High PEEP Increases Airway Dead Space and Decreases Alveolar Ventilation: A New Technique for Volumetric Capnography
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
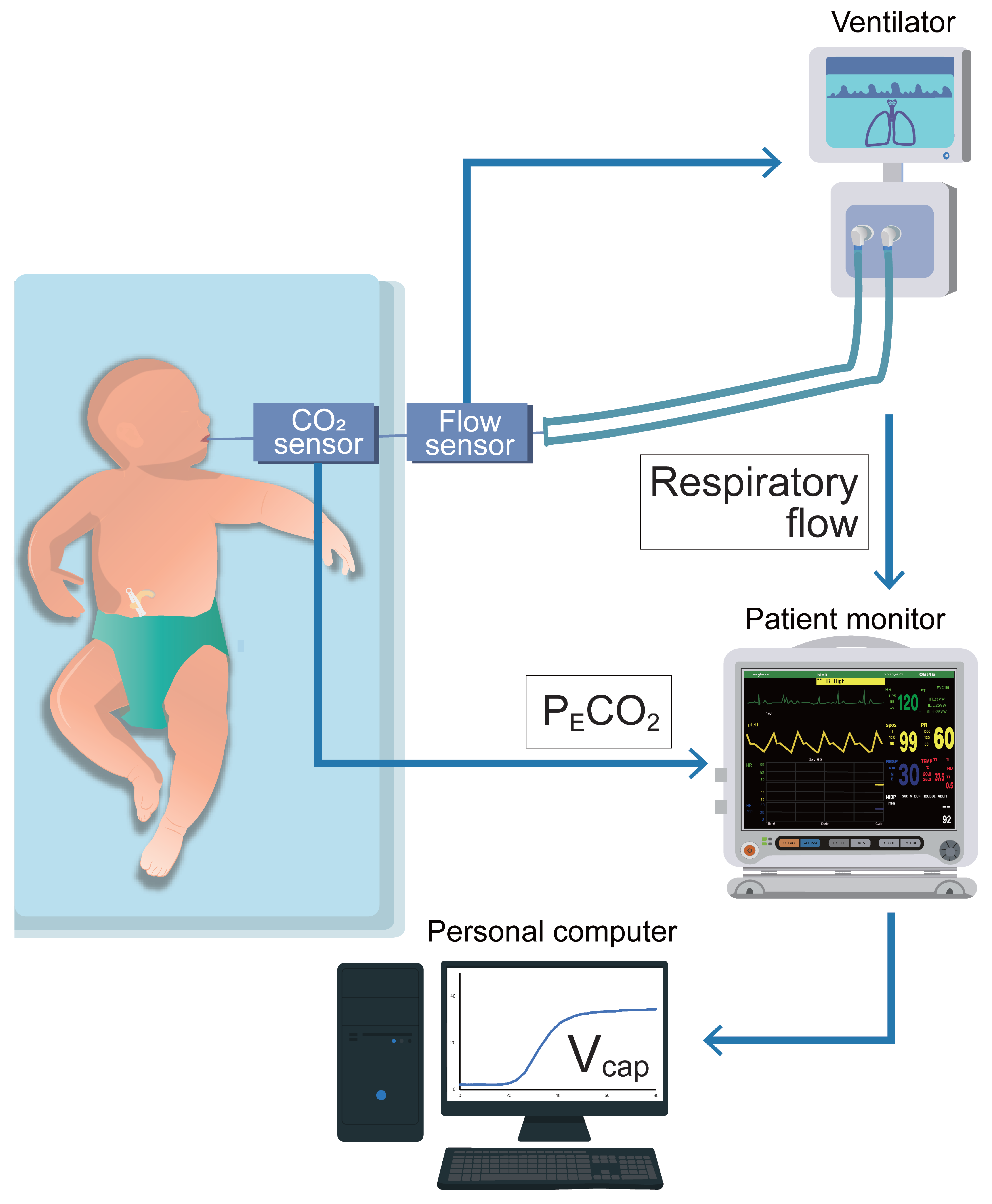
2.2. Methodology of Vcap,PM
2.3. Examination for Validity of Vcap,PM (Analysis 1)
2.4. Impact of PEEP on Term and Preterm Newborns (Analysis 2)
2.5. Statistical Analyses
3. Results
3.1. Analysis 1
3.2. Analysis 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CO2 | Carbon dioxide |
| FIO2 | Fraction of inspired oxygen |
| IQR | Interquartile range |
| KPIV | Capnographic index |
| NICU | Neonatal intensive care unit |
| PECO2 | Partial pressure expiratory CO2 |
| PEEP | Positive end-expiratory pressure |
| PICU | Pediatric ICU |
| SII | Capnographic slope of phase II |
| SIII | Capnographic slope of phase III |
| VILI | Ventilator-induced lung injury |
| Vcap | Volumetric capnography |
| Vcap,PM | Vcap based on the patient monitor |
| Vd,alv | Alveolar dead space |
| Vd,aw | Airway dead space volume |
| Vd,app | Apparatus dead space volume |
| Vd,Fowler | Fowler dead space volume |
| VT | tidal volume |
| VT,E | Expired tidal volume |
References
- Thekkeveedu, R.K.; El-Saie, A.; Prakash, V.; Katakam, L.; Shivanna, B. Ventilation-induced lung injury (VILI) in neonates: Evidence-based concepts and lung-protective strategies. J. Clin. Med. 2022, 11, 557. [Google Scholar] [CrossRef]
- Keszler, M. Mechanical ventilation strategies. Semin. Fetal. Neonatal. Med. 2017, 22, 267–274. [Google Scholar] [CrossRef]
- Rouby, J.J.; Ferrari, F.; Bouhemad, B.; Lu, Q. Positive end-expiratory pressure in acute respiratory distress syndrome: Should the ‘open lung strategy’ be replaced by a ‘protective lung strategy’? Crit. Care 2007, 11, 180. [Google Scholar] [CrossRef]
- Brochard, L. New goals for positive end-expiratory pressure in acute respiratory distress syndrome: A paradigm shift or the end of an area of uncertainty? Am. J. Respir. Crit. Care Med. 2010, 181, 528–530. [Google Scholar] [CrossRef]
- Terragni, P.P.; Rosboch, G.; Tealdi, A.; Corno, E.; Menaldo, E.; Davini, O.; Gandini, G.; Herrmann, P.; Mascia, L.; Quintel, M.; et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2007, 175, 160–666. [Google Scholar] [CrossRef]
- Vieillard-Baron, A.; Price, L.C.; Matthay, M.A. Acute cor pulmonale in ARDS. Intensive Care Med. 2013, 39, 1836–1838. [Google Scholar] [CrossRef] [PubMed]
- Bamat, N.; Fierro, J.; Wang, Y.; Millar, D.; Kirpalani, H. Positive end-expiratory pressure for preterm infants requiring conventional mechanical ventilation for respiratory distress syndrome or bronchopulmonary dysplasia. Cochrane Database Syst. Rev. 2019, 2, CD004500. [Google Scholar] [CrossRef] [PubMed]
- Verscheure, S.; Massion, P.B.; Verschuren, F.; Damas, P.; Magder, S. Volumetric capnography: Lessons from the past and current clinical applications. Crit. Care 2016, 20, 184. [Google Scholar] [CrossRef] [PubMed]
- Blankman, P.; Shono, A.; Hermans, B.J.; Wesselius, T.; Hasan, D.; Gommers, D. Detection of optimal PEEP for equal distribution of tidal volume by volumetric capnography and electrical impedance tomography during decreasing levels of PEEP in post cardiac-surgery patients. Br. J. Anaesth. 2016, 116, 862–869. [Google Scholar] [CrossRef]
- Tusman, G.; Gogniat, E.; Madorno, M.; Otero, P.; Dianti, J.; Ceballos, I.F.; Ceballos, M.; Verdier, N.; Böhm, S.H.; Rodriguez, P.O. Effect of PEEP on dead space in an Experimental model of ARDS. Respir. Care 2020, 65, 11–20. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Y.; Tang, R.; Chen, Q.; Hui, X.; Li, Y.; Yu, Q.; Zhao, H.; Qiu, H. Optimization of positive end-expiratory pressure by volumetric capnography variables in lavage-induced acute lung injury. Respiration 2014, 87, 75–83. [Google Scholar] [CrossRef]
- Schmalisch, G. Current methodological and technical limitations of time and volumetric capnography in newborns. Biomed. Eng. OnLine 2016, 15, 104. [Google Scholar] [CrossRef]
- Neumann, R.P.; Pillow, J.J.; Thamrin, C.; Larcombe, A.N.; Hall, G.L.; Schulzke, S.M. Influence of gestational age on dead space and alveolar ventilation in preterm infants ventilated with volume guarantee. Neonatology 2015, 107, 43–49. [Google Scholar] [CrossRef]
- Zuiki, M.; Yamano, A.; Kitamura, K.; Goda, T.; Oya, S.; Komatsu, H. Ventilated Infants Have Increased Dead Space and Lower alveolar Tidal Volumes during the Early versus Recovery Phase of Respiratory Distress. Neonatology 2020, 117, 189–192. [Google Scholar] [CrossRef]
- Zuiki, M.; Naito, Y.; Kitamura, K.; Tsurukawa, S.; Matsumura, U.; Kanayama, T.; Komatsu, H. Reduction in minute alveolar ventilation causes hypercapnia in ventilated neonates with respiratory distress. Eur. J. Pediatr. 2021, 180, 241–246. [Google Scholar] [CrossRef]
- Zuiki, M.; Kume, R.; Matsuura, A.; Mitsuno, K.; Kitamura, K.; Kanayama, T.; Komatsu, H. Large difference between Enghoff and Bohr dead space in ventilated infants with hypoxemic respiratory failure. Pediatr. Pulmonol. 2021, 56, 2102–2107. [Google Scholar] [CrossRef]
- Fowler, W.S. Lung function studies; the respiratory dead space. Am. J. Physiol. 1948, 154, 405–416. [Google Scholar] [CrossRef]
- Strömberg, N.O.; Gustafsson, P.M. Ventilation inhomogeneity assessed by nitrogen washout and ventilation-perfusion mismatch by capnography in stable and induced airway obstruction. Pediatr. Pulmonol. 2000, 29, 94–102. [Google Scholar] [CrossRef]
- Dassios, T.; Dixon, P.; Hickey, A.; Fouzas, S.; Greenough, A. Physiological and anatomical dead space in mechanically ventilated newborn infants. Pediatr. Pulmonol. 2018, 53, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, U.; Wauer, R.R.; Schmalisch, G. Comparison of different methods for dead space measurements in ventilated newborns using CO2-volume plot. Intensive Care Med 1999, 25, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Mereness, J.A.; Mariani, T.J. The critical role of collagen VI in lung development and chronic lung disease. Matrix Biol. Plus 2021, 10, 100058. [Google Scholar] [CrossRef] [PubMed]
- Bush, D.; Juliano, C.; Bowler, S.; Tiozzo, C. Development and disorders of the airway in bronchopulmonary dysplasia. Children 2023, 10, 1127. [Google Scholar] [CrossRef] [PubMed]
- Schittny, J.C. Development of the lung. Cell Tissue Res 2017, 367, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Krauss, B.; Deykin, A.; Lam, A.; Ryoo, J.J.; Hampton, D.R.; Schmitt, P.W.; Falk, J.L. Capnogram shape in obstructive lung disease. Anesth. Analg. 2005, 100, 884–888. [Google Scholar] [CrossRef]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European consensus guidelines on the management of respiratory distress syndrome-2019 update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef]
- Sahetya, S.K.; Goligher, E.C.; Brower, R.G. Fifty years of research in ARDS. Setting positive end-expiratory pressure in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1429–1438. [Google Scholar] [CrossRef]
- Vedrenne-Cloquet, M.; Khirani, S.; Khemani, R.; Lesage, F.; Oualha, M.; Renolleau, S.; Chiumello, D.; Demoule, A.; Fauroux, B. Pleural and transpulmonary pressures to tailor protective ventilation in children. Thorax 2023, 78, 97–105. [Google Scholar] [CrossRef]
- Pandey, M.; Gupta, D.; Gupta, N.; Sachdev, A. Use of transpulmonary pressure monitoring in the management of extrapulmonary pediatric acute respiratory distress syndrome with multi organ dysfunction syndrome (MODS): Are we Peepophobic? Clin. Med. Insights Case Rep. 2019, 12, 1179547619842183. [Google Scholar] [CrossRef]
- Gleich, S.J.; Schiltz, B.M.; Ouellette, Y.; Baker, J.E.; Aganga, D.O. Improvement in oxygenation utilizing transpulmonary pressure monitoring for optimal positive end-expiratory pressure in pediatric acute respiratory distress syndrome: A case report. A A Pract. 2019, 13, 114–117. [Google Scholar] [CrossRef]
- Ren, H.; Xie, L.; Wang, Z.; Tang, X.; Ning, B.; Teng, T.; Qian, J.; Wang, Y.; Fu, L.; Zhao, Z.; et al. Comparison of global and regional compliance-guided positive end-expiratory pressure titration on regional lung ventilation in moderate-to-severe pediatric acute respiratory distress syndrome. Front. Med. 2022, 9, 805680. [Google Scholar] [CrossRef]
- Pugh, C.P.; Ali, S.; Agarwal, A.; Matlock, D.N.; Sharma, M. Dynamic computed tomography for evaluation of tracheobronchomalacia in premature infants with bronchopulmonary dysplasia. Pediatr. Pulmonol. 2023, 58, 3255–3263. [Google Scholar] [CrossRef]
- Hysinger, E.; Friedman, N.; Jensen, E.; Zhang, H.; Piccione, J. Bronchoscopy in neonates with severe bronchopulmonary dysplasia in the NICU. J. Perinatol. 2019, 39, 263–268. [Google Scholar] [CrossRef]
- Dellacà, R.L.; Veneroni, C.; Vendettuoli, V.; Zannin, E.; Matassa, P.G.; Pedotti, A.; Pedotti, A.; Colnaghi, M.; Mosca, F. Relationship between respiratory impedance and positive end-expiratory pressure in mechanically ventilated neonates. Intensiv. Care Med. 2013, 39, 511–519. [Google Scholar] [CrossRef]



| Parameter | Clinical Data of the Study Population (n = 31) |
|---|---|
| Age, months | 9 (0–36) |
| Neonates, n (%) | 8 (26) |
| Infants, n (%) | 11 (35) |
| Children, n (%) | 12 (39) |
| Weight, kg | 6.0 (3.8–10.5) |
| Male/Female, n | 12/19 |
| Reason for intubation | |
| Asphyxia or encephalopathy, n (%) | 17 (55) |
| Respiratory failure, n (%) | 7 (23) |
| Operation, n (%) | 7 (23) |
| Ventilator settings | |
| FIO2 | 0.21 (0.21–0.35) |
| PIP, cmH2O | 17.6 ± 2.7 |
| PEEP, cmH2O | 5.9 ± 1.5 |
| RR, /min | 28 ± 7 |
| MAP, cmH2O | 9.0 ± 2.3 |
| VT, mL/kg | 9.8 ± 2.5 |
| Parameter | Term Infants (n = 28) | Preterm Infants (n = 21) |
|---|---|---|
| Gestational age, weeks | 38 (38–40) | 33 (31–34) |
| Birth weight, g | 2924 (2725–3109) | 1918 (1356–2186) |
| Male/Female, n | 12/16 | 8/13 |
| Cesarean section, n (%) | 20 (71) | 18 (86) |
| Twin birth, n (%) | 4 (14) | 4 (19) |
| Apgar score at 1 min | 5 (1–8) | 5 (4–6) |
| Apgar score at 5 min | 6 (4–9) | 7 (6–8) |
| Postnatal surfactant, n (%) | 4 (14) | 10 (48) |
| Days of measurements, days | 2 (1–4) | 4 (3–5) |
| Ventilator settings at baseline | ||
| FIO2 | 0.22 ± 0.01 | 0.23 ± 0.02 |
| PIP, cmH2O | 11.8 ± 1.4 | 12.9 ± 1.3 |
| PEEP, cmH2O | 5.4 ± 0.6 | 5.4 ± 0.7 |
| RR, /min | 38 ± 4 | 41 ± 6 |
| MAP, cmH2O | 6.7 ± 0.8 | 7.2 ± 0.9 |
| Parameter | Term (n = 28) | Preterm (n = 21) | ||||
|---|---|---|---|---|---|---|
| Mild | Moderate | High | Mild | Moderate | High | |
| PEEP, cmH2O | 5.0 ± 0 ††† | 7.0 ± 0 ††† | 9.8 ± 0.4 | 5.0 ± 0 ††† | 7.0 ± 0 ††† | 9.6 ± 0.5 |
| PIP, cmH2O | 11.4 ± 1.0 ††† | 15.3 ± 1.2 ††† | 22.1 ± 1.8 | 12.4 ± 0.9 ††† | 15.4 ± 0.8 ††† | 22.5 ± 1.8 |
| MAP, cmH2O | 6.4 ± 0.3 ††† | 8.8 ± 0.4 ††† | 12.6 ± 0.5 | 6.8 ± 0.3 ††† | 9.0 ± 0.4 ††† | 13.0 ± 0.5 |
| FIO2 | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.25 ± 0.01 | 0.25 ± 0.01 | 0.25 ± 0.01 |
| SpO2, % | 98.8 ± 1.4 | 98.7 ± 1.5 | 98.5 ± 1.4 | 96.7 ± 1.8 | 96.8 ± 1.8 | 96.9 ± 1.7 |
| VT/kg, mL/kg | 6.5 (5.5–6.6) | 6.2 (5.4–6.6) | 6.5 (5.5–6.7) | 6.1 (5.6–6.8) | 6.3 (5.6–6.5) | 6.2 (5.4–6.5) |
| Vd,aw, mL/kg | 2.0 (1.8–2.2) ††† | 2.1 (1.8–2.4) ††† | 2.4 (2.2–2.7) | 2.6 (2.2–2.8) ††† | 2.8 (2.4–3.1) ††† | 3.1 (2.5–3.4) |
| Vd,aw/VT | 0.34 (0.27–0.39) ††† | 0.36 (0.30–0.42) ††† | 0.40 (0.34–0.46) | 0.40 (0.36–0.44) ††† | 0.42 (0.39–0.45) ††† | 0.48 (0.44–0.50) |
| VA, mL/kg | 3.6 (3.2–4.2) †† | 3.4 (3.1–3.9) | 3.0 (2.7–3.8) | 3.1 (2.7–3.7) ††† | 2.9 (2.6–3.6) ††† | 2.6 (2.2–3.0) |
| VA/VT | 0.59 (0.53–0.64) ††† | 0.56 (0.50–0.62) †† | 0.52 (0.46–0.58) | 0.50 (0.46–0.55) †††† | 0.49 (0.45–0.52) ††† | 0.43 (0.40–0.48) |
| SII, mmHg/mL | 8.4 (6.4–9.5) | 9.3 (6.1–10.5) | 9.2 (6.6–10.6) | 12.5 (9.8–15.1) | 13.1 (10.3–18.2) | 13.0 (10.5–18.3) |
| SnII, mmHg | 150 (123–173) | 158 (124–174) | 166 (139–194) | 135 (117–161) | 146 (138–174) | 156 (138–174) |
| SIII, mmHg/mL | 0.17 (0.12–0.26) | 0.18 (0.08–0.26) | 0.2 (0.11–0.33) | 0.37 (0.18–0.68) | 0.35 (0.20–0.71) | 0.22 (0.09–0.44) |
| SnIII, mmHg | 3.9 (1.9–4.5) | 3.2 (1.6–4.1) | 3.6 (2.2–5.0) | 4.7 (2.7–8.8) † | 4.2 (2.7–9.8) | 2.3 (1.5–3.7) |
| KPlV | 24 (12–35) | 20 (11–30) | 22 (11–33) | 35 (17–54) † | 28 (17–58) | 16 (9–26) |
| Parameter | Term (n = 28) | Preterm (n = 21) | ||||
|---|---|---|---|---|---|---|
| High | Moderate | Mild | High | Moderate | Mild | |
| PEEP, cmH2O | 9.8 ± 0.4 | 7.0 ± 0 ††† | 5.0 ± 0 ††† | 9.6 ± 0.5 | 7.0 ± 0 ††† | 5.0 ± 0 ††† |
| PIP, cmH2O | 22.0 ± 1.6 | 15.3 ± 1.0 ††† | 11.4 ± 0.9 ††† | 22.4 ± 1.7 | 15.2 ± 0.7 ††† | 12.2 ± 0.8 ††† |
| MAP, cmH2O | 12.7 ± 0.5 | 8.9 ± 0.4 ††† | 6.4 ± 0.3 ††† | 13.0 ± 0.5 | 9.0 ± 0.3 ††† | 6.7 ± 0.3 ††† |
| FIO2 | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.23 ± 0.01 | 0.23 ± 0.01 | 0.23 ± 0.01 |
| SpO2, % | 99.0 ± 1.3 | 98.7 ± 1.6 | 98.7 ± 1.6 | 98.1 ± 2.0 | 97.7 ± 2.0 | 97.1 ± 2.0 |
| VT/kg, mL/kg | 6.5 (5.5–6.6) | 6.5 (5.5–6.8) | 6.5 (5.6–6.9) | 6.2 (5.7–6.9) | 6.9 (6.3–7.5) | 6.1 (5.5–7.4) |
| Vd,aw, mL/kg | 2.4 (2.2–2.4) | 2.1 (1.9–2.3) ††† | 2.0 (1.8–2.2) ††† | 3.2 (2.5–3.5) | 2.6 (2.3–3.0) ††† | 2.5 (2.1–2.7) ††† |
| Vd,aw/VT | 0.40 (0.34–0.46) | 0.36 (0.31–0.39) ††† | 0.33 (0.29–0.37) ††† | 0.48 (0.44–0.5) | 0.39 (0.33–0.44) ††† | 0.39 (0.34–0.42) ††† |
| VA, mL/kg | 2.9 (2.6–3.7) | 3.1 (2.9–3.9) | 3.4 (2.9–4.1) † | 2.6 (2.2–3.0) | 3.5 (2.7–3.7) ††† | 3.3 (2.8–4.0) ††† |
| VA/VT | 0.52 (0.46–0.58) | 0.55 (0.52–0.60) †† | 0.56 (0.51–0.62) ††† | 0.42 (0.40–0.47) | 0.52 (0.47–0.57) ††† | 0.52 (0.48–0.57) ††† |
| SII, mmHg/mL | 9.2 (6.6–10.8) | 8.6 (6.1–10.3) | 8.5 (7.0–9.2) | 13.0 (10.4–18.3) | 13.5 (9.5–15.7) | 12.8 (9.3–15.4) |
| SnII, mmHg | 166 (139–193) | 155 (124–176) | 152 (125–164) | 156 (138–174) | 162 (130–191) | 137 (125–164) |
| SIII, mmHg/mL | 0.2 (0.11–0.32) | 0.15 (0.09–0.19) | 0.17 (0.06–0.29) | 0.22 (0.09–0.44) | 0.28 (0.12–0.62) | 0.42 (0.25–0.77) |
| SnIII, mmHg | 3.6 (2.2–4.9) | 3.0 (1.7–4.0) | 3.5 (1.1–5.2) | 2.3 (1.5–3.7) | 4.3 (1.7–7.1) | 4.7 (3.1–10.1) † |
| KPlV | 22 (11–34) | 20 (11–23) | 24 (7–34) | 16 (9–27) | 20 (10–59) | 33 (23–58) † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuiki, M.; Watanabe, K.; Iwata, N.; Mitsuno, R.; Konishi, M.; Yamano, A.; Ichise, E.; Morimoto, H.; Hashiguchi, K.; Hasegawa, T.; et al. High PEEP Increases Airway Dead Space and Decreases Alveolar Ventilation: A New Technique for Volumetric Capnography. Biomedicines 2025, 13, 2275. https://doi.org/10.3390/biomedicines13092275
Zuiki M, Watanabe K, Iwata N, Mitsuno R, Konishi M, Yamano A, Ichise E, Morimoto H, Hashiguchi K, Hasegawa T, et al. High PEEP Increases Airway Dead Space and Decreases Alveolar Ventilation: A New Technique for Volumetric Capnography. Biomedicines. 2025; 13(9):2275. https://doi.org/10.3390/biomedicines13092275
Chicago/Turabian StyleZuiki, Masashi, Kazunori Watanabe, Norihiro Iwata, Rika Mitsuno, Madoka Konishi, Akio Yamano, Eisuke Ichise, Hidechika Morimoto, Kanae Hashiguchi, Tatsuji Hasegawa, and et al. 2025. "High PEEP Increases Airway Dead Space and Decreases Alveolar Ventilation: A New Technique for Volumetric Capnography" Biomedicines 13, no. 9: 2275. https://doi.org/10.3390/biomedicines13092275
APA StyleZuiki, M., Watanabe, K., Iwata, N., Mitsuno, R., Konishi, M., Yamano, A., Ichise, E., Morimoto, H., Hashiguchi, K., Hasegawa, T., & Iehara, T. (2025). High PEEP Increases Airway Dead Space and Decreases Alveolar Ventilation: A New Technique for Volumetric Capnography. Biomedicines, 13(9), 2275. https://doi.org/10.3390/biomedicines13092275





