Decoding TRIP13’s Role in Gastric Cancer: Implications for Prognosis and Immune Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of TRIP13 mRNA Expression
2.2. GC Specimens and Immunohistochemistry (IHC) Staining
2.3. Survival Prognosis Assessment
2.4. Function and Pathway Analysis
2.5. Immune Correlation Analysis
2.6. Statistical Analysis
3. Results
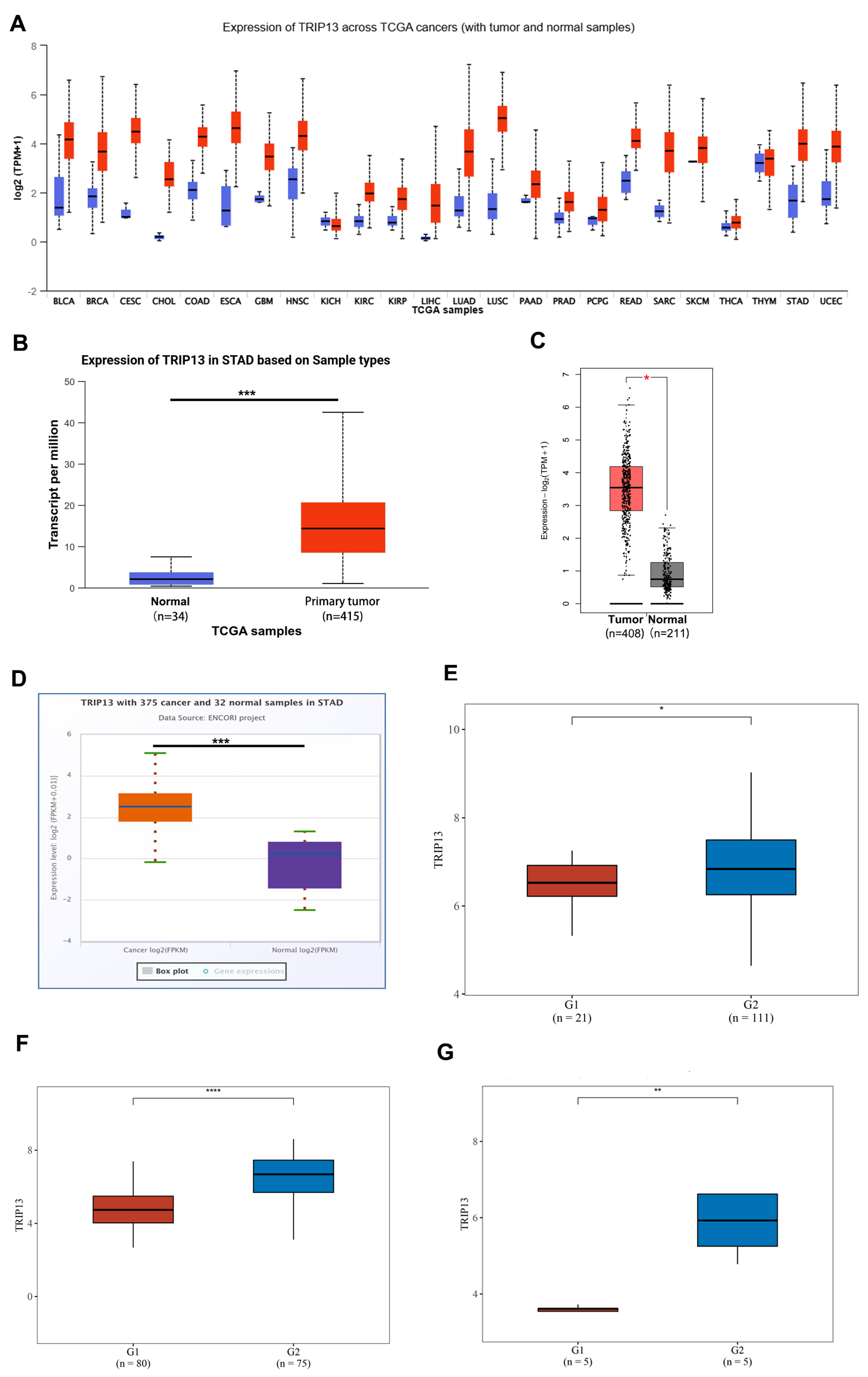
3.1. Transcriptional Levels of TRIP13 in GC Tissues
3.2. Elevated TRIP13 Expression Correlates with Clinical Pathological Characteristics and Survival in GC Patients
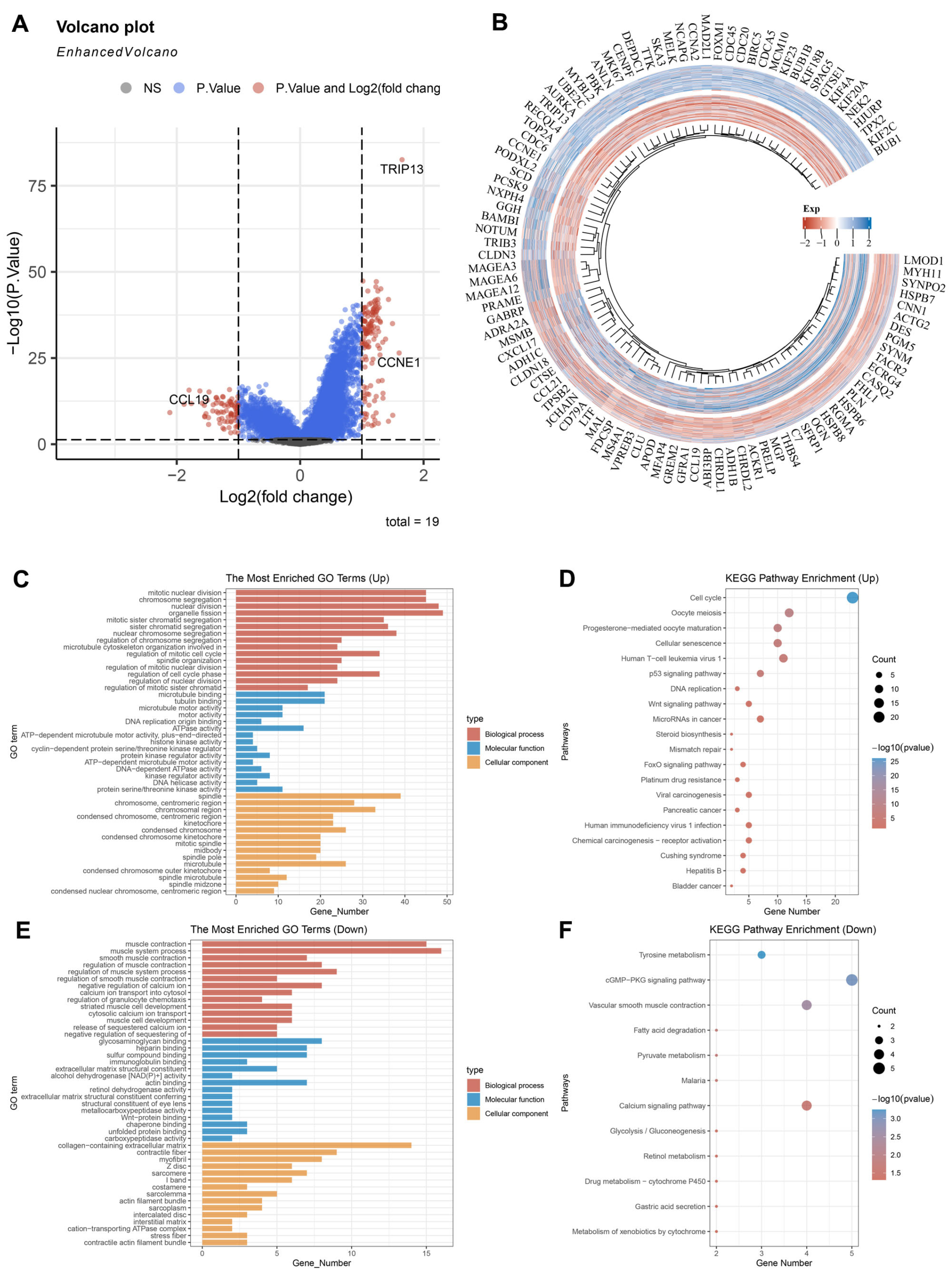
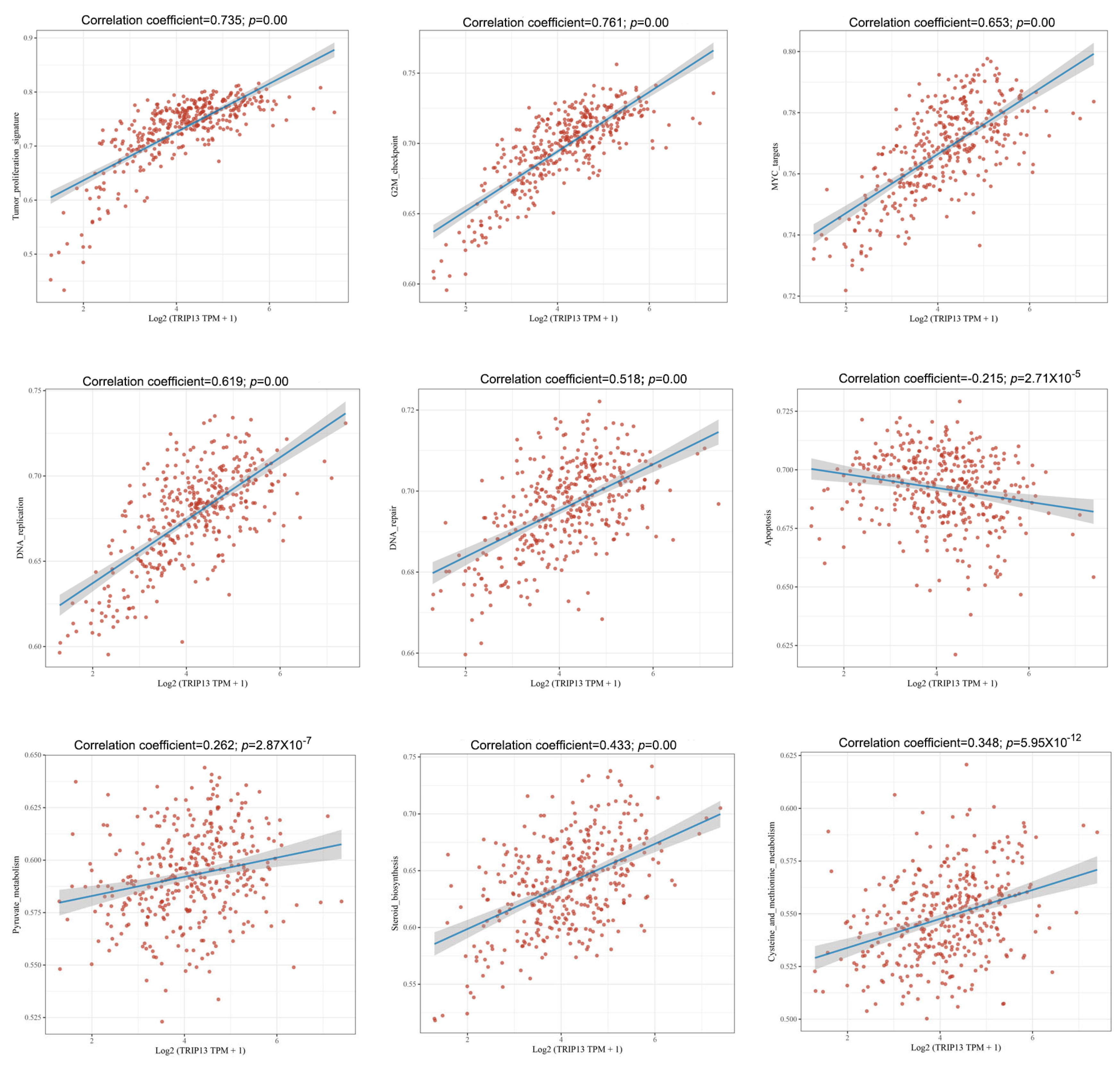
3.3. The Potential Biological Role of TRIP13 in GC
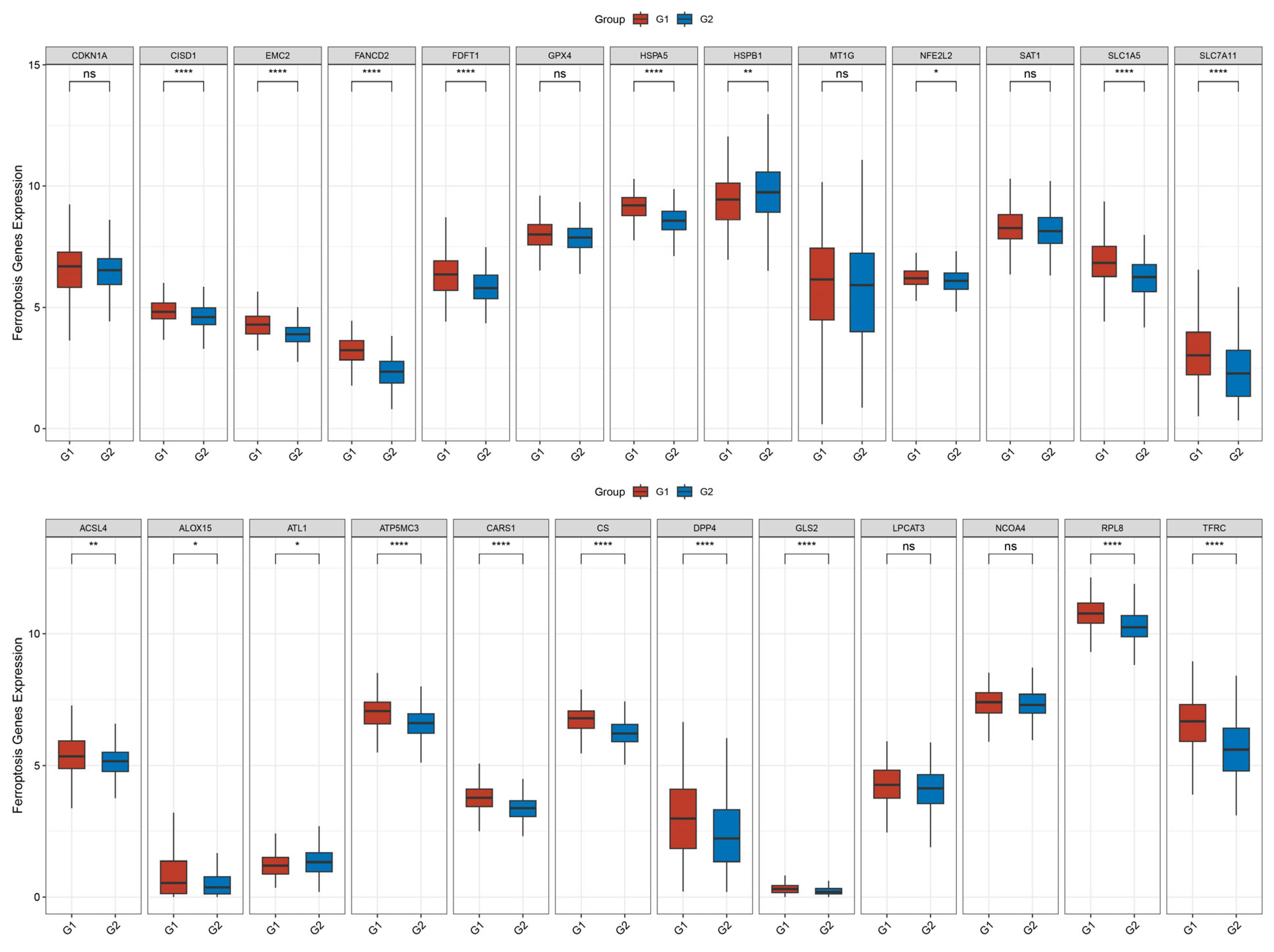
3.4. Relationship Between TRIP13 Expression and Ferroptosis
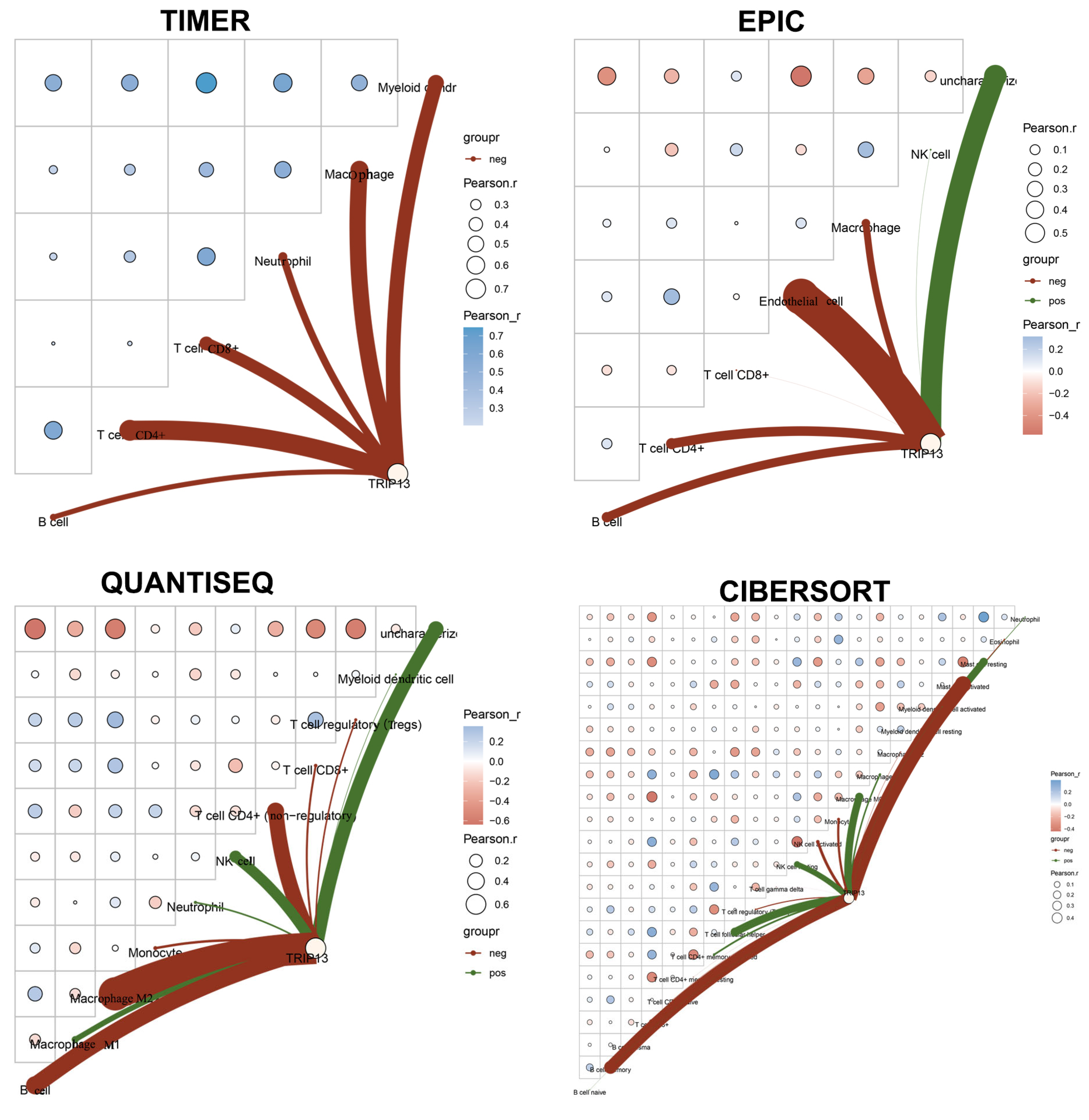
3.5. The Association Between TRIP13 Expression and Infiltrating Immune Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GC | gastric cancer |
| TRIP13 | thyroid hormone receptor-interacting protein 13 |
| TMA | tissue microarray |
| IHC | immunohistochemistry |
| NAT | non-cancerous adjacent tissues |
| LN | lymph node |
| DEGs | differentially expressed genes |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
References
- Chen, Y.C.; Malfertheiner, P.; Yu, H.T.; Kuo, C.L.; Chang, Y.Y.; Meng, F.T.; Wu, Y.X.; Hsiao, J.L.; Chen, M.J.; Lin, K.P.; et al. Global Prevalence of Helicobacter pylori Infection and Incidence of Gastric Cancer Between 1980 and 2022. Gastroenterology 2024, 166, 605–619. [Google Scholar] [CrossRef]
- Ilic, M.; Ilic, I. Epidemiology of stomach cancer. World J. Gastroenterol. 2022, 28, 1187–1203. [Google Scholar] [CrossRef]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef]
- Ramachandran, R.; Grantham, T.; Parvataneni, S.; Budh, D.; Gollapalli, S.; Reddy, M.; Gaduputi, V. Gastric Cancer: Clinical Features, Screening, Diagnosis, Treatment, and Prevention. J. Community Hosp. Intern. Med. Perspect. 2024, 14, 49–57. [Google Scholar] [CrossRef]
- Jing, S.; Zhao, L.; Zhao, L.; Gao, Y.J.; He, T. TRIP13: A promising cancer immunotherapy target. Cancer Innov. 2024, 3, e147. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, R.; Wang, B.; Yan, Q.; Zhao, P.; Zhang, J.; Su, W.; Yang, L.; Cui, H. TRIP13 regulates progression of gastric cancer through stabilising the expression of DDX21. Cell Death Dis. 2024, 15, 622. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, M.; Zhang, X.; Rong, X.; Xu, H. TRIP13 overexpression promotes gefitinib resistance in non-small cell lung cancer via regulating autophagy and phosphorylation of the EGFR signaling pathway. Oncol. Rep. 2023, 49, 84. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, H.; Zhu, J.; Yang, K.; Zhang, G.; Gu, Y.; Shi, T.; Chen, W. Gastric cancer derived exosomal THBS1 enhanced Vgamma9Vdelta2 T-cell function through activating RIG-I-like receptor signaling pathway in a N6-methyladenosine methylation dependent manner. Cancer Lett. 2023, 576, 216410. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Y.; Xia, L.; Wang, J.; Zhu, J.; Li, J.; Wang, K.; Shen, K.; Zhang, D.; Zhang, G.; et al. TRIM69 suppressed the anoikis resistance and metastasis of gastric cancer through ubiquitin-proteasome-mediated degradation of PRKCD. Oncogene 2023, 42, 3619–3632. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar]
- Wei, J.; Huang, K.; Chen, Z.; Hu, M.; Bai, Y.; Lin, S.; Du, H. Characterization of Glycolysis-Associated Molecules in the Tumor Microenvironment Revealed by Pan-Cancer Tissues and Lung Cancer Single Cell Data. Cancers 2020, 12, 1788. [Google Scholar] [CrossRef]
- Hanzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Xiao, Z.; Dai, Z.; Locasale, J.W. Metabolic landscape of the tumor microenvironment at single cell resolution. Nat. Commun. 2019, 10, 3763. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Q.; Zuo, Z.X.; Yuan, S.Q.; Yu, K.; Zhang, Q.; Zhang, X.; Sheng, H.; Ju, H.Q.; Cheng, H.; et al. Systematic Analysis of the Aberrances and Functional Implications of Ferroptosis in Cancer. iScience 2020, 23, 101302. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Wu, G.; Guo, L.; Zou, X.; Huang, P. Comprehensive Analysis of the PD-L1 and Immune Infiltrates of m(6)A RNA Methylation Regulators in Head and Neck Squamous Cell Carcinoma. Mol. Ther. Nucleic Acids 2020, 21, 299–314. [Google Scholar]
- Wen, T.; Xie, P.; Yang, S.; Niu, G.; Liu, X.; Ding, Z.; Xue, C.; Liu, Y.X.; Shen, Q.; Yuan, J. ggClusterNet: An R package for microbiome network analysis and modularity-based multiple network layouts. Imeta 2022, 1, e32. [Google Scholar] [PubMed]
- Hoadley, K.A.; Yau, C.; Wolf, D.M.; Cherniack, A.D.; Tamborero, D.; Ng, S.; Leiserson, M.D.M.; Niu, B.; McLellan, M.D.; Uzunangelov, V.; et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell 2014, 158, 929–944. [Google Scholar] [CrossRef] [PubMed]
- Iglesia, M.D.; Parker, J.S.; Hoadley, K.A.; Serody, J.S.; Perou, C.M.; Vincent, B.G. Genomic Analysis of Immune Cell Infiltrates Across 11 Tumor Types. J. Natl. Cancer Inst. 2016, 108, djw144. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2 0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar]
- Chen, C.; Li, P.; Fan, G.; Yang, E.; Jing, S.; Shi, Y.; Gong, Y.; Zhang, L.; Wang, Z. Role of TRIP13 in human cancer development. Mol. Biol. Rep. 2024, 51, 1088. [Google Scholar] [CrossRef]
- Sun, Y.J.; Zhang, Q.; Cao, S.J.; Sun, X.H.; Zhang, J.C.; Zhang, B.Y.; Shang, Z.B.; Zhao, C.Y.; Cao, Z.Y.; Zhang, Q.J.; et al. Tetrahydrocurcumin targets TRIP13 inhibiting the interaction of TRIP13/USP7/c-FLIP to mediate c-FLIP ubiquitination in triple-negative breast cancer. J. Adv. Res. 2024, 75, 591–605. [Google Scholar] [CrossRef]
- Sheng, N.; Yan, L.; Wu, K.; You, W.; Gong, J.; Hu, L.; Tan, G.; Chen, H.; Wang, Z. TRIP13 promotes tumor growth and is associated with poor prognosis in colorectal cancer. Cell Death Dis. 2018, 9, 402. [Google Scholar] [CrossRef]
- Li, Z.H.; Lei, L.; Fei, L.R.; Huang, W.J.; Zheng, Y.W.; Yang, M.Q.; Wang, Z.; Liu, C.C.; Xu, H.T. TRIP13 promotes the proliferation and invasion of lung cancer cells via the Wnt signaling pathway and epithelial-mesenchymal transition. J. Mol. Histol. 2021, 52, 11–20. [Google Scholar] [CrossRef]
- Banerjee, R.; Russo, N.; Liu, M.; Basrur, V.; Bellile, E.; Palanisamy, N.; Scanlon, C.S.; van Tubergen, E.; Inglehart, R.C.; Metwally, T.; et al. TRIP13 promotes error-prone nonhomologous end joining and induces chemoresistance in head and neck cancer. Nat. Commun. 2014, 5, 4527. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Huang, J.; Tao, X.; Gao, Y.; Zhang, L.; Huang, W.; Luo, J.; Liu, C.; Deng, Y.; Liu, L.; et al. Evaluation of the TRIP13 level in breast cancer and insights into potential molecular pathways. J. Cell Mol. Med. 2022, 26, 2673–2685. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhang, X.; Li, J.; Zhao, D.; Gao, B.; Zhou, H.; Gao, S.; Zhang, L. Silencing TRIP13 inhibits cell growth and metastasis of hepatocellular carcinoma by activating of TGF-beta1/smad3. Cancer Cell Int. 2018, 18, 208. [Google Scholar] [CrossRef]
- Dong, L.; Ding, H.; Li, Y.; Xue, D.; Li, Z.; Liu, Y.; Zhang, T.; Zhou, J.; Wang, P. TRIP13 is a predictor for poor prognosis and regulates cell proliferation, migration and invasion in prostate cancer. Int. J. Biol. Macromol. 2019, 121, 200–206. [Google Scholar] [CrossRef]
- Liu, X.; Shen, X.; Zhang, J. TRIP13 exerts a cancer-promoting role in cervical cancer by enhancing Wnt/beta-catenin signaling via ACTN4. Environ. Toxicol. 2021, 36, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Behring, M.; Kim, H.G.; Chandrashekar, D.S.; Chakravarthi, B.; Gupta, N.; Bajpai, P.; Elkholy, A.; Al Diffalha, S.; Datta, P.K.; et al. TRIP13 promotes metastasis of colorectal cancer regardless of p53 and microsatellite instability status. Mol. Oncol. 2020, 14, 3007–3029. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xiao, Y.; Zhou, X.; Wang, J.; Chen, S.; Peng, T.; Zhu, X. TRIP13 interference inhibits the proliferation and metastasis of thyroid cancer cells through regulating TTC5/p53 pathway and epithelial-mesenchymal transition related genes expression. Biomed. Pharmacother. 2019, 120, 109508. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.; Wang, G.; Guo, S.; Yu, D.; Feng, Q.; Hu, K.; Chen, G.; Li, B.; Xu, Z.; et al. Aberrant activation of TRIP13-EZH2 signaling axis promotes stemness and therapy resistance in multiple myeloma. Leukemia 2023, 37, 1576–1579. [Google Scholar] [CrossRef]
- Liu, G.; Wang, H.; Ran, R.; Wang, Y.; Li, Y. TRIP13 Activates Glycolysis to Promote Cell Stemness and Strengthen Doxorubicin Resistance of Colorectal Cancer Cells. Curr. Med. Chem. 2024, 31, 3397–3411. [Google Scholar] [CrossRef]
- Gu, R.; Xia, Y.; Li, P.; Zou, D.; Lu, K.; Ren, L.; Zhang, H.; Sun, Z. Ferroptosis and its Role in Gastric Cancer. Front. Cell Dev. Biol. 2022, 10, 860344. [Google Scholar] [CrossRef]
- Le, J.; Pan, G.; Zhang, C.; Chen, Y.; Tiwari, A.K.; Qin, J.J. Targeting ferroptosis in gastric cancer: Strategies and opportunities. Immunol. Rev. 2024, 321, 228–245. [Google Scholar] [CrossRef]
- Lee, J.Y.; Nam, M.; Son, H.Y.; Hyun, K.; Jang, S.Y.; Kim, J.W.; Kim, M.W.; Jung, Y.; Jang, E.; Yoon, S.J.; et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 32433–32442. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Y.; Dong, C.; Chen, T.; Dong, A.; Ren, J.; Li, W.; Shu, G.; Yang, J.; Shen, W.; et al. CST1 inhibits ferroptosis and promotes gastric cancer metastasis by regulating GPX4 protein stability via OTUB1. Oncogene 2023, 42, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Li, H.; Lou, L.; Huang, Q.; Zhang, Z.; Mo, J.; Li, M.; Lu, J.; Zhu, K.; Chu, Y.; et al. Inhibition of STAT3-ferroptosis negative regulatory axis suppresses tumor growth and alleviates chemoresistance in gastric cancer. Redox Biol. 2022, 52, 102317. [Google Scholar] [CrossRef]
- Xue, J.; Wu, H.; Shi, Y.; Li, Z. TRIP13 overexpression in hepatocellular carcinoma: Implications for poor prognosis and immune cell infiltration. Discov. Oncol. 2023, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Li, C. TRIP13 Is a Potential Prognostic Marker and Therapeutic Target for Endometrial Cancer. Crit. Rev. Eukaryot. Gene Expr. 2025, 35, 23–41. [Google Scholar] [CrossRef]
- Li, C.; Zhou, Z.; Liu, Y.; Guo, Y.; Liu, T.; Teng, J.; Chen, P.; Hu, A.; Wu, L.; Qiao, D.; et al. Pan-cancer analysis shows that TRIP13 as a potential prognostic and immunotherapeutic biomarker for multiple cancer types including LIHC and LUAD. Medicine 2025, 104, e42588. [Google Scholar] [CrossRef]
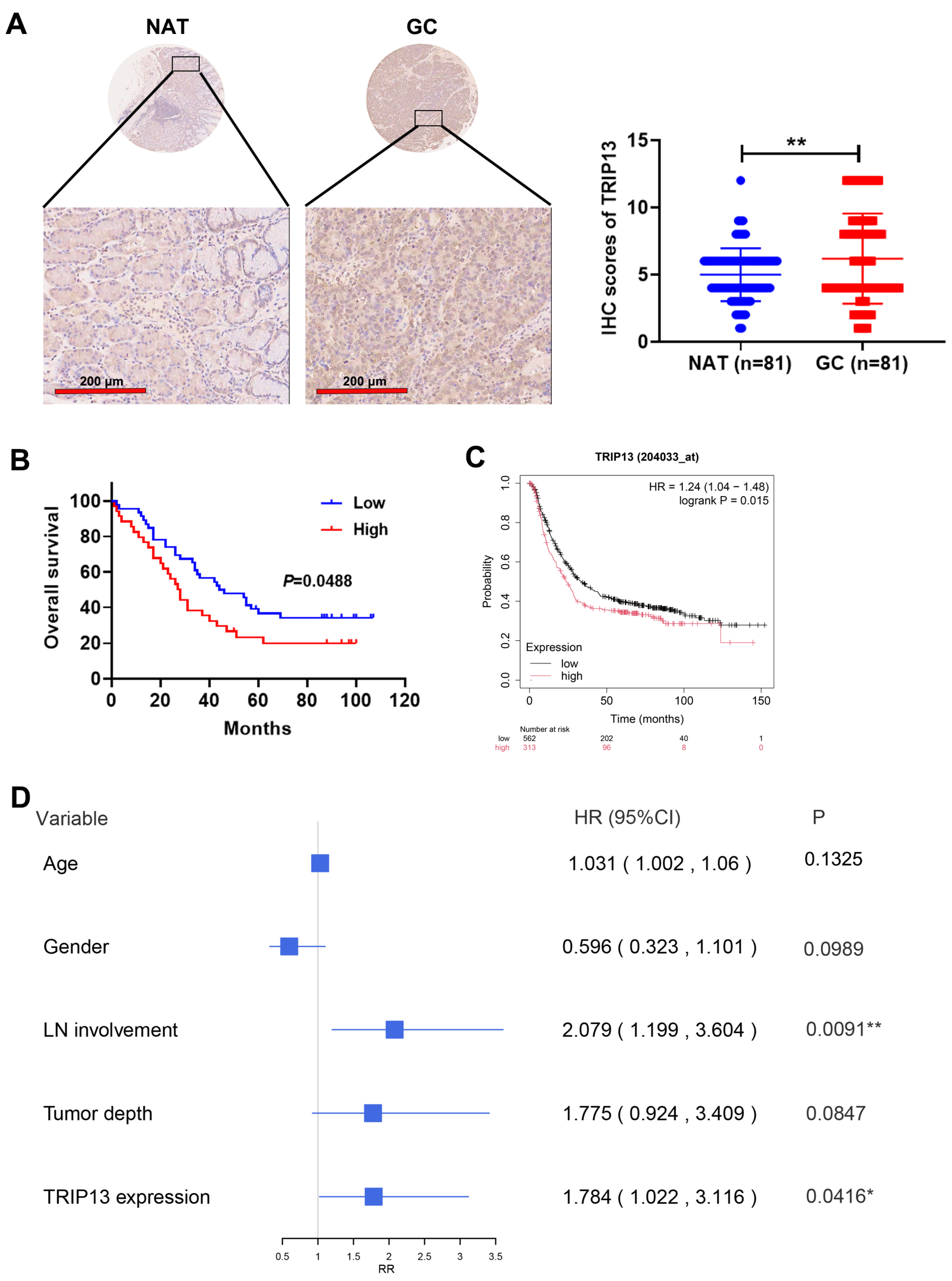

| Characteristic | Total No. | TRIP13 Expression | p Value | |
|---|---|---|---|---|
| Low | High | |||
| All cases | 81 | 46 | 35 | |
| Age | 0.5646 | |||
| ≤65 | 46 | 24 | 22 | |
| >65 | 35 | 16 | 19 | |
| Gender | 0.9103 | |||
| Male | 55 | 31 | 24 | |
| Female | 26 | 15 | 11 | |
| LN involvement | 0.8884 | |||
| N0 | 47 | 27 | 20 | |
| N1/N2 | 34 | 19 | 15 | |
| Tumor depth | 0.0253 | |||
| T1/T2 | 46 | 8 | 38 | |
| T3/T4 | 35 | 13 | 22 | |
| AJCC stage | 0.1937 | |||
| IA/IB | 4 | 7 | ||
| IIA/IIB | 42 | 28 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.; Shen, Y.; Zhao, A.; Dong, R.; Chen, F.; Xia, S. Decoding TRIP13’s Role in Gastric Cancer: Implications for Prognosis and Immune Response. Biomedicines 2025, 13, 2268. https://doi.org/10.3390/biomedicines13092268
Shi T, Shen Y, Zhao A, Dong R, Chen F, Xia S. Decoding TRIP13’s Role in Gastric Cancer: Implications for Prognosis and Immune Response. Biomedicines. 2025; 13(9):2268. https://doi.org/10.3390/biomedicines13092268
Chicago/Turabian StyleShi, Tongguo, Yu Shen, Anjing Zhao, Rufang Dong, Fan Chen, and Suhua Xia. 2025. "Decoding TRIP13’s Role in Gastric Cancer: Implications for Prognosis and Immune Response" Biomedicines 13, no. 9: 2268. https://doi.org/10.3390/biomedicines13092268
APA StyleShi, T., Shen, Y., Zhao, A., Dong, R., Chen, F., & Xia, S. (2025). Decoding TRIP13’s Role in Gastric Cancer: Implications for Prognosis and Immune Response. Biomedicines, 13(9), 2268. https://doi.org/10.3390/biomedicines13092268





