The Effect of Neurorehabilitation of the Cognitive Symptoms of Long COVID Evaluated with Neuropsi Atención y Memoria-III and BANFE-III
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Design and Participants
2.2. The Demographic Data and Neuropsychological Tests
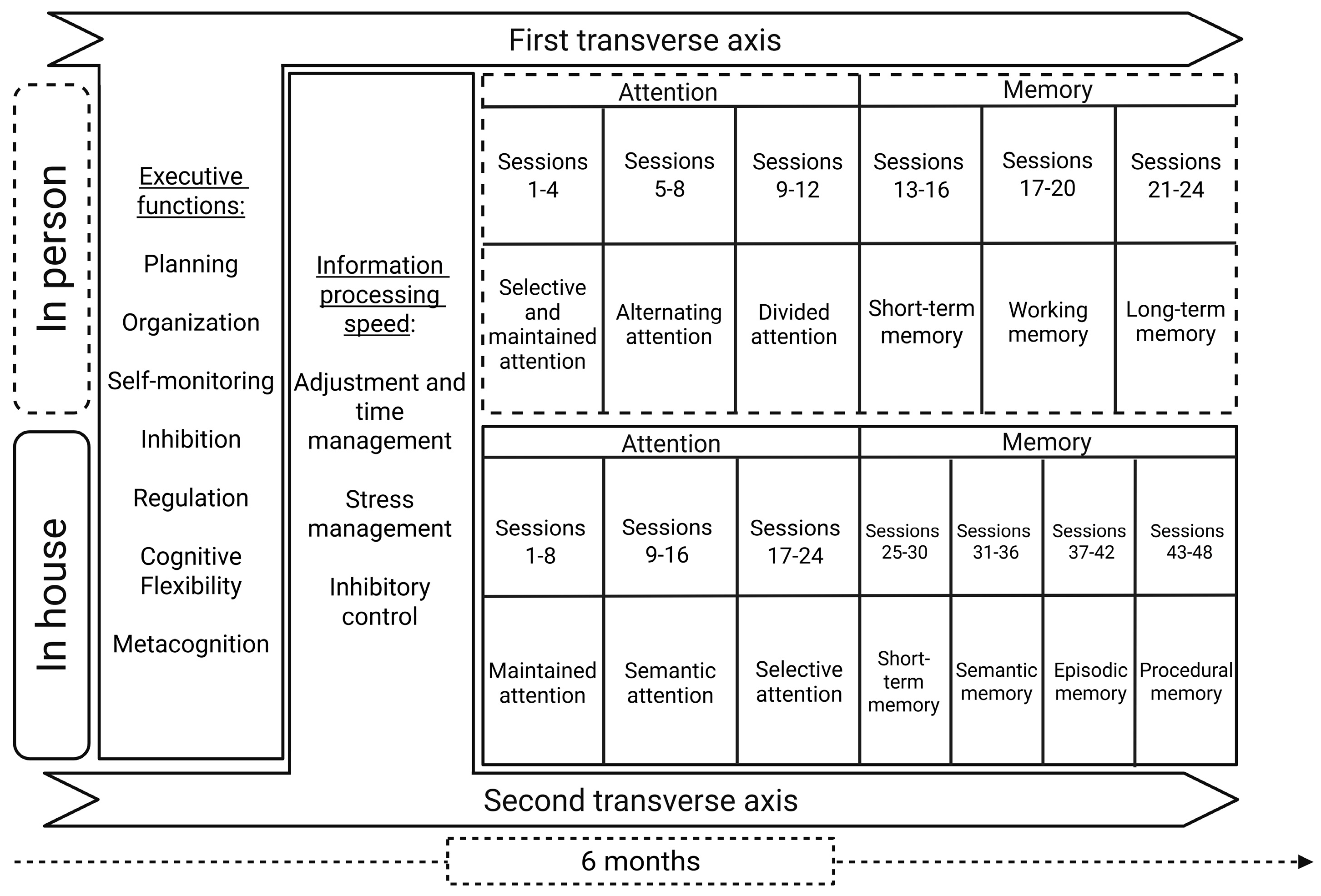
2.3. Intervention
2.4. Statistical Analysis
3. Results
3.1. Clinical and Demographic Data
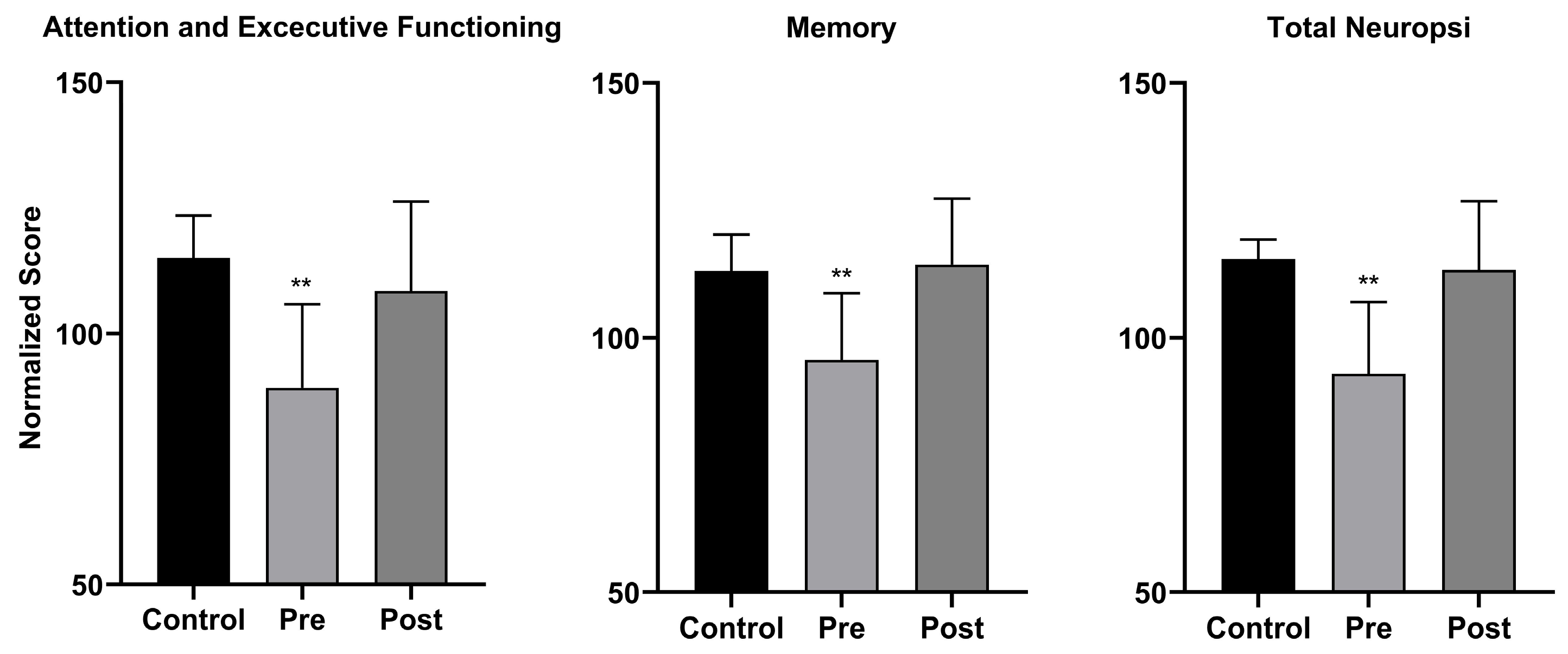
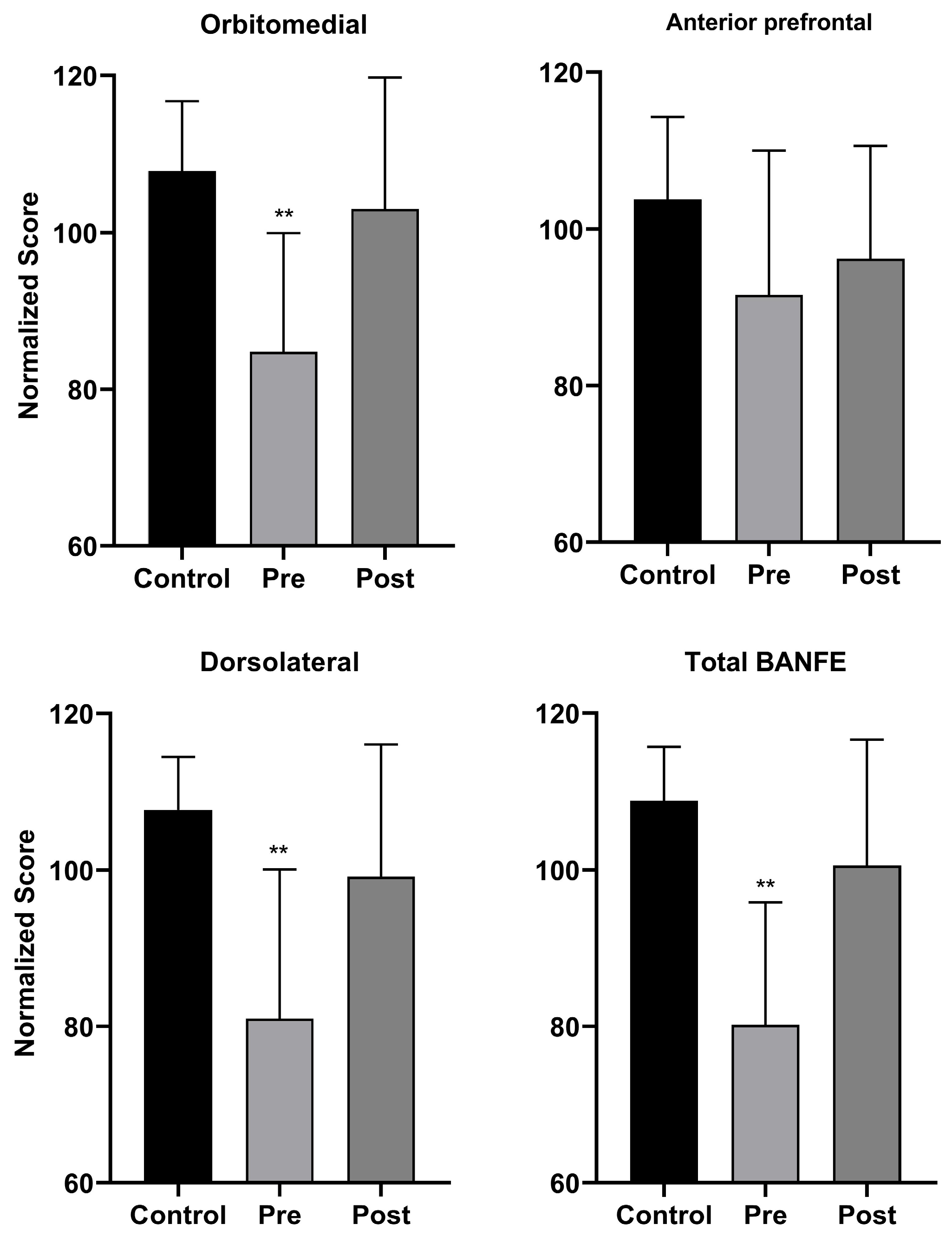
3.2. Neuropsychological Assessment Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and Predictors of Long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Hampshire, A.; Azor, A.; Atchison, C.; Trender, W.; Hellyer, P.J.; Giunchiglia, V.; Husain, M.; Cooke, G.S.; Cooper, E.; Lound, A.; et al. Cognition and Memory after COVID-19 in a Large Community Sample. N. Engl. J. Med. 2024, 390, 806–818. [Google Scholar] [CrossRef]
- Fahriani, M.; Ilmawan, M.; Fajar, J.K.; Maliga, H.A.; Frediansyah, A.; Masyeni, S.; Yusuf, H.; Nainu, F.; Rosiello, F.; Sirinam, S.; et al. Persistence of Long COVID Symptoms in COVID-19 Survivors Worldwide and Its Potential Pathogenesis—A Systematic Review and Meta-Analysis. Narra J. 2021, 1, e36. [Google Scholar] [CrossRef]
- Haverty, R.; McCormack, J.; Evans, C.; Purves, K.; O’Reilly, S.; Gautier, V.; Rochfort, K.; Fabre, A.; Fletcher, N.F. SARS-CoV-2 Infects Neurons, Astrocytes, Choroid Plexus Epithelial Cells and Pericytes of the Human Central Nervous System In Vitro. J. Gen. Virol. 2024, 105, 002009. [Google Scholar] [CrossRef]
- Greene, C.; Connolly, R.; Brennan, D.; Laffan, A.; O’Keeffe, E.; Zaporojan, L.; O’Callaghan, J.; Thomson, B.; Connolly, E.; Argue, R.; et al. Blood–Brain Barrier Disruption and Sustained Systemic Inflammation in Individuals with Long COVID-Associated Cognitive Impairment. Nat. Neurosci. 2024, 27, 421–432. [Google Scholar] [CrossRef]
- Rong, Z.; Mai, H.; Ebert, G.; Kapoor, S.; Puelles, V.G.; Czogalla, J.; Hu, S.; Su, J.; Prtvar, D.; Singh, I.; et al. Persistence of Spike Protein at the Skull-Meninges-Brain Axis May Contribute to the Neurological Sequelae of COVID-19. Cell Host Microbe 2024, 32, 2112–2130.e10. [Google Scholar] [CrossRef]
- Higgins, V.; Sohaei, D.; Diamandis, E.P.; Prassas, I. COVID-19: From an Acute to Chronic Disease? Potential Long-Term Health Consequences. Crit. Rev. Clin. Lab. Sci. 2021, 58, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 Long-Term Effects of COVID-19: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.A.; Safavynia, S.A.; Evered, L.A. The ‘Third Wave’: Impending Cognitive and Functional Decline in COVID-19 Survivors. Br. J. Anaesth. 2021, 126, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Bruno, D.; Fernandez, V.; Portillo, F.; Llarena Nuñez, S. Valores normativos para el Cuestionario de Quejas Cognitivas para adultos entre 30 y 90 años. Neurol. Argent. 2023, 15, 271–278. [Google Scholar] [CrossRef]
- Ostrosky-Solís, F.; Esther Gómez-Pérez, M.; Matute, E.; Rosselli, M.; Ardila, A.; Pineda, D. NEUROPSI ATTENTION AND MEMORY: A Neuropsychological Test Battery in Spanish with Norms by Age and Educational Level. Appl. Neuropsychol. 2007, 14, 156–170. [Google Scholar] [CrossRef]
- Flores, J.C.; Castillo-Preciado, R.E.; Jiménez-Miramonte, N.A. Desarrollo de funciones ejecutivas, de la niñez a la juventud. Ann. Psychol. 2014, 30, 463–473. [Google Scholar] [CrossRef]
- García-Molina, A.; García-Carmona, S.; Espiña-Bou, M.; Rodríguez-Rajo, P.; Sánchez-Carrión, R.; Enseñat-Cantallops, A. Rehabilitación neuropsicológica en el síndrome post-COVID-19: Resultados de un programa clínico y seguimiento a los 6 meses. Neurología 2022, 39, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Altuna, M.; Sánchez-Saudinós, M.B.; Lleó, A. Cognitive Symptoms after COVID-19. Neurol. Perspect. 2021, 1, S16–S24. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent Fatigue Following SARS-CoV-2 Infection Is Common and Independent of Severity of Initial Infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Townsend, L.; Dowds, J.; O’Brien, K.; Sheill, G.; Dyer, A.H.; O’Kelly, B.; Hynes, J.P.; Mooney, A.; Dunne, J.; Ni Cheallaigh, C.; et al. Persistent Poor Health After COVID-19 Is Not Associated with Respiratory Complications or Initial Disease Severity. Ann. Am. Thorac. Soc. 2021, 18, 997–1003. [Google Scholar] [CrossRef]
- Casasola-Rivera, W. La importancia de la psicoeducación en la intervención de personas adultas mayores con trastorno neurocognitivo leve. Rev. Costarric. De Psicol. 2024, 43, 1–13. [Google Scholar] [CrossRef]
- Stanton, J.D.; Sebesta, A.J.; Dunlosky, J. Fostering Metacognition to Support Student Learning and Performance. CBE—Life Sci. Educ. 2021, 20, fe3. [Google Scholar] [CrossRef]
- Paz-Rodríguez, F.; Lozano-Tovar, S.; Rodríguez-Agudelo, Y.; Cruz-Narciso, B.; Rodríguez-Rodríguez, M.; García-Santos, A.; López-González, D.; Soto-Moreno, F.-J.; González-Navarro, M.; González-Alonso, K.; et al. Assessment of Visuospatial Functions in Post-COVID 19 Patients: Beyond the Traditional Paradigm. Behav. Brain Res. 2024, 471, 115095. [Google Scholar] [CrossRef]
- Becker, J.H.; Lin, J.J.; Doernberg, M.; Stone, K.; Navis, A.; Festa, J.R.; Wisnivesky, J.P. Assessment of Cognitive Function in Patients After COVID-19 Infection. JAMA Netw. Open 2021, 4, e2130645. [Google Scholar] [CrossRef]
- Zhou, H.; Lu, S.; Chen, J.; Wei, N.; Wang, D.; Lyu, H.; Shi, C.; Hu, S. The Landscape of Cognitive Function in Recovered COVID-19 Patients. J. Psychiatr. Res. 2020, 129, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Beaud, V.; Crottaz-Herbette, S.; Dunet, V.; Vaucher, J.; Bernard-Valnet, R.; Du Pasquier, R.; Bart, P.-A.; Clarke, S. Pattern of Cognitive Deficits in Severe COVID-19. J. Neurol. Neurosurg. Psychiatry 2021, 92, 567–568. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The Diagnosis of Dementia Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; Leurgans, S.E.; Boyle, P.A.; Bennett, D.A. Cognitive Decline in Prodromal Alzheimer Disease and Mild Cognitive Impairment. Arch. Neurol. 2011, 68, 351–356. [Google Scholar] [CrossRef]
- Damasio, A.R.; Everitt, B.J.; Bishop, D. The Somatic Marker Hypothesis and the Possible Functions of the Prefrontal Cortex. Philos. Trans. R. Soc. Lond. B 1996, 351, 1413–1420. [Google Scholar] [CrossRef]
- Bechara, A. Emotion, Decision Making and the Orbitofrontal Cortex. Cereb. Cortex 2000, 10, 295–307. [Google Scholar] [CrossRef]
- Daroische, R.; Hemminghyth, M.S.; Eilertsen, T.H.; Breitve, M.H.; Chwiszczuk, L.J. Cognitive Impairment After COVID-19—A Review on Objective Test Data. Front. Neurol. 2021, 12, 699582. [Google Scholar] [CrossRef]
- Woo, M.S.; Malsy, J.; Pöttgen, J.; Seddiq Zai, S.; Ufer, F.; Hadjilaou, A.; Schmiedel, S.; Addo, M.M.; Gerloff, C.; Heesen, C.; et al. Frequent Neurocognitive Deficits after Recovery from Mild COVID-19. Brain Commun. 2020, 2, fcaa205. [Google Scholar] [CrossRef]
- Davies, S.R.; Field, A.R.J.; Andersen, T.; Pestell, C. The Ecological Validity of the Rey–Osterrieth Complex Figure: Predicting Everyday Problems in Children with Neuropsychological Disorders. J. Clin. Exp. Neuropsychol. 2011, 33, 820–831. [Google Scholar] [CrossRef]
- Robledo-Castro, C.; Ramírez-Suarez, G.R.; Rodríguez-Rodríguez, L.H. Effects of Computer-Based Cognitive Training vs. Paper-and-Pencil-Based Training on the Cognitive Development of Typically Developing Children: Protocol for a Randomized Controlled Trial. MethodsX 2024, 13, 102877. [Google Scholar] [CrossRef]
| Variable | Subjects (n = 18) | Controls (n = 15) |
|---|---|---|
| Sex | ||
| Female | 13 (72.22) | 10 (66.67) |
| Male | 5 (27.78) | 5 (33.33) |
| Age (Mean ± SD) | 47.94 ± 11.12 | 47.80 ± 10.09 |
| Manual Dominance (Right-Handed) | 17 | 15 |
| Comorbidities | ||
| Overweight | 7 (38.89) | 2 (13.33) |
| Diabetes Type 1 | 1 (5.55) | 1 (6.67) |
| Hypertension | 1 (5.55) | 0 |
| COVID-19 Symptoms | ||
| Cough | 9 (50.00) | 5 (33.33) |
| Headache | 13 (72.22) | 9 (60.00) |
| Joint Pain | 12 (66.67) | 5 (33.33) |
| Anosmia | 10 (55.56) | 4 (26.67) |
| Ageusia | 9 (50.00) | 4 (29.67) |
| Diarrhea | 5 (27.78) | 2 (13.33) |
| Shortness of Breath | 3 (16.67) | 2 (13.33) |
| Generalized Pain | 13 (72.22) | 4 (26.67) |
| Chest Pain | 5 (27.78) | 7 (46.67) |
| Throat Pain | 9 (50.00) | 8 (53.33) |
| Domain | Control | Pre | Post | p | Normative Values |
|---|---|---|---|---|---|
| Attention | 2.04 ± 0.58 | 3.73 ± 0.53 | 2.79 ± 0.53 | <0.001 | 4.73 ± 3.286 |
| Orientation | 1.33 ± 0.44 | 1.89 ± 0.49 | 1.46 ± 0.38 | 0.04 | 0.76 ± 1.048 |
| Executive function | 1.92 ± 0.82 | 2.85 ± 0.64 | 1.88 ± 0.58 | <0.001 | 2.44 ± 2.892 |
| Memory | 1.83 ± 0.75 | 3.35 ± 0.63 | 2.21 ± 0.64 | <0.001 | 4.13 ± 3.892 |
| Praxis and gnosis | 1.17 ± 0.20 | 2.42 ± 0.62 | 1.69 ± 0.67 | 0.005 | 1.66 ± 1.919 |
| Language | 1.42 ± 0.38 | 2.77 ± 0.91 | 1.88 ± 0.42 | 0.001 | 3.30 ± 2.856 |
| Total | 9.71 ± 2.75 | 17.00 ± 2.66 | 11.88 ± 2.024 | <0.001 | 16.90 ± 11.948 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotor-Llerena, A.L.; Reyes-Long, S.; Najera-García, L.; Hernández-Corral, S.; Arechaga-Ocampo, E.; Avendaño-Ortiz, K.; Trejo-Martínez, D.; Rosell-Becerril, H.; Cortes-Altamirano, J.L.; Alfaro-Rodríguez, A. The Effect of Neurorehabilitation of the Cognitive Symptoms of Long COVID Evaluated with Neuropsi Atención y Memoria-III and BANFE-III. Biomedicines 2025, 13, 2267. https://doi.org/10.3390/biomedicines13092267
Dotor-Llerena AL, Reyes-Long S, Najera-García L, Hernández-Corral S, Arechaga-Ocampo E, Avendaño-Ortiz K, Trejo-Martínez D, Rosell-Becerril H, Cortes-Altamirano JL, Alfaro-Rodríguez A. The Effect of Neurorehabilitation of the Cognitive Symptoms of Long COVID Evaluated with Neuropsi Atención y Memoria-III and BANFE-III. Biomedicines. 2025; 13(9):2267. https://doi.org/10.3390/biomedicines13092267
Chicago/Turabian StyleDotor-Llerena, Ana Lilia, Samuel Reyes-Long, Leilani Najera-García, Sandra Hernández-Corral, Elena Arechaga-Ocampo, Karina Avendaño-Ortiz, David Trejo-Martínez, Humberto Rosell-Becerril, Jose Luis Cortes-Altamirano, and Alfonso Alfaro-Rodríguez. 2025. "The Effect of Neurorehabilitation of the Cognitive Symptoms of Long COVID Evaluated with Neuropsi Atención y Memoria-III and BANFE-III" Biomedicines 13, no. 9: 2267. https://doi.org/10.3390/biomedicines13092267
APA StyleDotor-Llerena, A. L., Reyes-Long, S., Najera-García, L., Hernández-Corral, S., Arechaga-Ocampo, E., Avendaño-Ortiz, K., Trejo-Martínez, D., Rosell-Becerril, H., Cortes-Altamirano, J. L., & Alfaro-Rodríguez, A. (2025). The Effect of Neurorehabilitation of the Cognitive Symptoms of Long COVID Evaluated with Neuropsi Atención y Memoria-III and BANFE-III. Biomedicines, 13(9), 2267. https://doi.org/10.3390/biomedicines13092267






