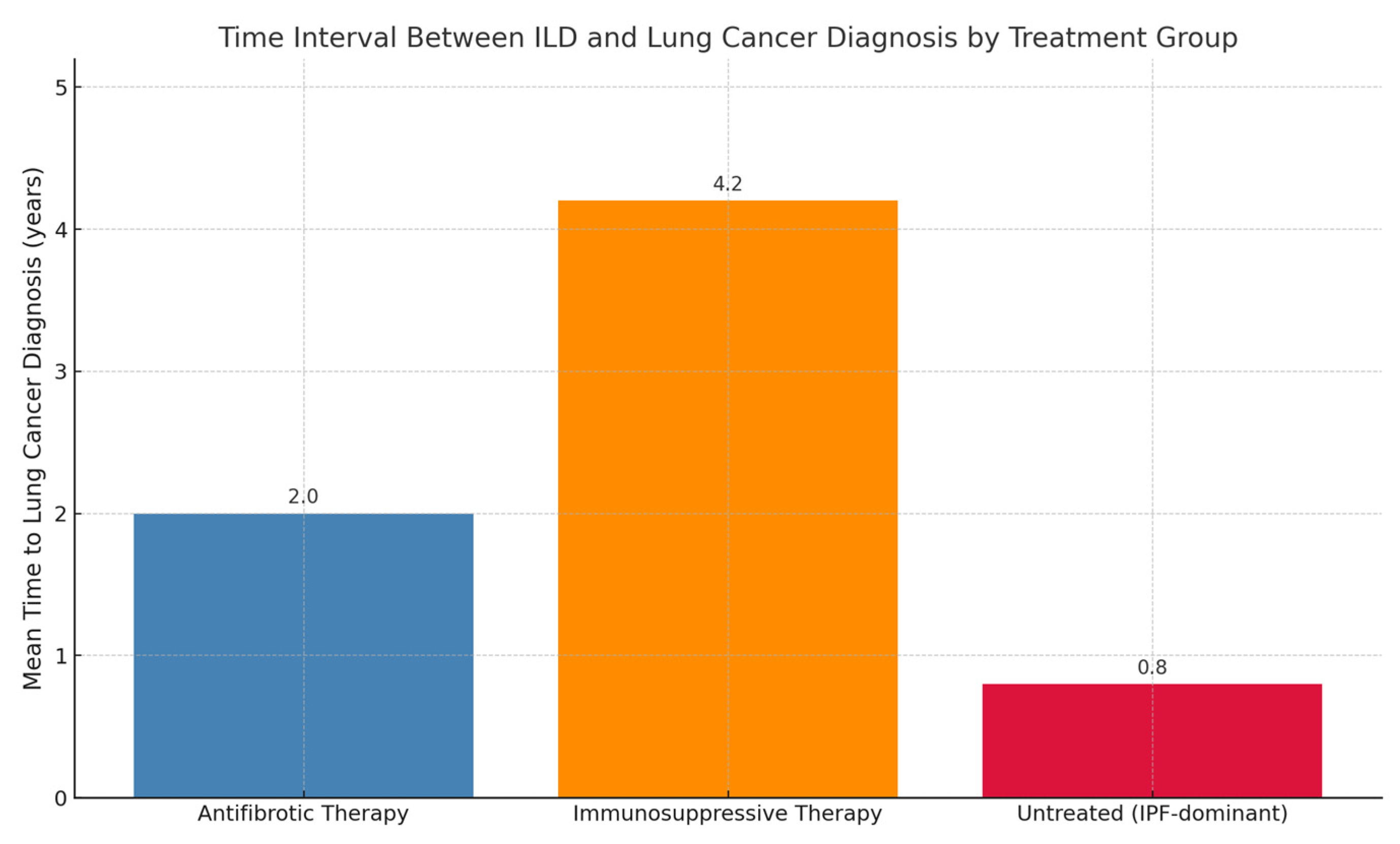
3.3. Role of Antifibrotic Therapy in the Timing of Lung Cancer Onset
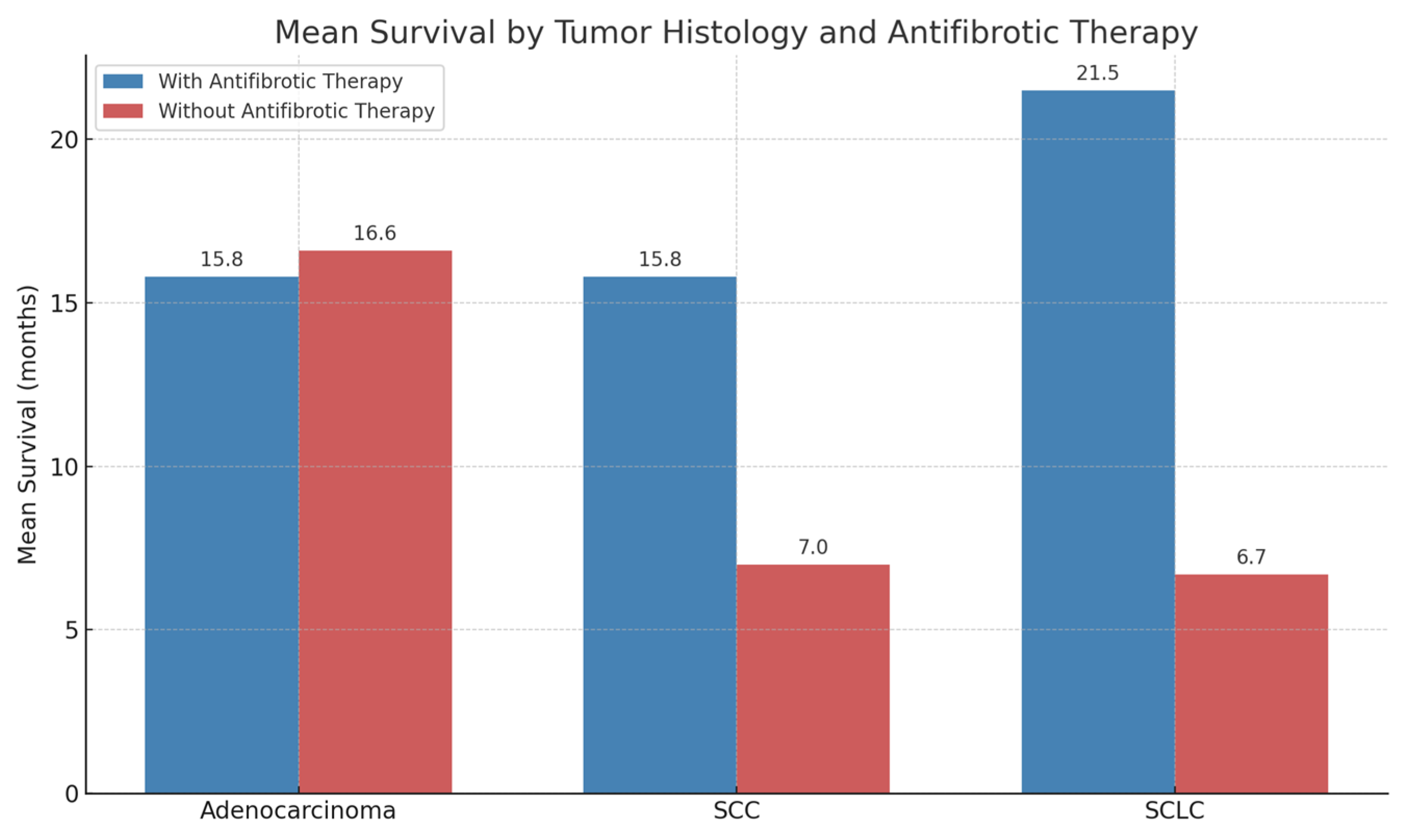
3.4. Effects of Antifibrotic Therapy According to Tumor Histology
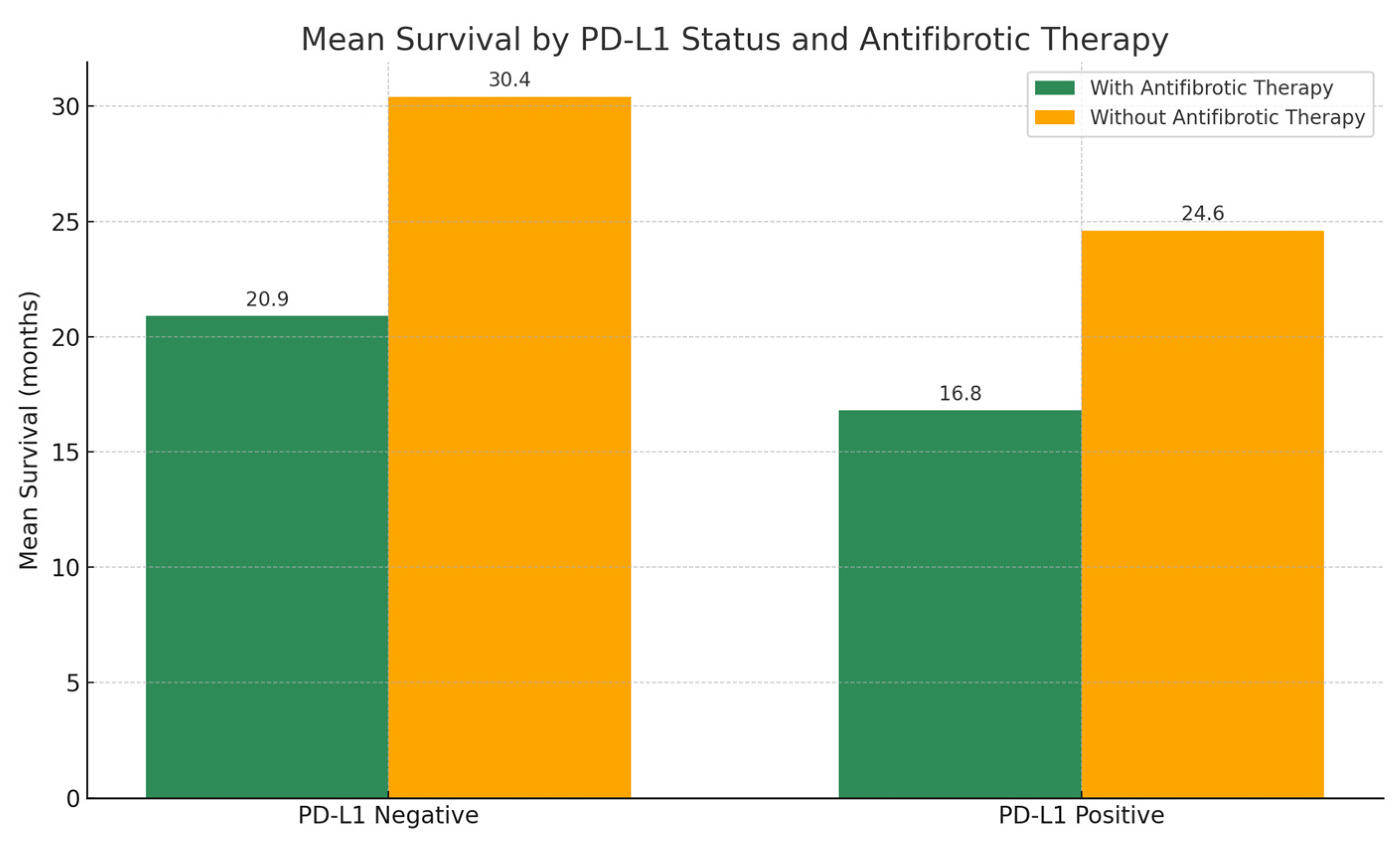
3.5. Role of PD-L1
3.6. Role of Autoimmunity
4. Discussion
4.1. Clinical Implications
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kinoshita, T.; Goto, T. Molecular Mechanisms of Pulmonary Fibrogenesis and Its Progression to Lung Cancer: A Review. Int. J. Mol. Sci. 2019, 20, 1461. [Google Scholar] [CrossRef]
- Bonella, F.; Spagnolo, P.; Ryerson, C. Current and Future Treatment Landscape for Idiopathic Pulmonary Fibrosis. Drugs 2023, 83, 1581–1593. [Google Scholar] [CrossRef]
- Drakopanagiotakis, F.; Krauss, E.; Michailidou, I.; Drosos, V.; Anevlavis, S.; Günther, A.; Steiropoulos, P. Lung Cancer and Interstitial Lung Diseases. Cancers 2024, 16, 2837. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Horie, M.; Nagase, T. TGF-β Signaling in Lung Health and Disease. Int. J. Mol. Sci. 2018, 19, 2460. [Google Scholar] [CrossRef]
- Kornepati, A.V.R.; Vadlamudi, R.K.; Curiel, T.J. Programmed Death Ligand 1 Signals in Cancer Cells. Nat. Rev. Cancer 2022, 22, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Khalili-Tanha, G.; Radisky, E.S.; Radisky, D.C.; Shoari, A. Matrix Metalloproteinase-Driven Epithelial-Mesenchymal Transition: Implications in Health and Disease. J. Transl. Med. 2025, 23, 436. [Google Scholar] [CrossRef] [PubMed]
- Naoi, H.; Suzuki, Y.; Mori, K.; Aono, Y.; Kono, M.; Hasegawa, H.; Yokomura, K.; Inoue, Y.; Hozumi, H.; Karayama, M.; et al. Impact of Antifibrotic Therapy on Lung Cancer Development in Idiopathic Pulmonary Fibrosis. Thorax 2022, 77, 727–730. [Google Scholar] [CrossRef]
- Wang, J.; Li, K.; Hao, D.; Li, X.; Zhu, Y.; Yu, H.; Chen, H. Pulmonary Fibrosis: Pathogenesis and Therapeutic Strategies. MedComm 2024, 5, e744. [Google Scholar] [CrossRef]
- Xu, H.; Yin, L.; Bian, W.; Kang, M.; Adami, H.-O.; Ye, W. Interstitial Lung Disease and Risk of Lung Cancer. JAMA Netw. open 2025, 8, e2519630. [Google Scholar] [CrossRef]
- Kewalramani, N.; Machahua, C.; Poletti, V.; Cadranel, J.; Wells, A.U.; Funke-Chambour, M. Lung Cancer in Patients with Fibrosing Interstitial Lung Diseases: An Overview of Current Knowledge and Challenges. ERJ Open Res. 2022, 8, 00115–2022. [Google Scholar] [CrossRef]
- Sampsonas, F.; Bosgana, P.; Bravou, V.; Tzouvelekis, A.; Dimitrakopoulos, F.-I.; Kokkotou, E. Interstitial Lung Diseases and Non-Small Cell Lung Cancer: Particularities in Pathogenesis and Expression of Driver Mutations. Genes 2024, 15, 934. [Google Scholar] [CrossRef]
- Watanabe, S.; Saeki, K.; Waseda, Y.; Murata, A.; Takato, H.; Ichikawa, Y.; Yasui, M.; Kimura, H.; Hamaguchi, Y.; Matsushita, T.; et al. Lung Cancer in Connective Tissue Disease-Associated Interstitial Lung Disease: Clinical Features and Impact on Outcomes. J. Thorac. Dis. 2018, 10, 799–807. [Google Scholar] [CrossRef]
- Kuramochi, J.; Inase, N.; Miyazaki, Y.; Kawachi, H.; Takemura, T.; Yoshizawa, Y. Lung Cancer in Chronic Hypersensitivity Pneumonitis. Respiration. 2011, 82, 263–267. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, L.; Wang, Q. Analysis of Clinical Characteristics and Prognosis of Lung Cancer Patients with CPFE or COPD: A Retrospective Study. BMC Pulm. Med. 2024, 24, 274. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Kim, H.H.; Hyun, D.; Ji, W.; Choi, C.-M.; Lee, J.C.; Kim, H.C. Clinical Characteristics and Outcome of Lung Cancer in Patients with Fibrosing Interstitial Lung Disease. BMC Pulm. Med. 2024, 24, 136. [Google Scholar] [CrossRef]
- Kurimoto, R.; Ebata, T.; Iwasawa, S.; Ishiwata, T.; Tada, Y.; Tatsumi, K.; Takiguchi, Y. Pirfenidone May Revert the Epithelial-to-Mesenchymal Transition in Human Lung Adenocarcinoma. Oncol. Lett. 2017, 14, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Xue, Q.; Hu, X.; Yang, J. Inhibitor of PD-1/PD-L1: A New Approach May Be Beneficial for the Treatment of Idiopathic Pulmonary Fibrosis. J. Transl. Med. 2024, 22, 95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Luo, D.; Cheng, Z.; Zeng, Q.; Wang, G.; Chen, M.; Zhang, S.; Luo, P. Pirfenidone Inhibits TGF-Β1-Induced Metabolic Reprogramming during Epithelial-Mesenchymal Transition in Non-Small Cell Lung Cancer. J. Cell. Mol. Med. 2024, 28, e18059. [Google Scholar] [CrossRef]
- Fujiwara, A.; Shintani, Y.; Funaki, S.; Kawamura, T.; Kimura, T.; Minami, M.; Okumura, M. Pirfenidone Plays a Biphasic Role in Inhibition of Epithelial-Mesenchymal Transition in Non-Small Cell Lung Cancer. Lung Cancer 2017, 106, 8–16. [Google Scholar] [CrossRef]
- Mediavilla-Varela, M.; Boateng, K.; Noyes, D.; Antonia, S.J. The Anti-Fibrotic Agent Pirfenidone Synergizes with Cisplatin in Killing Tumor Cells and Cancer-Associated Fibroblasts. BMC Cancer 2016, 16, 176. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Farrales, P.T.; Kunda, N.K.; Muth, A.; Gupta, V. Systematic Development and Optimization of Inhalable Pirfenidone Liposomes for Non-Small Cell Lung Cancer Treatment. Pharmaceutics 2020, 12, 206. [Google Scholar] [CrossRef]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- Sato, S.; Shinohara, S.; Hayashi, S.; Morizumi, S.; Abe, S.; Okazaki, H.; Chen, Y.; Goto, H.; Aono, Y.; Ogawa, H.; et al. Anti-Fibrotic Efficacy of Nintedanib in Pulmonary Fibrosis via the Inhibition of Fibrocyte Activity. Respir. Res. 2017, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Otsubo, K.; Kishimoto, J.; Ando, M.; Kenmotsu, H.; Minegishi, Y.; Horinouchi, H.; Kato, T.; Ichihara, E.; Kondo, M.; Atagi, S.; et al. Nintedanib plus Chemotherapy for Nonsmall Cell Lung Cancer with Idiopathic Pulmonary Fibrosis: A Randomised Phase 3 Trial. Eur. Respir. J. 2022, 60, 2200380. [Google Scholar] [CrossRef] [PubMed]
- Syrios, J.; Nintos, G.; Georgoulias, V. Nintedanib in Combination with Docetaxel for Second-Line Treatment of Advanced Non-Small-Cell Lung Cancer. Expert Rev. Anticancer Ther. 2015, 15, 875–884. [Google Scholar] [CrossRef]
- Alhadeethi, A.; Adel Awwad, S.; Abed, M.; Amin, A.M.; Aboelkhier, M.M.; Yassin, M.N.A.; Morsi, M.H.; Kashbour, M.O. Nintedanib in Combination With Chemotherapy in the Treatment of Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e53812. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Xu, H.; Ma, L.; Li, C.; Qin, W.; Chen, X.; Yi, M.; Sun, L.; Liu, B.; Yuan, X. Nintedanib Enhances the Efficacy of PD-L1 Blockade by Upregulating MHC-I and PD-L1 Expression in Tumor Cells. Theranostics 2022, 12, 747–766. [Google Scholar] [CrossRef]
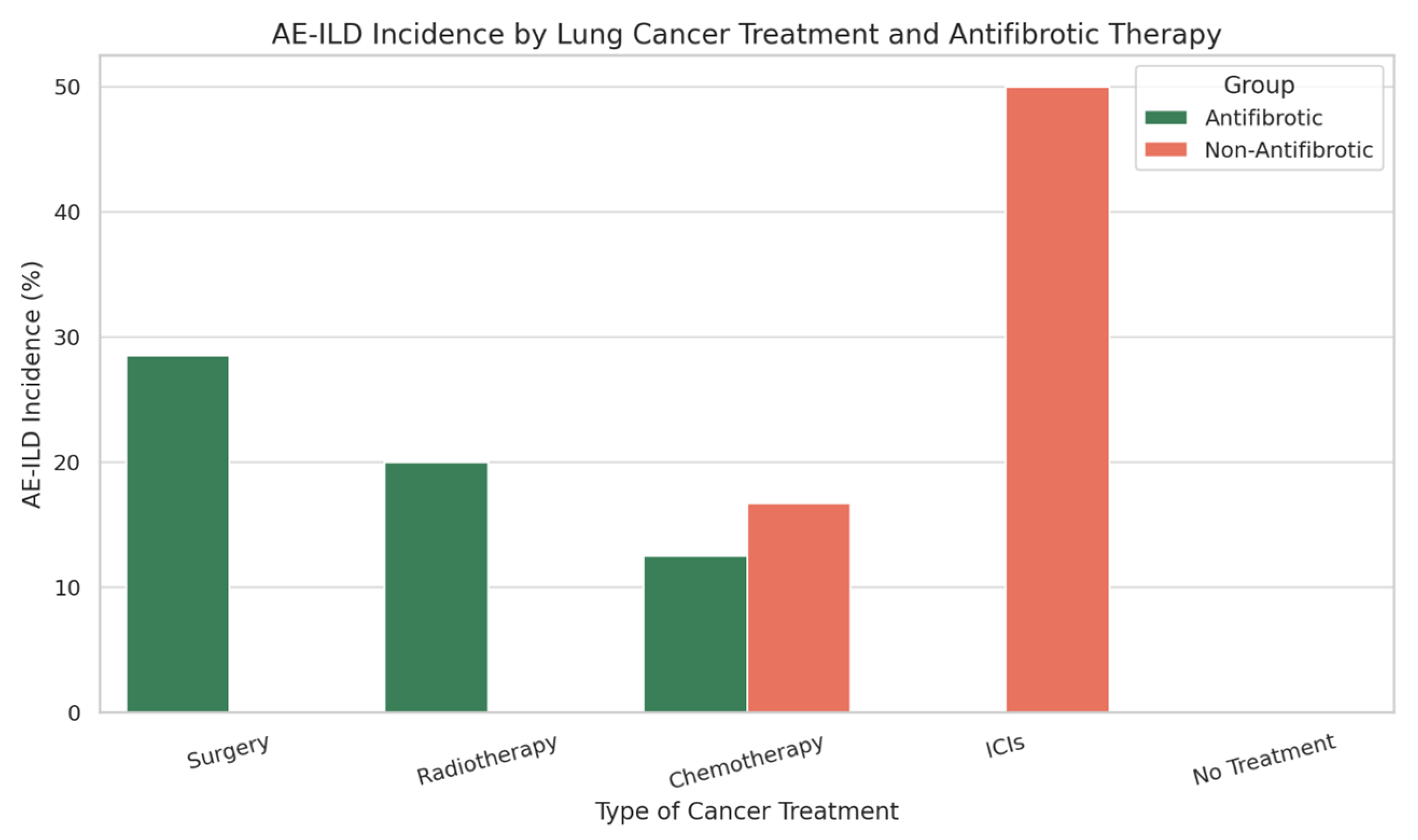
| Cancer Treatment Type | AE-ILD Incidence (%)—Antifibrotic Group | AE-ILD Incidence (%)—Non-Antifibrotic Group |
|---|---|---|
| Surgery | 28.5% | 0% |
| Radiotherapy | 20% | 0% |
| Chemotherapy | 12.5% | 16.7% |
| Immune Checkpoint Inhibitors (ICIs) | 0% | 50% |
| No Cancer Treatment | 0% | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertuccio, F.R.; Baio, N.; Perrotta, F.; Lacedonia, D.; D’Agnano, V.; Bianco, A.; Scioscia, G.; Tondo, P.; Foschino Barbaro, M.P.; Bortolotto, C.; et al. The Role of Antifibrotic Therapy in Pulmonary Fibrosis and Lung Cancer: A Multicenter Retrospective Analysis. Biomedicines 2025, 13, 2310. https://doi.org/10.3390/biomedicines13092310
Bertuccio FR, Baio N, Perrotta F, Lacedonia D, D’Agnano V, Bianco A, Scioscia G, Tondo P, Foschino Barbaro MP, Bortolotto C, et al. The Role of Antifibrotic Therapy in Pulmonary Fibrosis and Lung Cancer: A Multicenter Retrospective Analysis. Biomedicines. 2025; 13(9):2310. https://doi.org/10.3390/biomedicines13092310
Chicago/Turabian StyleBertuccio, Francesco Rocco, Nicola Baio, Fabio Perrotta, Donato Lacedonia, Vito D’Agnano, Andrea Bianco, Giulia Scioscia, Pasquale Tondo, Maria Pia Foschino Barbaro, Chandra Bortolotto, and et al. 2025. "The Role of Antifibrotic Therapy in Pulmonary Fibrosis and Lung Cancer: A Multicenter Retrospective Analysis" Biomedicines 13, no. 9: 2310. https://doi.org/10.3390/biomedicines13092310
APA StyleBertuccio, F. R., Baio, N., Perrotta, F., Lacedonia, D., D’Agnano, V., Bianco, A., Scioscia, G., Tondo, P., Foschino Barbaro, M. P., Bortolotto, C., Corsico, A. G., & Stella, G. M. (2025). The Role of Antifibrotic Therapy in Pulmonary Fibrosis and Lung Cancer: A Multicenter Retrospective Analysis. Biomedicines, 13(9), 2310. https://doi.org/10.3390/biomedicines13092310








